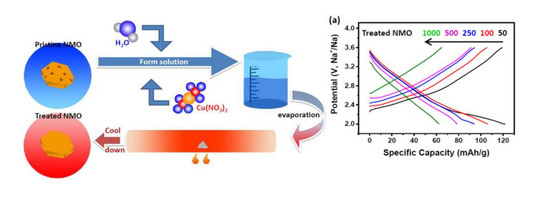
Smoothing the Surface and Improving the Electrochemical Properties of NaxMnO2 by a Wet Chemical Method
Abstract
:1. Introduction
2. Experimental Details
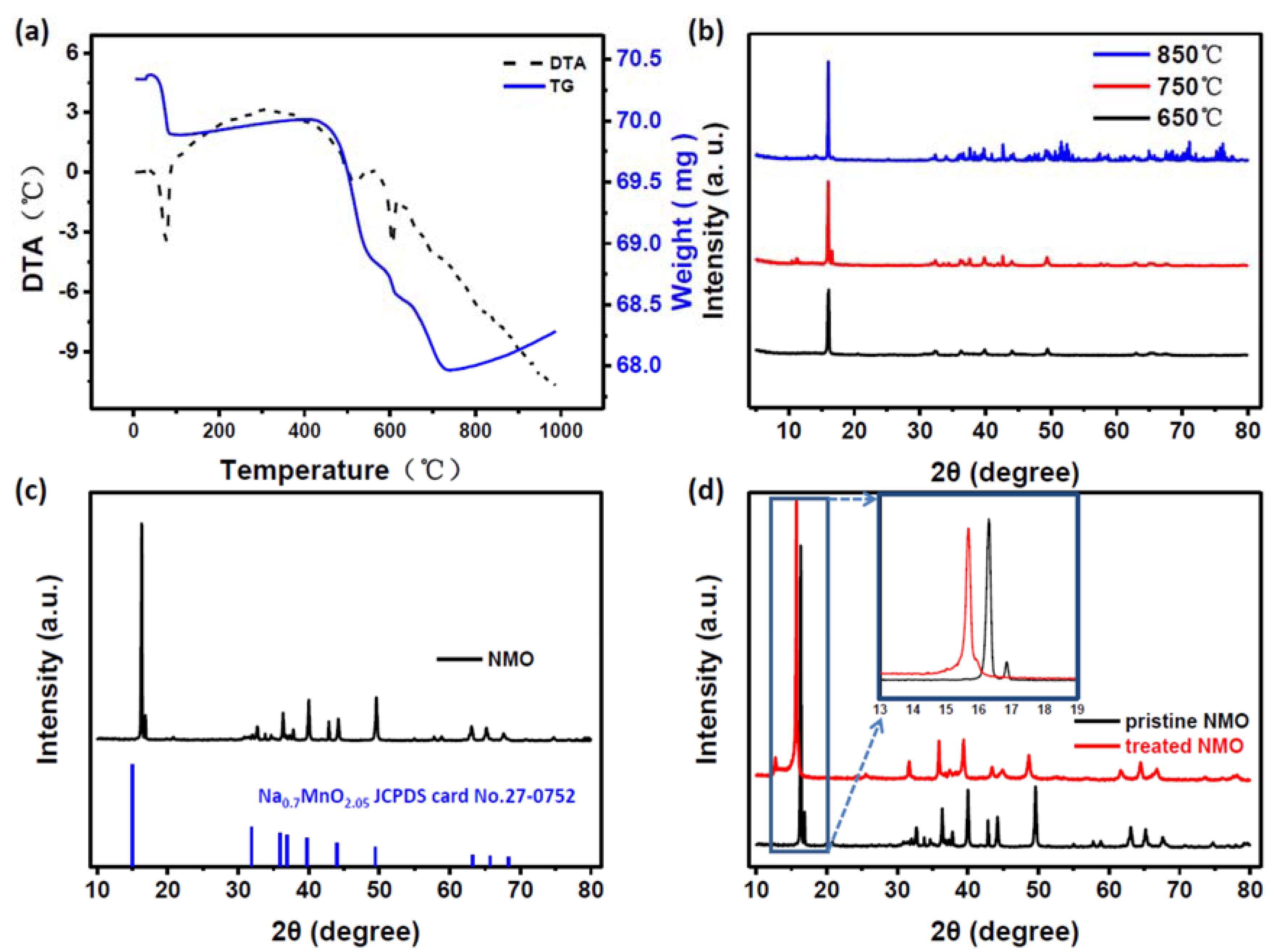
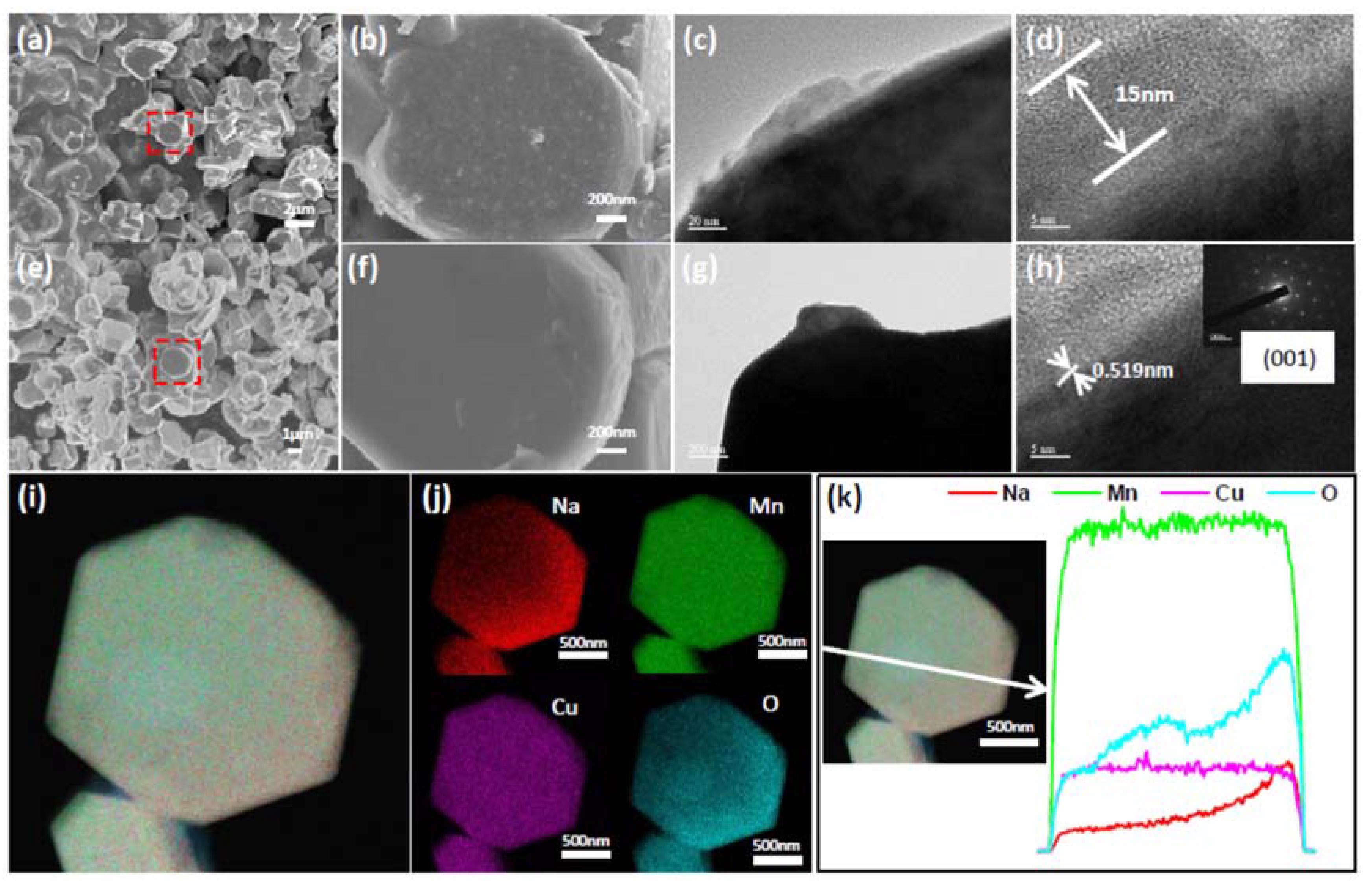
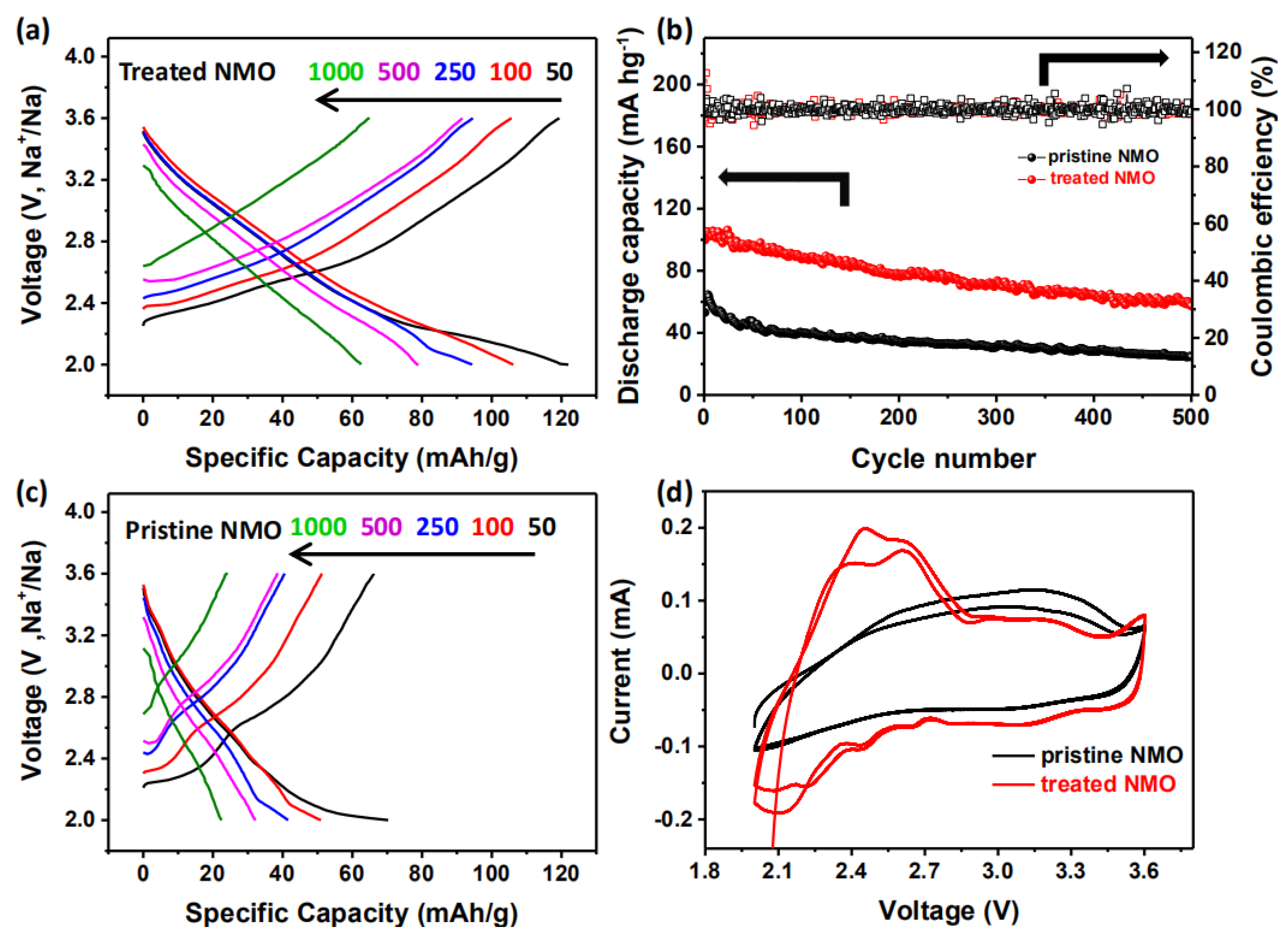
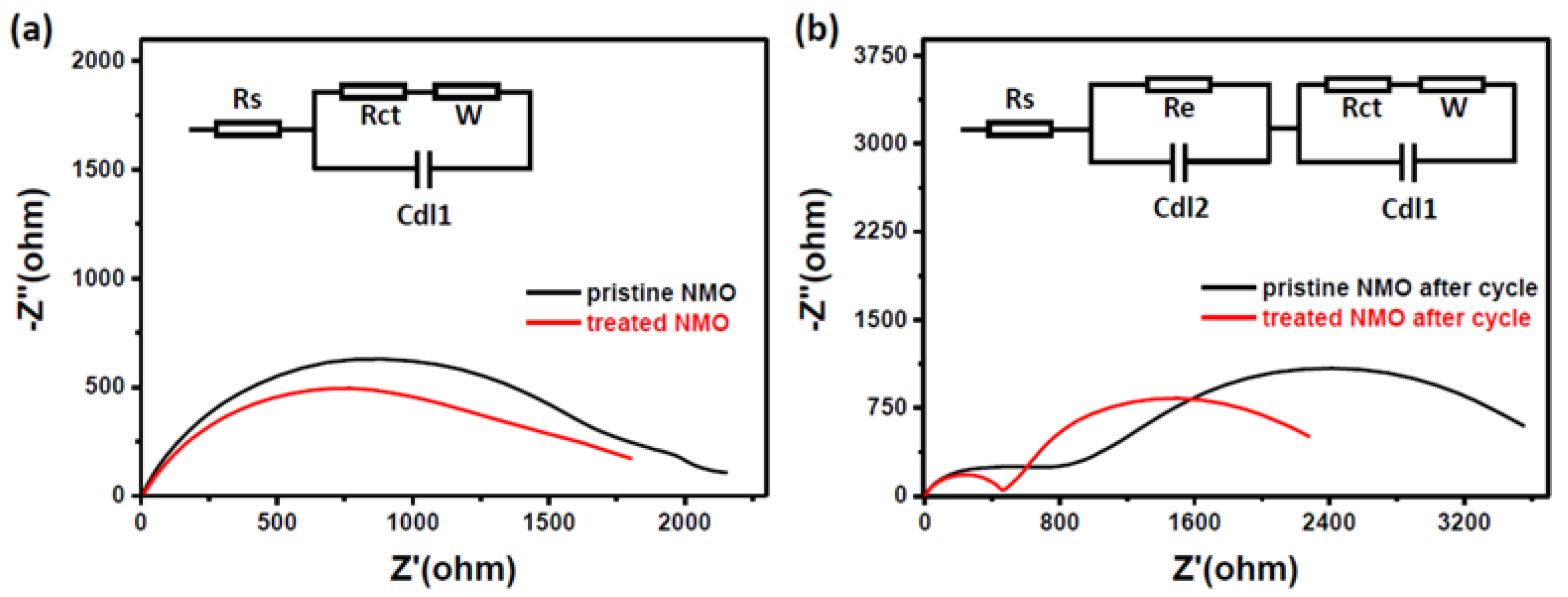
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Sou, L.M.; Hu, Y.S.; Li, H.; Armand, M.; Chen, L.Q. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481–1489. [Google Scholar]
- Nölle, R.; Beltrop, K.; Holtstiege, F.; Kasnatscheew, J.; Placke, T.; Winter, M. A reality check and tutorial on electrochemical characterization of battery cell materials: How to choose the appropriate cell setup. Mater. Today 2019. [CrossRef]
- Ha, K.H.; Woo, S.H.; Mok, D.; Choi, N.S.; Park, Y.; Oh, S.M.; Kim, Y.; Kim, J.; Lee, J.; Nazar, L.F.; et al. Na4-αM2+α/2(P2O7)2 (2/3≤α≤7/8, M=Fe, Fe0.5Mn0.5, Mn): A promising sodium ion cathode for Na-ion batteries. Adv. Energy Mater. 2013, 3, 770–776. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, A.; Wong, K.H.; Balaya, P. The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv. Energy Mater. 2012, 3, 444–450. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Rousse, G.; Arroyo-de Dompablo, M.E.; Vezin, H.; Tarascon, J.M.; Palacin, M.R. Low-potential sodium insertion in a NASICON-type structure through the Ti(Ⅲ)/Ti(Ⅱ) redox couple. J. Am. Chem. Soc. 2013, 135, 3897–3903. [Google Scholar] [CrossRef]
- Ma, X.H.; Chen, H.L.; Ceder, G. Electrochemical properties of monoclnic NaMnO2. J. Electrochem. Soc. 2011, 158, A1307–A1312. [Google Scholar] [CrossRef]
- Ding, J.J.; Zhou, Y.N.; Sun, Q.; Fu, Z.Y. Cycle performance improvement of NaCrO2 cathode by carbon coating for sodium ion batteries. Electrochem. Commun. 2012, 22, 85–88. [Google Scholar] [CrossRef]
- Oh, S.M.; Myung, S.T.; Yoon, C.S.; Lu, J.; Hassoun, J.; Scrosati, B.; Amine, K.; Sun, Y.K. Advanced Na[Ni0.25Fe0.5Mn0.25]O2/C-Fe3O4 sodium-ion batteries using EMS electrolyte for energy storage. Nano Lett. 2014, 14, 1620–1626. [Google Scholar] [CrossRef]
- Mendiboure, A.; Delmas, C.; Hagenmuller, P. Electrochemical intercalation and deintercalation of NaxMnO2 bronzes. J. Solid State Chem. 1985, 57, 323–331. [Google Scholar] [CrossRef]
