Ultra-Uniform and Very Thin Ag Nanowires Synthesized via the Synergy of Cl−, Br− and Fe3+ for Transparent Conductive Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AgNWs and Preparation of TCFs
2.3. Characterization
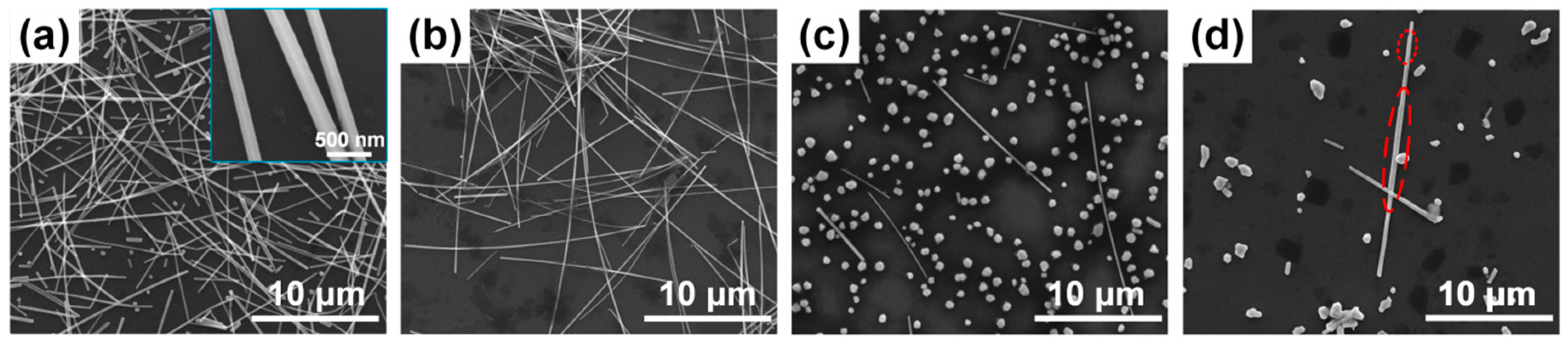
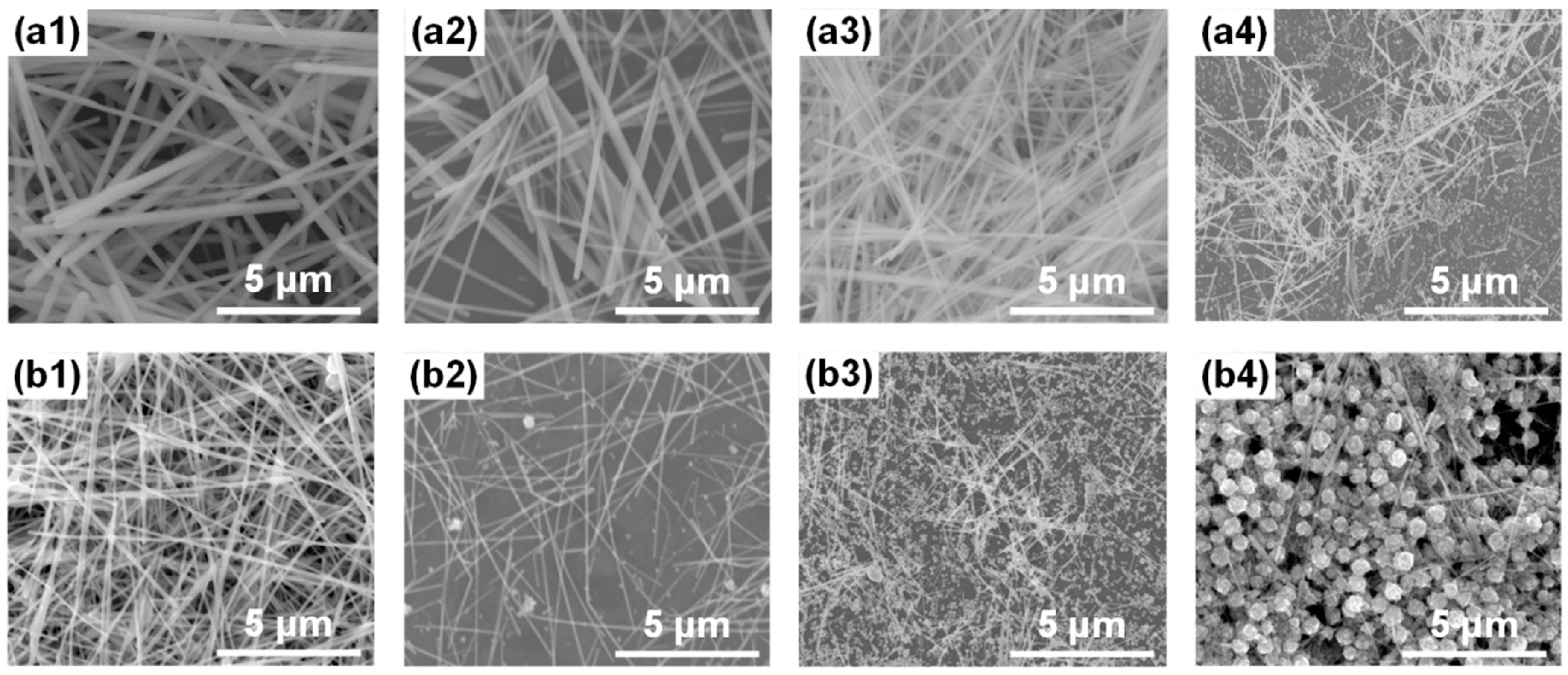
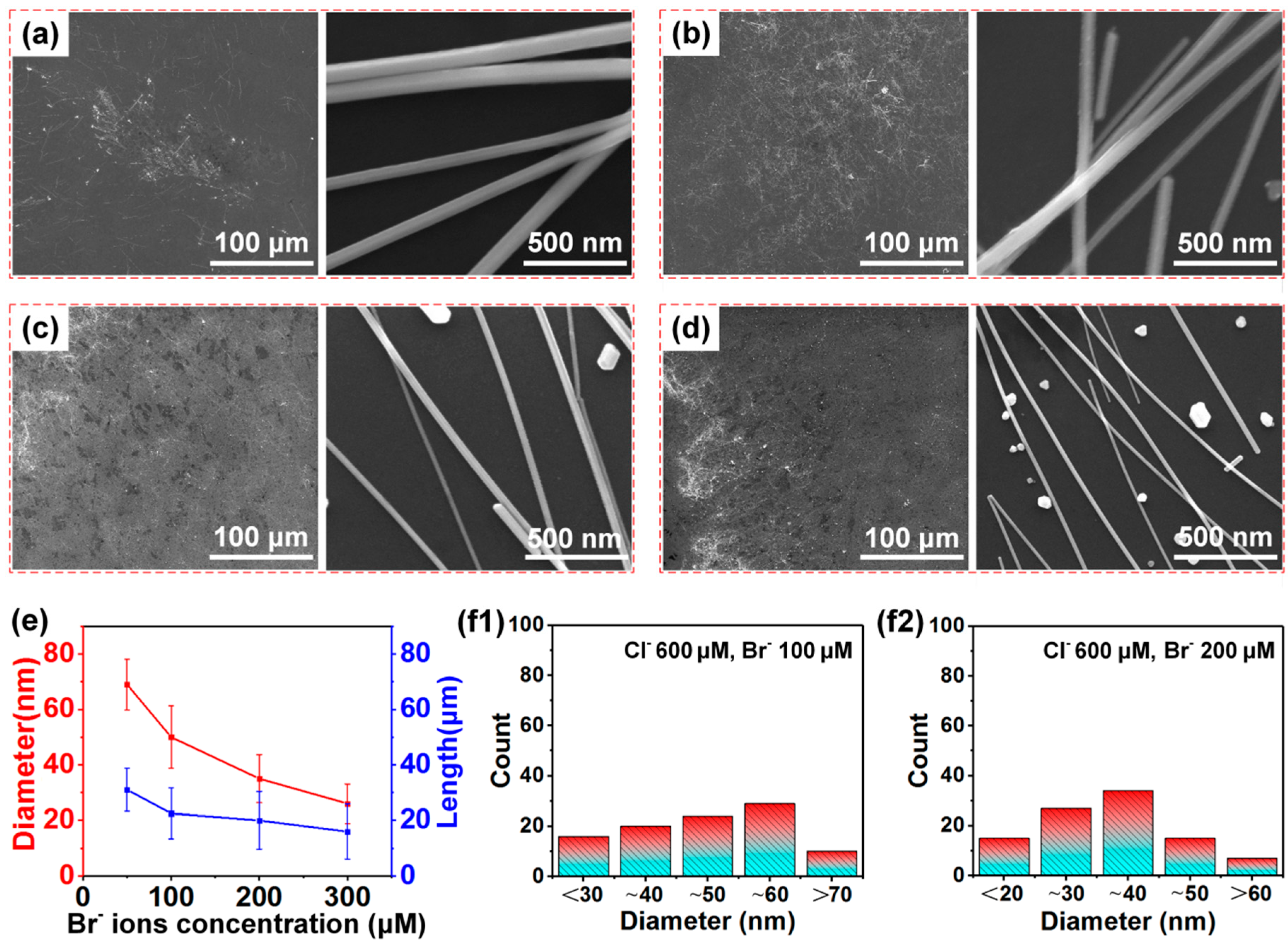
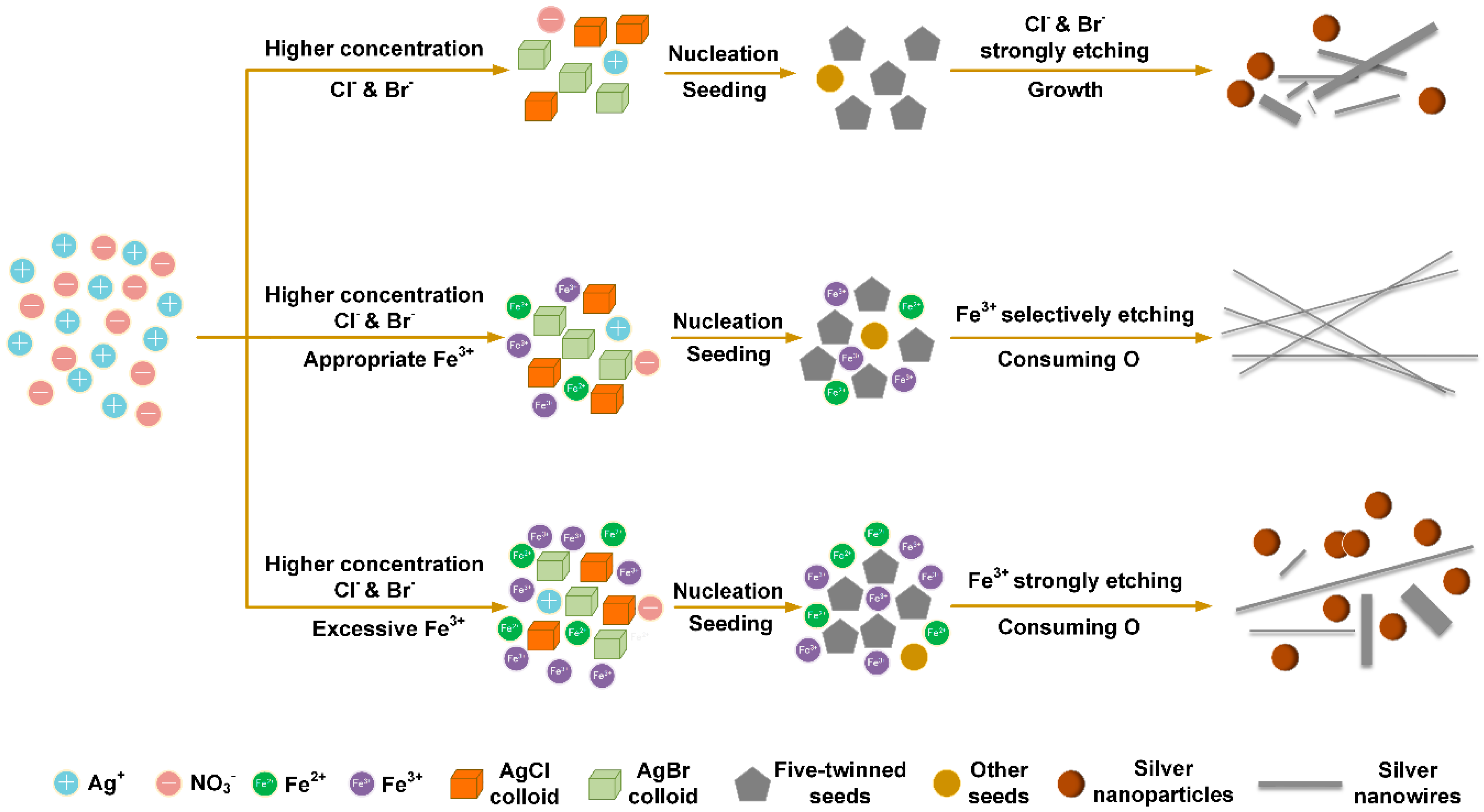
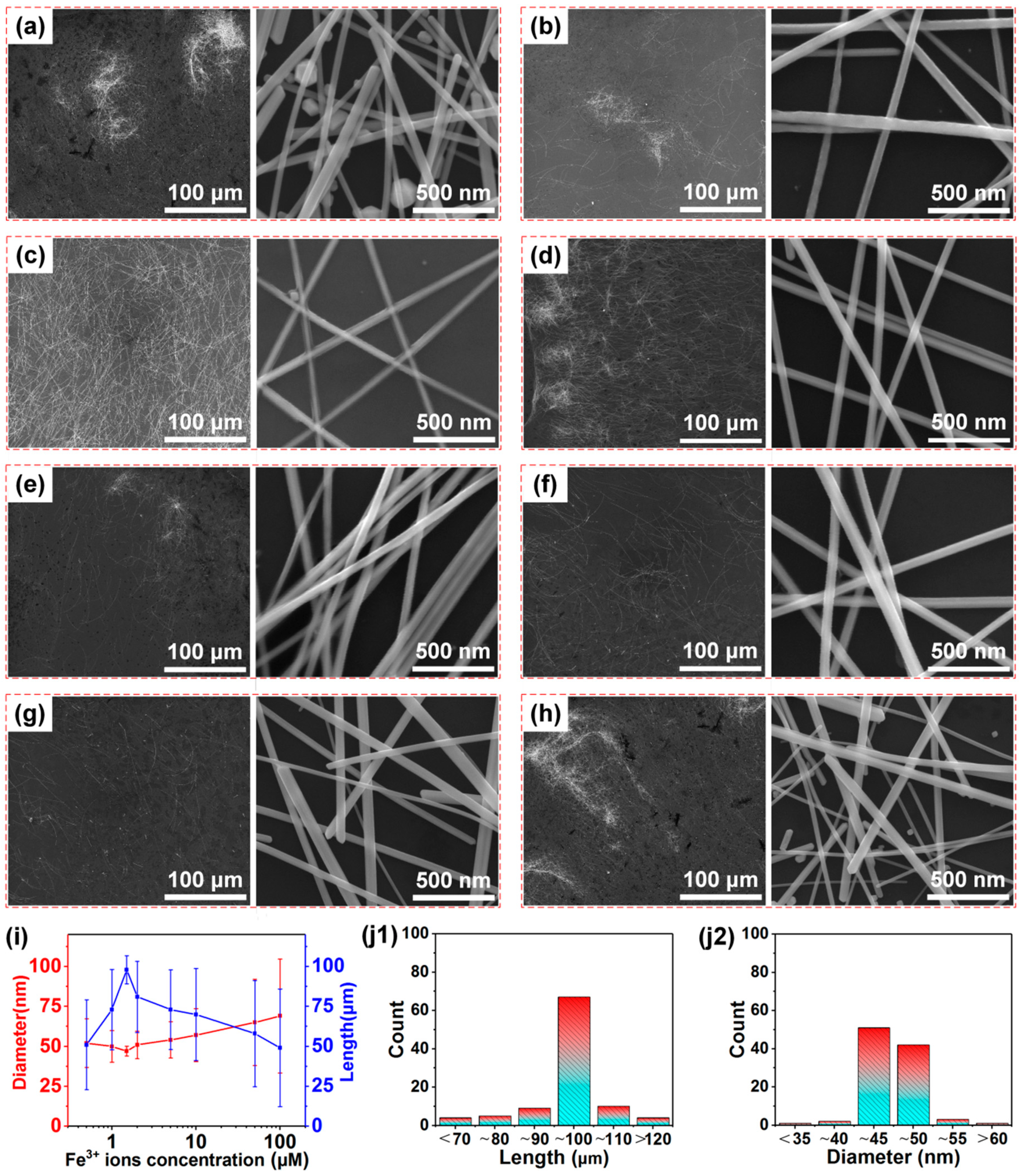
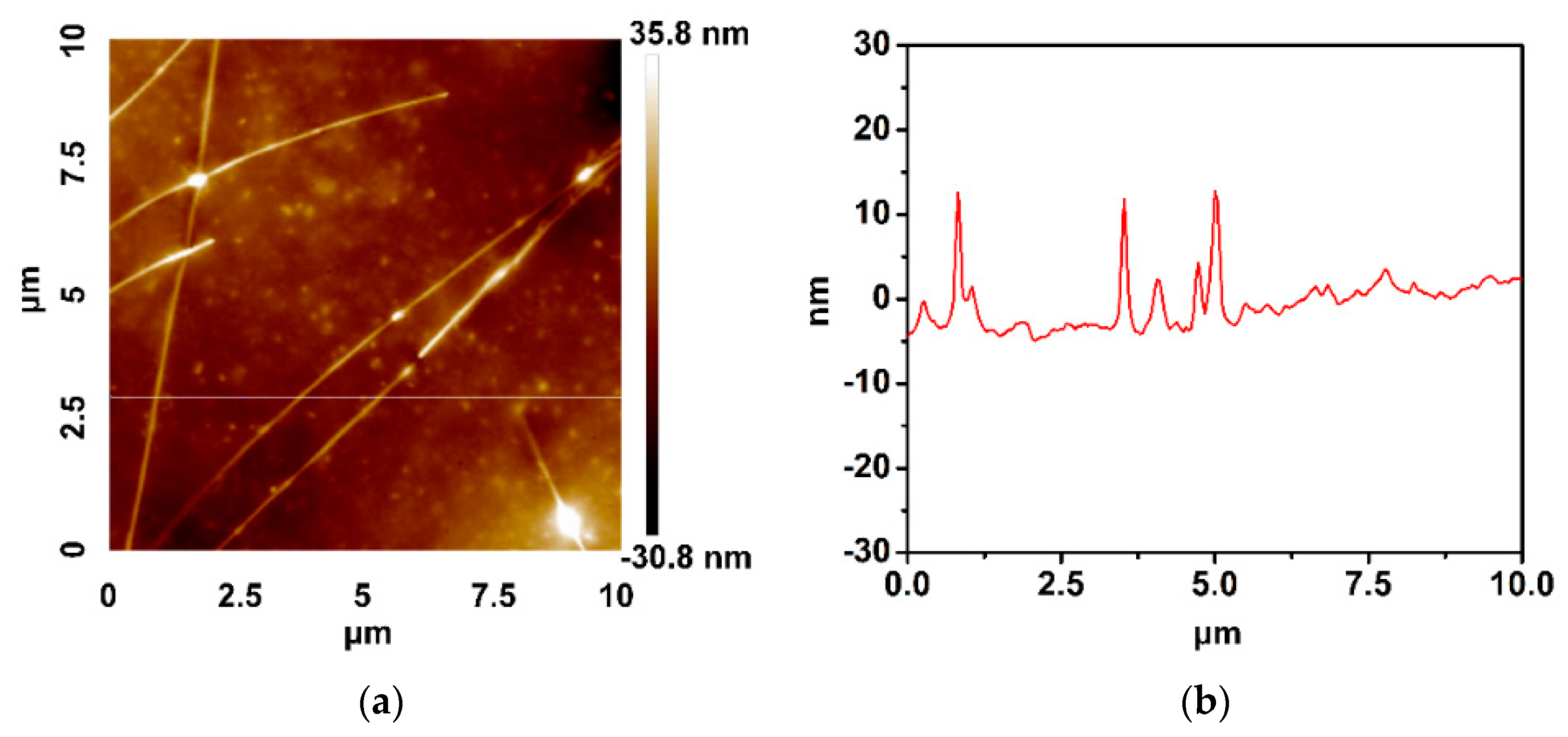
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Guo, J.N.; Xu, D.; Sun, Y.; Yan, F. One-pot synthesis and purification of ultralong silver nanowires for flexible transparent conductive electrodes. ACS Appl. Mater. Interfaces 2017, 9, 25465–25473. [Google Scholar] [CrossRef]
- Steega, E.; Kirmsea, H.; Rabea, J.P.; Kirsteina, S. Silver iodide nanowires grown within tubular J-aggregates. J. Colloid. Interf. Sci. 2018, 503, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, P.; Wang, L. Highly bendable, conductive, and transparent film by an enhanced adhesion of silver nanowires. ACS Appl. Mater. Interfaces 2013, 5, 9155–9160. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Ellis, A.V.; Voelcker, N.H. Electrochemical synthesis of silver oxide nanowires, microplatelets and application as SERS substrate precursors. Electrochim. Acta 2012, 59, 346–353. [Google Scholar] [CrossRef]
- Wang, D.M.; Hua, H.M.; Liu, Y.; Tang, H.R.; Li, Y.X. Single Ag nanowire electrodes and single Pt@Ag nanowire electrodes: Fabrication, electrocatalysis, and surface-enhanced raman scattering applications. Anal. Chem. 2019, 91, 4291–4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, E.H.; Mun, C.W.; Kim, C.T.; Park, S.G.; Choi, E.J.; Kim, S.H.; Dang, J.; Choo, J.; Oh, J.W.; Kim, D.H.; et al. M13 bacteriophage/silver nanowire surface-enhanced raman scattering sensor for sensitive and selective pesticide detection. ACS Appl. Mater. Interfaces 2018, 10, 10388–10397. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, I.; Harada, Y. Optical and surface-enhanced raman scattering properties of black and metallic silver micro/nano structures fabricated on cicada wings by silver mirror reaction. J. Nanosci. Nanotechnol. 2019, 19, 7853–7858. [Google Scholar] [CrossRef]
