Vascular Wall–Mesenchymal Stem Cells Differentiation on 3D Biodegradable Highly Porous CaSi-DCPD Doped Poly (?-hydroxy) Acids Scaffolds for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffolds Preparation
2.2. Surface Porosity Evaluated by the Morphometric Analysis on ESEM Images
2.3. Isolation and Culture of Primary Porcine Vascular Wall Mesenchymal Stem Cells
2.4. Cell Seeding Efficiency Assay
2.5. Metabolic Cell Activity Assay
2.6. RNA Extraction and qPCR
2.7. DAPI Staining and Immunofluorescence
2.8. Statistical Analysis
3. Results
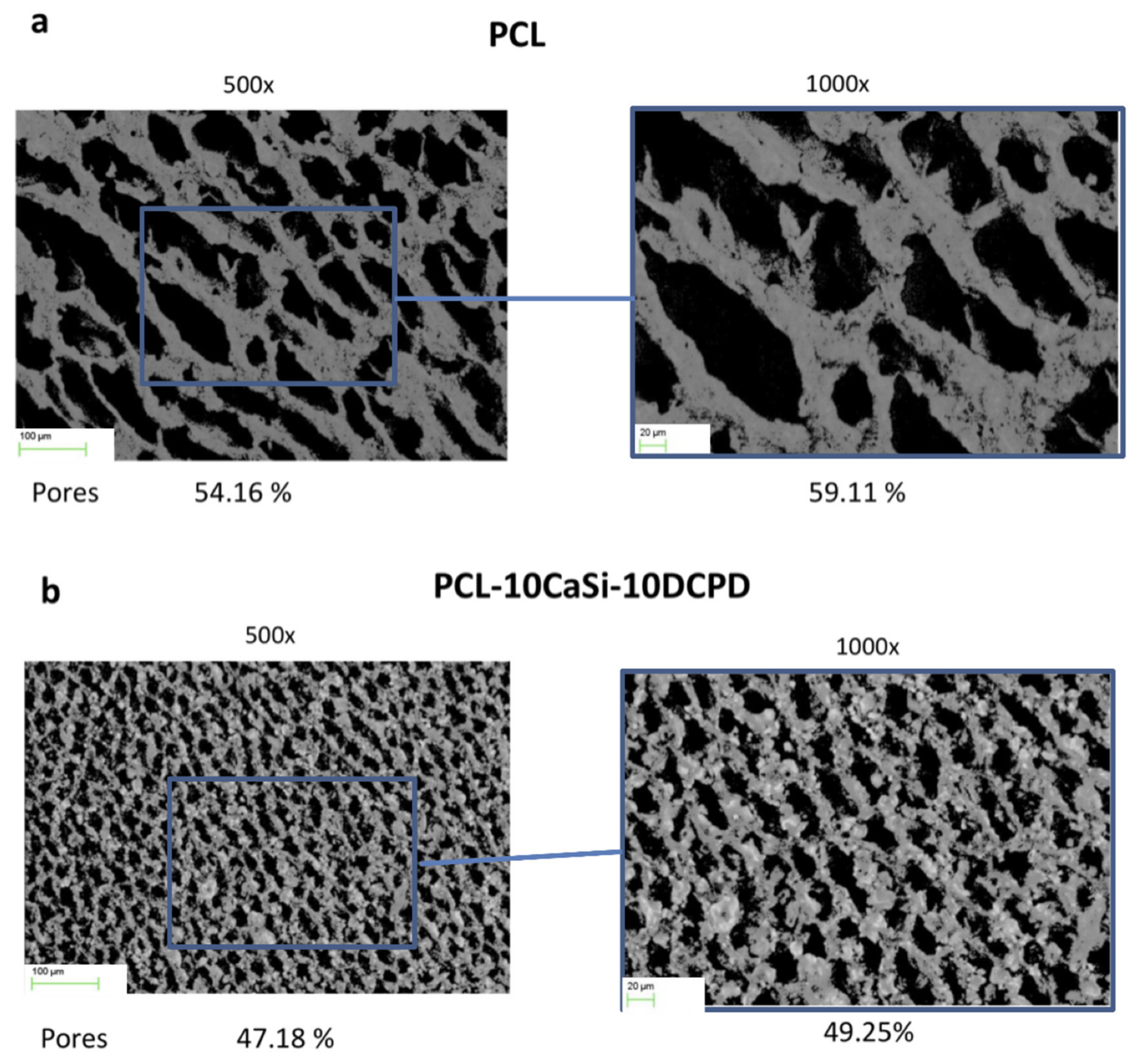
3.1. Surface Porosity Evaluated by the Morphometric Analysis on ESEM Images
3.2. Cytocompatibility
3.3. Metabolic Activity
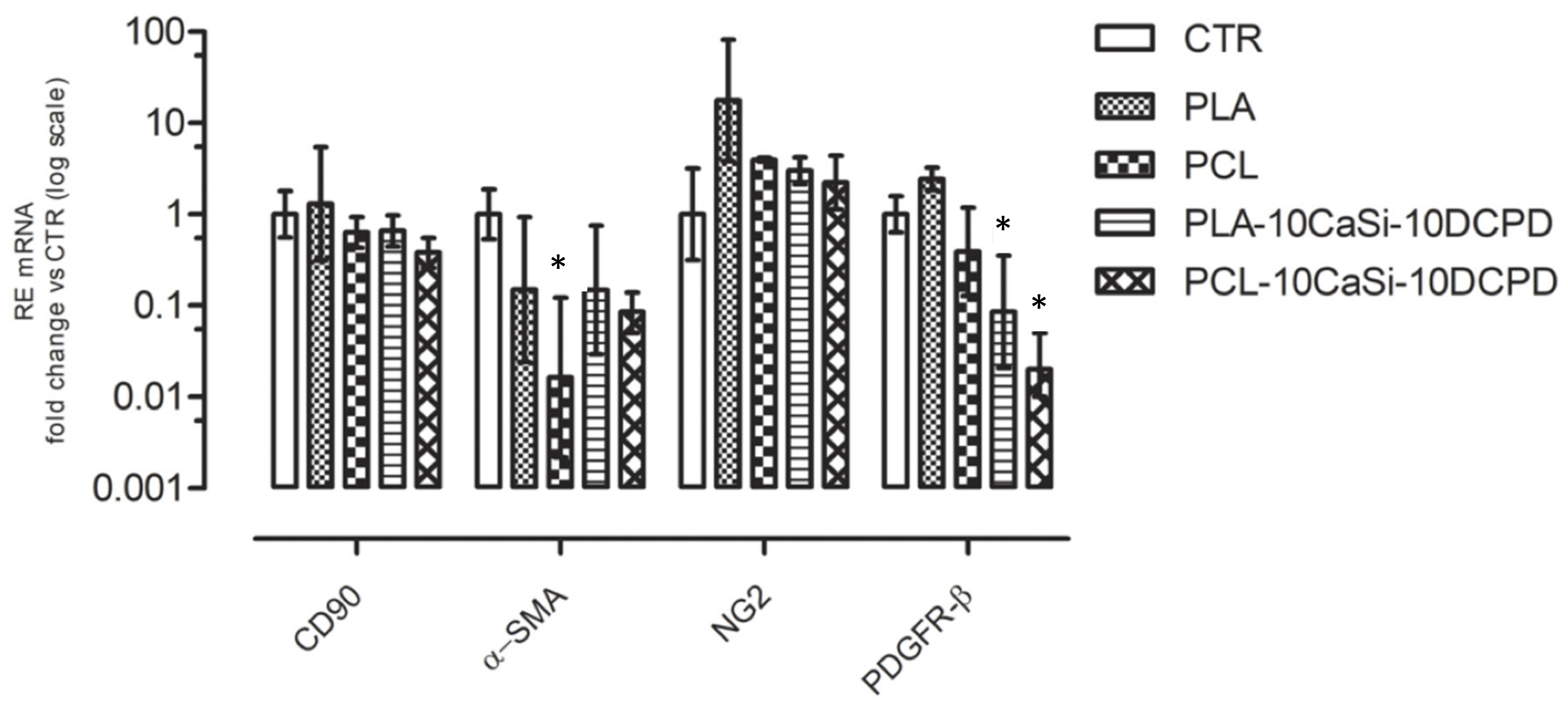
3.4. Effect of Biomaterials on VW-MSCs Markers
3.5. VW-MSCs Distribution on Different Scaffolds
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Chen, S.; Zheng, L.; Qin, L. Angiogenesis Assays for the Evaluation of Angiogenic Properties of Orthopaedic Biomaterials—A General Review. Adv. Healthcare Mat. 2017, 6, 1600434. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, S.; Kawazoe, N.; Chen, G. Promoted Angiogenesis and Osteogenesis by Dexamethasone-loaded Calcium Phosphate Nanoparticles/Collagen Composite Scaffolds with Microgroove Networks. Sci. Rep. 2018, 20, 14143. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. A review of bioactive silicate ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef]
- Lin, C.-S.; Lue, T.F. Defining vascular stem cells. Stem Cells Dev. 2013, 22, 1018–1026. [Google Scholar] [CrossRef] [Green Version]
- Pasquinelli, G.; Pacilli, A.; Alviano, F.; Foroni, L.; Ricci, F.; Valente, S.; Orrico, C.; Lanzoni, G.; Buzzi, M.; Tazzari, P.L.; et al. Multidistrict human mesenchymal vascular cells: Pluripotency and stemness characteristics. Cytotherapy 2010, 12, 275–287. [Google Scholar] [CrossRef]
- Pasquinelli, G.; Tazzari, P.L.; Vaselli, C.; Foroni, L.; Buzzi, M.; Storci, G.; Alviano, F.; Ricci, F.; Bonafè, M.; Orrico, C.; et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells 2007, 25, 1627–1634. [Google Scholar] [CrossRef]
- Psaltis, P.J.; Simari, R.D. Vascular wall progenitor cells in health and disease. Circ. Res. 2015, 116, 1392–1412. [Google Scholar] [CrossRef] [Green Version]
- Zaniboni, A.; Bernardini, C.; Alessandri, M.; Mangano, C.; Zannoni, A.; Bianchi, F.; Sarli, G.; Calzà, L.; Bacci, M.L.; Forni, M. Cells derived from porcine aorta tunica media show mesenchymal stromal-like cell properties in in vitro culture. Am. J. Physiol.-Cell Phys. 2014, 306, 322–333. [Google Scholar] [CrossRef] [Green Version]
- Zaniboni, A.; Bernardini, C.; Bertocchi, M.; Zannoni, A.; Bianchi, F.; Avallone, G.; Mangano, C.; Sarli, G.; Calzà, L.; Bacci, M.L.; et al. In vitro differentiation of porcine aortic vascular precursor cells to endothelial and vascular smooth muscle cells. Am. J. Physiol.-Cell Phys. 2015, 309, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, C.; Bertocchi, M.; Zannoni, A.; Salaroli, R.; Tubon, I.; Dothel, G.; Fernandez, M.; Bacci, M.L.; Calzà, L.; Forni, M. Constitutive and LPS-stimulated secretome of porcine Vascular Wall-Mesenchymal Stem Cells exerts effects on in vitro endothelial angiogenesis. BMC Vet. Res. 2019, 27, 123. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 31, 5744–5794. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 897–912. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Moers-Carpi, M.M.; Sherwood, S. Polycaprolactone for the correction of nasolabial folds: A 24-month, prospective, randomized, controlled clinical trial. Dermatol. Surg. 2013, 39, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ciapetti, G.; Prati, C. Development of the Foremost Light-Curable Calcium-Silicate MTA cement as Root-End in Oral Surgery. Chemical-Physical Properties, Bioactivity and Biological Behaviour. Dent. Mat. 2011, 27, 134–157. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Chen, L.; Lin, X.; Huang, Y.; Dai, K.; Naoki, K.; Chen, G.; Chang, G. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 2012, 8, 341–349. [Google Scholar] [CrossRef]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; Dorigo, E.D.S.; Prati, C. Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1412–1422. [Google Scholar] [CrossRef]
- Gandolfi, T.P.; Modena, E.; Siboni, F.; Prati, C. Biointeractivity-related versus chemi/physisorption-related apatite precursor-forming ability of current root end filling materials. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Shah, S.N.; Feng, R.; Prati, C.; Akintoye, S.O. Biomimetic Calcium-Silicate Cements Support Differentiation of Human Orofacial Mesenchymal Stem Cells. J. Endod. 