- Zhu, R.; Chung, C.H.; Cha, K.C.; Yang, W.B.; Zheng, Y.B.; Zhou, H.P.; Song, T.B.; Chen, C.C.; Weiss, P.S.; Li, G.; et al. Fused silver nanowires with metal oxide nanoparticles and organic polymers for highly transparent conductors. ACS Nano 2011, 5, 9877–9882. [Google Scholar] [CrossRef]
- Wang, Y.H.; Du, D.X.; Yang, X.; Zhang, X.F.; Zhao, Y.Z. Optoelectronic and electrothermal properties of transparent conductive silver nanowires films. Nanomaterials 2019, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.B.; Luo, J.; Liu, Y.S.; Zhong, Y.C.; Wang, J.; Zhang, Y. Highly performance flexible polymer solar cells by flipping the bilayer film of Ag nanowires and polyvinyl alcohol on polyethylene terephthalate as transparent conductive electrodes. IEEE J. Photovolt. 2019, 9, 710–714. [Google Scholar] [CrossRef]
- Chu, X.K.; Wang, K.; Tao, J.Q.; Li, S.X.; Ji, S.L.; Ye, C.H. Tackling the stability issues of silver nanowire transparent conductive films through FeCl3 dilute solution treatment. Nanomaterials 2019, 9, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Du, D.X.; Wang, Y.H. Length-dependent electro-optical properties of silver nanowires-based transparent conducting films. J. Mater. Sci. - Mater. Electron. 2019, 30, 6838–6845. [Google Scholar] [CrossRef]
- Kurowska, E.; Brzozka, A.; Jarosz, M.; Sulka, G.D.; Jaskula, M. Silver nanowire array sensor for sensitive and rapid detection of H2O2. Electrochim. Acta 2013, 104, 439–447. [Google Scholar] [CrossRef]
- Hu, W.L.; Niu, X.N.; Zhao, R.; Pei, Q.B. Elastomeric transparent capacitive sensors based on an interpenetrating composite of silver nanowires and polyurethane. Appl. Phys. Lett. 2013, 102, 083303. [Google Scholar] [CrossRef]
- Shi, R.L.; Lou, Z.; Chen, S. Flexible and transparent capacitive pressure sensor with patterned microstructured composite rubber dielectric for wearable touch keyboard application. Sci. Chin. Mater. 2018, 61, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Xuan, S.H.; Ding, L. Stretchable and magneto-sensitive strain sensor based on silver nanowire-polyurethane sponge enhanced magnetorheological elastomer. Mater. Design 2018, 156, 528–537. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.S.; Sharma, A. Transparent AgNW-CoNPs conducting film for heat sensor. Microelectron. Eng. 2019, 205, 37–43. [Google Scholar] [CrossRef]