2011, 37, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prati, C.; Azizi, A.; Pirani, C.; Zamparini, F.; Iacono, F.; Montebugnoli, L.; Gandolfi, M.G. Apical surgery vs apical surgery with simultaneous orthograde retreatment: A prospective cohort clinical study of teeth affected by persistent periapical lesion. G. Ital. Endod. 2018, 32, 2–8. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Spagnuolo, G.; Siboni, F.; Procino, A.; Rivieccio, V.; Pelliccioni, G.A.; Prati, C.; Rengo, S. Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: Chemical-physical properties and human pulp cells response. Clin. Oral. Investig. 2015, 19, 2075–2089. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, M.G.; Ciapetti, G.; Taddei, P.; Perut, F.; Tinti, A.; Cardoso, M.V.; Van Meerbeek, B.; Prati, C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010, 26, 974–992. [Google Scholar] [CrossRef]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Degli Esposti, M.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-Based Mineral-Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. Materials 2019, 16, 597. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Aparicio, C.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Polylactic acid-based porous scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 163–181. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly porous polycaprolactone scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 341–361. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castilla Bolaños, M.A.; Buttigieg, J.; Briceño Triana, J.C. Development and characterization of a novel porous small intestine submucosa-hydroxyapatite scaffold for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Dothel, G.; Bernardini, C.; Zannoni, A.; Spirito, M.R.; Salaroli, R.; Bacci, M.L.; Forni, M.; De Ponti, F. Ex vivo effect of vascular wall stromal cells secretome on enteric ganglia. World J. Gastroenterol. 2019, 25, 4892–4903. [Google Scholar] [CrossRef]
- Dozza, B.; Lesci, I.G.; Duchi, S.; Bella, E.D.; Martini, L.; Salamanna, F.; Falconi, M.; Cinotti, S.; Fini, M.; Lucarelli, E.; et al. When size matters: Differences in demineralized bone matrix particles affect collagen structure, mesenchymal stem cell behavior, and osteogenic potential. J. Biomed. Mater. Res. A 2017, 105, 1019–1033. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Degli Esposti, M.; Parchi, G.; Batur, E.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-doped poly(L-lactide) acid scaffolds enriched with exosomes improve osteogenic commitment of human adipose-derived mesenchymal stem cells. Nanomatials 2020, submitted. [Google Scholar]