- Finn, D.J.; Lotya, M.; Coleman, J.N. Inkjet printing of silver nanowire networks. ACS Appl. Mater. Interfaces 2015, 7, 9254–9261. [Google Scholar] [CrossRef]
- Liang, X.W.; Zhao, T.; Jiang, W.; Yu, X.C.; Hu, Y.G.; Zhu, P.L.; Zheng, H.R.; Sun, R.; Wong, C.P. Highly transparent triboelectric nanogenerator utilizing in-situ chemically welded silver nanowire network as electrode for mechanical energy harvesting and body motion monitoring. Nano Energy 2019, 59, 508–516. [Google Scholar] [CrossRef]
- Kim, S.W.; Kwon, S.N.; Na, S.I. Stretchable and electrically conductive polyurethane-silver/graphene composite fibers prepared by wet-spinning process. Compos. Part. B-Eng. 2019, 167, 573–581. [Google Scholar] [CrossRef]
- Dudem, B.; Kim, D.H.; Bharat, L.K.; Yu, J.S. Highly-flexible piezoelectric nanogenerators with silver nanowires and barium titanate embedded composite films for mechanical energy harvesting. Appl. Energ. 2018, 230, 865–874. [Google Scholar] [CrossRef]
- Kim, I.; Jeon, H.; Kim, D.; You, J.M.; Kim, D.W. All-in-one cellulose based triboelectric nanogenerator for electronic paper using simple filtration process. Nano Energy 2018, 53, 975–981. [Google Scholar] [CrossRef]
- Bergin, S.M.; Chen, Y.H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagrange, M.; Langley, D.P.; Giusti, G.; Jimeneza, C.; Bréchetd, Y.; Bellet, D. Optimization of silver nanowire-based transparent electrodes: Effects of density, size and thermal annealing. Nanoscale 2015, 7, 17410–17423. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.D.; Zhu, Q.S.; Zhao, Y. Flexible silver nanowire transparent conductive films prepared by an electrostatic adsorption self-assembly process. J. Mater. Sci. 2019, 54, 5802–5812. [Google Scholar] [CrossRef]
- Wang, H.A.; Wang, Y.; Chen, X.M. Synthesis of uniform silver nanowires from AgCl seeds for transparent conductive films via spin-coating at variable spin-speed. Colloid. Surface. A 2019, 565, 154–161. [Google Scholar] [CrossRef]
- Li, Y.X.; Yuan, X.M.; Yang, H.W.; Chao, Y.X.; Guo, S.L.; Wang, C. One-step synthesis of silver nanowires with ultra-long length and thin diameter to make flexible transparent conductive films. Materials 2019, 12, 401. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, M.; Matsumoto, K.; Miyamae, N.; Tsuji, T.; Zhang, X. Rapid preparation of silver nanorods and nanowires by a microwave-polyol method in the presence of Pt catalyst and polyvinylpyrrolidone. Cryst. Growth. Des. 2006, 7, 311–320. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, B.S.; Zhang, H.C.; Wan, P.B.; Luo, L.; Kuang, Y. Probing the seeded protocol for high-concentration preparation of silver nanowires. Nano Res. 2016, 9, 1532–1542. [Google Scholar] [CrossRef]
- Tang, Y.X.; Jiang, Z.L.; Deng, J.Y.; Gong, D.G.; Lai, Y.K.; Tay, H.T.; Joo, L.T.; Lau, T.H.; Dong, Z.L.; Chen, Z. Synthesis of nanostructured silver/silver halides on titanate surfaces and their visible-light photocatalytic performance. ACS Appl. Mater. Interfaces 2012, 4, 438–446. [Google Scholar] [CrossRef]
- Zhan, K.; Su, R.; Bai, S.H.; Yu, Z.H.; Cheng, N.; Wang, C.L.; Xu, S.; Liu, W.; Guo, S.S.; Zhao, X.Z. One-pot stirring-free synthesis of silver nanowires with tunable lengths and diameters via a Fe3+ & Cl- co-mediated polyol method and their application as transparent conductive films. Nanoscale 2016, 8, 18121–18133. [Google Scholar] [PubMed]
- Liu, C.; Wang, B.J.; Han, T.; Shi, D.M.; Wang, G.F. Fe foil-guided fabrication of uniform Ag@AgX nanowires for sensitive detection of leukemia DNA. ACS Appl. Mater. Interfaces 2019, 11, 4820–4825. [Google Scholar] [CrossRef] [PubMed]
- Schuette, W.M.; Buhro, W.E. Silver chloride as a heterogeneous nucleant for the growth of silver nanowires. ACS Nano 2019, 7, 3844–3853. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Shang, T.M.; Zhou, Q.F.; Feng, K. Sodium chloride assisted synthesis of silver nanowires. Micro. Nano. Lett. 2011, 6, 90. [Google Scholar] [CrossRef]
- Zhang, K.L.; Du, Y.G.; Chen, S.M. Sub 30nm silver nanowire synthesized using KBr as co-nucleant through one-pot polyol method for optoelectronic applications. Org. Electron. 2015, 26, 380–385. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.T.; Jin, L.F. Solution-possessed vertical photodetectors based on composition dependent cesium lead halide (CsPbX3, X=Cl, Br, and I) perovskite quantum dots. Opt. Comp. Mater. 2019, 10914. [Google Scholar]