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The effects of Biodentine/polycaprolactone three-dimensional-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2018, 51, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wei, L.; Liu, X.; Li, J.; Li, B.; Wang, G. Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater. 2009, 5, 1284–1293. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A possible factor in bone calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef]
- Bernardini, C.; Zannoni, A.; Bertocchi, M.; Bianchi, F.; Salaroli, R.; Botelho, G.; Baccia, M.L.; Ventrella, V.; Forni, M. Deleterious effects of tributyltin on porcine vascular stem cells physiology. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 186, 38–44. [Google Scholar] [CrossRef]
- Nesci, S.; Bernardini, C.; Salaroli, R.; Zannoni, A.; Trombetti, F.; Ventrella, V.; Pagliarani, A.; Forni, M. Characterization of metabolic profiles and lipopolysaccharide effects on porcine vascular wall mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 16685–16691. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Celetta, G.; Tomasek, J.T.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancopoulos, G.D.; Davis, S.; Gale, N.; Rudge, J.; Wiegand, S.; Holash, J. Vascular specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Mukouyama, Y. Tissue Specific Origin, Development, and Pathological Perspectives of Pericytes. Front. Cardiovasc. Med. 2018, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Michelis, K.C.; Nomura-Kitabayashi, A.; Lecce, L.; Franzén, O.; Koplev, S.; Xu, Y.; Santini, M.P.; D’Escamard, V.; Lee, J.T.L.; Fuster, V.; et al. CD90 Identifies Adventitial Mesenchymal Progenitor Cells in Adult Human Medium- and Large-Sized Arteries. Stem Cell Rep. 2018, 11, 242–257. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhang, X.; Pei, M. Significance of Cellular Cross-Talk in Stromal Vascular Fraction of Adipose Tissue in Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1034–1044. [Google Scholar] [CrossRef]