- Wan, M.J.; Tao, J.Q.; Jia, D.; Chu, X.K.; Li, S.X.; Ji, S.L.; Ye, C.H. High-yield very thin silver nanowires obtained by ostwald ripening-driven coarsening and sedimentation of nanoparticles. Cryst. Eng. Comm. 2018, 20, 2834–2840. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.L.; Ou, M.; Huang, Z.Z.; Lin, S.D.; Tu, Y.Y.; Hu, J.W. Behind the role of bromide ions in the synthesis of ultrathin silver nanowires. Mater. Lett. 2018, 213, 23–26. [Google Scholar] [CrossRef]
- Lei, B.W.; Wang, J.; Du, Y.G.; Zhang, K.L. Controlling the size of silver nanowires through one-pot polyol method with trace halide and its effect on kinetic process. Mater. Res. Express. 2017, 4, 551–561. [Google Scholar] [CrossRef]
- Wiley, B.J.; Sun, Y.G.; Xia, Y.N. Synthesis of silver nanostructures with controlled shape and properties. Acc. Chem. Res. 2007, 40, 1067–1076. [Google Scholar] [CrossRef]
- Wiley, B.J.; Xiong, Y.; Li, Z.Y.; Yin, Y.D.; Xia, Y.N. Right bipyramids of silver: A new shape derived from single twinned seeds. Nano Lett. 2006, 6, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.M.; Sun, Y.G.; Xia, Y.N. Polyol synthesis of silver nanostructures control of product morphology with Fe(II) or Fe(III) species. Langmur 2005, 21, 8076–8080. [Google Scholar] [CrossRef] [PubMed]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y.N. Rapid synthesis of silver nanowires through a CuCl-or CuCl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhan, M.S. Rapid production of silver nanowires based on high concentration of AgNO3 precursor and use of FeCl3 as reaction promoter. RSC Adv. 2014, 4, 21060. [Google Scholar] [CrossRef]
- Johan, M.R.; Aznan, N.A.K.; Yee, S.T.; Ho, L.H.; Ooi, S.W.; Singho, N.D.; Aplop, F. Synthesis and growth mechanism of silver nanowires through different mediated agents (CuCl2 and NaCl) polyol process. J. Nanomater. 2014, 13, 1–7. [Google Scholar] [CrossRef]
- Amirjani, A.; Marashi, P.; Fatmehsari, D.H. Effect of AgNO3 addition rate on aspect ratio of CuCl2-mediated synthesized silver nanowires using response surface methodology. Colloid. Surf. A 2014, 444, 33–39. [Google Scholar] [CrossRef]