- Sweeney, M.; Foldes, G. It Takes Two: Endothelial-Perivascular Cell Cross-Talk in Vascular Development and Disease. Front. Cardiovasc. Med. 2018, 30, 154. [Google Scholar] [CrossRef]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.U.; Looman, C.; Ahgren, A.; Wu, Y.; Claesson-Welsh, L.; Heuchel, R.L. Platelet-derived growth factor receptor-beta constitutive activity promotes angiogenesis in vivo and in vitro. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2142–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozerdem, U.; Grako, K.A.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 2001, 222, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ozerdem, U.; Stallcup, W.B. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis 2003, 6, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef]
- Zamparini, F.; Siboni, F.; Prati, C.; Taddei, P.; Gandolfi, M.G. Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin. Oral. Investig. 2019, 23, 445–457. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forni, M.; Bernardini, C.; Zamparini, F.; Zannoni, A.; Salaroli, R.; Ventrella, D.; Parchi, G.; Degli Esposti, M.; Polimeni, A.; Fabbri, P.; et al. Vascular Wall–Mesenchymal Stem Cells Differentiation on 3D Biodegradable Highly Porous CaSi-DCPD Doped Poly (?-hydroxy) Acids Scaffolds for Bone Regeneration. Nanomaterials 2020, 10, 243. https://doi.org/10.3390/nano10020243
Forni M, Bernardini C, Zamparini F, Zannoni A, Salaroli R, Ventrella D, Parchi G, Degli Esposti M, Polimeni A, Fabbri P, et al. Vascular Wall–Mesenchymal Stem Cells Differentiation on 3D Biodegradable Highly Porous CaSi-DCPD Doped Poly (?-hydroxy) Acids Scaffolds for Bone Regeneration. Nanomaterials. 2020; 10(2):243. https://doi.org/10.3390/nano10020243
Chicago/Turabian StyleForni, Monica, Chiara Bernardini, Fausto Zamparini, Augusta Zannoni, Roberta Salaroli, Domenico Ventrella, Greta Parchi, Micaela Degli Esposti, Antonella Polimeni, Paola Fabbri, and et al. 2020. "Vascular Wall–Mesenchymal Stem Cells Differentiation on 3D Biodegradable Highly Porous CaSi-DCPD Doped Poly (?-hydroxy) Acids Scaffolds for Bone Regeneration" Nanomaterials 10, no. 2: 243. https://doi.org/10.3390/nano10020243
APA StyleForni, M., Bernardini, C., Zamparini, F., Zannoni, A., Salaroli, R., Ventrella, D., Parchi, G., Degli Esposti, M., Polimeni, A., Fabbri, P., Fava, F., Prati, C., & Gandolfi, M. G. (2020). Vascular Wall–Mesenchymal Stem Cells Differentiation on 3D Biodegradable Highly Porous CaSi-DCPD Doped Poly (?-hydroxy) Acids Scaffolds for Bone Regeneration. Nanomaterials, 10(2), 243. https://doi.org/10.3390/nano10020243