- Chen, J.J.; Liu, S.L.; Wu, H.B.; Sowade, E.; Baumann, R.R.; Wang, Y.; Gu, F.Q.; Liu, S.L.; Feng, Z.S. Structural regulation of silver nanowires and their application in flexible electronic thin films. Mater. Design 2018, 154, 266–274. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, P.; Liu, D.F.; Yuan, H.J.; Yan, X.Q.; Zhou, Z.P.; Wang, J.X.; Song, L.; Liu, L.F.; Zhou, W.Y.; et al. Evidence for the monolayer assembly of poly (vinylpyrrolidone) on the surfaces of silver. J. Phys. Chem. B 2004, 108, 12877–12881. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol synthesis of silver nanowires: An extensive parametric study. Cryst. Growth. Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- Chang, S.; Chen, S.; Hua, Q.; Ma, Y.; Huang, W.J. Evidence for the growth mechanisms of silver nanocubes and nanowires. J. Phys. Chem. C 2011, 115, 7979–7986. [Google Scholar] [CrossRef]
- Hu, M.; Gao, J.; Dong, Y.; Yang, S.; Li, R.K.Y. Rapid controllable high-concentration synthesis and mutual attachment of silver nanowires. RSC Adv. 2012, 2, 2055–2060. [Google Scholar] [CrossRef]
- Oliveira, C.C.S.; Ando, R.A.; Camargo, P.H.C. Size-controlled synthesis of silver micro/nanowires as enabled by HCl oxidative etching. Phys. Chem. Chem. Phys. 2013, 15, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Lee, Y.T.; Wiley, B.; Xia, Y. Large scale synthesis of silver nanocubes: The role of HCl in promoting cube perfection and monodispersity. Angew. Chem. Int. Ed. 2005, 44, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Chipara, M.; Zaleski, J.M. Convenient rapid synthesis of silver nanowires. Chem. Mater. 2007, 19, 1755–1760. [Google Scholar] [CrossRef]
- Lee, E.J.; Chang, M.H.; Kim, Y.S.; Kim, J.Y. High-pressure polyol synthesis of ultrathin silver nanowires: Electrical and optical properties. APL Mater. 2013, 1, 042118. [Google Scholar] [CrossRef] [Green Version]
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y.N. Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- De Silva, R.R.; Yang, M.; Choi, S.I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.Y.; Camargo, P.H.C.; Ribeiro, S.J.L.; Xia, Y. Facile synthesis of sub-20 nm silver nanowires through a bromide-mediated polyol method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef]
- Jiu, J.; Murai, K.; Kim, D.; Kim, K.; Suganuma, K. Preparation of Ag nanorods with high yield by polyol process. Mater. Chem. Phys. 2009, 114, 333–338. [Google Scholar] [CrossRef]
- Jiang, P.; Li, S.Y.; Xie, S.S.; Gao, Y.; Song, Y. Machinable long PVP-stabilized silver nanowires. Chem. Eur. J. 2004, 10, 4817–4821. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.W.; Chen, X.Y.; Wan, J.X.; Qian, Y.T. A simple hydrothermal route to large-scale synthesis of uniform silver nanowires. Chem. Eur. J. 2005, 11, 160–163. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Xu, F.; Xu, W.; Mao, B.X.; Shen, W.F.; Yu, Y.; Tan, R.Q.; Song, W.J. Preparation and cold welding of silver nanowire based transparent electrodes with optical transmittances >90% and sheet resistances <10 ohm/sq. J. Colloid. Interf. Sci. 2018, 512, 201–208. [Google Scholar]
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver nanowire networks as flexible, transparent, conducting films: Extremely high DC to optical conductivity ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hecht, D.S.; Gruner, G. Percolation in transparent and conducting carbonnanotube networks. Nano Lett. 2004, 4, 2513–2517. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Grüner, G. A method of printing carbon nanotube thin films. Appl. Phys. Lett. 2006, 88, 123109. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, P.; Lee, D.G.; Lee, S.S.; Ko, S.H. Large-scale synthesis and characterization of very long silver nanowires via successive multistep growth. Cryst. Growth. Des. 2012, 12, 5598–5605. [Google Scholar] [CrossRef]
- Jiu, J.; Araki, T.; Wang, J.; Nagao, S.; Koga, H.; Suganuma, K.; Nakazawa, E.; Hara, M.; Uchidab, H.; Shinozakic, K. Facile synthesis of very-long silver nanowires for transparent electrodes. J. Mater. Chem. 2014, 2, 6326–6330. [Google Scholar] [CrossRef]
- Lee, B.; Ye, S.R.; Stewart, L.E.; Alvarez, S.; Wiley, B.J. Synthesis and purification of silver nanowires to make conducting films with a transmittance of 99%. Nano Lett. 2015, 15, 6722–6726. [Google Scholar]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved thermal oxidationStability of solution-processable silver nanowire transparent electrode by reduced graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, H.; Hur, K.; Kim, W.C.; Lee, H. 2D graphene oxide nanosheets as an adhesive over-coating layer for flexible transparent conductive electrodes. Sci. Rep. 2013, 3, 1112. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.S.; Niec, A.; Carmichael, R.S.; Carmichael, T.B. Silver nanowire/optical adhesive coatings as transparent electrodes for flexible electronics. ACS Appl. Mater. Interfaces 2013, 5, 10165–10172. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, W.; Burkhard, G.F.; McGehee, M.D.; Peumans, P. Smooth nanowire/polymer composite transparent electrodes. Adv. Mater. 2011, 23, 2909–2910. [Google Scholar]
- Hauger, T.C.; Al-Rafia, S.M.I.; Buriak, J.M. Rolling silver nanowire electrodes: Simultaneously addressing adhesion, roughness, and conductivity. ACS Appl. Mater. Interface 2013, 5, 12663–12671. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.X.; Pi, Z.C.; Chen, C.Y.; Shen, X.Q. Scalable fabrication of silver nanowires-polyethylene terephthalate transparent conductive films by rolling-pressing transfer technique at room temperature. Sci. Adv. Mater. 2015, 7, 1599–1604. [Google Scholar] [CrossRef]
- Ye, N.; Yan, J.L.; Xie, S.; Kong, Y.H.; Liang, T.; Chen, H.Z.; Xu, M.S. Silver nanowires-graphene hybrid transparent conductive electrodes for highly efficientinverted organic solar cells. Nanotechnology 2017, 28, 305402. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-M.; Chen, L.; Sowade, E.; Rodriguez, R.D.; Sheremet, E.; Yu, C.-M.; Baumann, R.R.; Chen, J.-J. Ultra-Uniform and Very Thin Ag Nanowires Synthesized via the Synergy of Cl−, Br− and Fe3+ for Transparent Conductive Films. Nanomaterials 2020, 10, 237. https://doi.org/10.3390/nano10020237
Wang X-M, Chen L, Sowade E, Rodriguez RD, Sheremet E, Yu C-M, Baumann RR, Chen J-J. Ultra-Uniform and Very Thin Ag Nanowires Synthesized via the Synergy of Cl−, Br− and Fe3+ for Transparent Conductive Films. Nanomaterials. 2020; 10(2):237. https://doi.org/10.3390/nano10020237
Chicago/Turabian StyleWang, Xiao-Ming, Long Chen, Enrico Sowade, Raul D. Rodriguez, Evgeniya Sheremet, Chun-Mei Yu, Reinhard R. Baumann, and Jin-Ju Chen. 2020. "Ultra-Uniform and Very Thin Ag Nanowires Synthesized via the Synergy of Cl−, Br− and Fe3+ for Transparent Conductive Films" Nanomaterials 10, no. 2: 237. https://doi.org/10.3390/nano10020237
APA StyleWang, X.-M., Chen, L., Sowade, E., Rodriguez, R. D., Sheremet, E., Yu, C.-M., Baumann, R. R., & Chen, J.-J. (2020). Ultra-Uniform and Very Thin Ag Nanowires Synthesized via the Synergy of Cl−, Br− and Fe3+ for Transparent Conductive Films. Nanomaterials, 10(2), 237. https://doi.org/10.3390/nano10020237