- Billaud, J.; Clément, R.J.; Armstrong, A.R.; Canales-Vázquez, J.; Rozier, P.; Clare, P.G.; Bluce, P.G. β-NaMnO2: A high-performance cathode for sodium-ion batteries. J. Am. Chem. Soc. 2014, 136, 17243–17248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisse de, B.M.; Carlier, D.; Guignard, D.M.; Bourgeois, L.; Delmas, C. P2-NaxMn0.5Fe0.5O2 phase used as positive electrode in Na batteries: Structural changes induced by the electrochemical (de) intercalation process. Inorg. Chem. 2014, 53, 11197–11205. [Google Scholar] [CrossRef] [PubMed]
- Sathiya, M.; Hemalatha, K.; Ramesha, K.; Tarascon, J.M.; Prakash, A.S. Synthesis, structure, and electrochemical properties of the layered sodium insertion cathode material: NaNi1/3Mn1/3Co1/3O2. Chem. Mater. 2012, 24, 1846–1853. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lee, Y.H.; Jo, E.; Chung, K.Y.; Choi, W.C.; Kim, S.M.; Chang, W.Y. Structure and electrochemical properties of layered sodium manganese oxide NaxMnO2. ACS Appl. Mater. Interfaces 2017, 9, 18883. [Google Scholar] [CrossRef]
- Caballero, A.; Hernán, L.; Morales, J.; Sánchez, L.; Santos Peña, J.; Aranda, M.A.G. Synthesis and characterization of high-temperature hexagonal P2-Na0.6MnO2 and its electrochemical behaviour as cathode in sodium cells. J. Mater. Chem. 2002, 12, 1142–1147. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Y.; Zhang, X.P.; Liu, J.Q.; Zhu, X.; Zhong, S.K. Synthesis of carbon-coated Na2MnPO4F hollow spheres as a potential cathode material for Na-ion batteries. J. Power Sources 2018, 374, 40–47. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.F.; Xiong, D.B.; Hao, Y.; Kou, H.; Yan, B.; Li, D.J.; Lu, S.G.; Koo, A.; Adair, K.; et al. Significantily improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 2018, 44, 111–120. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Meng, X.; Tang, Y.; Banis, M.N.; Yang, J.; Hu, Y.; Li, R.; Cai, M.; Sun, X. Signficant impact on cathode performance of lithium-ion batteries by precisely controlled metal oxide nanocoatings via atomic layer deposition. J. Power Sources 2014, 247, 57–69. [Google Scholar] [CrossRef]
- Kong, J.Z.; Ren, C.; Tai, G.A.; Zhang, X.; Li, A.D.; Wu, D.; Li, H.; Zhou, F. Ultrathin ZnO coating for improved electrochemical performance of LiNi0.5Co0.5Mn0.3O2 cathode material. J. Power Sources 2014, 266, 433–439. [Google Scholar] [CrossRef]
- Su, Y.; Cui, S.; Zhuo, Z.; Yang, W.; Wang, X.; Pan, F. Enhancing the high-voltage cycling performance of LiNi0.5Mn0.3Co0.2O2 by retarding its interfacial reaction with an electrolyte by atomic-layer-deposited Al2O3. ACS Appl. Mater. Interfaces 2015, 7, 25105–25112. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Lim, J.C.; Lee, J.K. Improving the performance of silicon anode in lithium-ion batteries by Cu2O coating layer. J. Appl. Electrochem. 2014, 44, 353–360. [Google Scholar] [CrossRef]
- Dong, R.B.; Li, Q.; Chen, M.M.; Hu, Z.B.; Xiao, X.L. CuO-coated and Cu2+ doped Co-modified P2-type Na2/3[Ni1/3Mn2/3]O2 for sodium-ion batteries. Phys. Chem. Chem. Phys. 2019, 21, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Jo, Y.N.; Kim, T.H.; Do, S.J.; Santhoshkumar, P.; Sivagami, I.N.; Lee, C.W. Physical and electrochemical propertied of CuO-coating Li[Ni0.8Co0.1Mn0.1]O2 cathodes at elevated temperature for lithium ion batteries. J. Nanosci. NanoTechnol. 2017, 17, 8093–8099. [Google Scholar] [CrossRef]
- House, R.A.; Maitra, U.; Pérez-Osorio, M.A.; Juan, G.L.; Jin, L.Y.; Somerville, J.W.; Duda, L.C.; Nag, A.; Walters, A.; Zhou, K.J.; et al. Superstucture control of first-cycle voltage hysteresis in O-redox cathodes. Nature 2020, 577, 502–508. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Lin, Z.; Wu, F.; Xiao, F.; Xu, J. Smoothing the Surface and Improving the Electrochemical Properties of NaxMnO2 by a Wet Chemical Method. Nanomaterials 2020, 10, 246. https://doi.org/10.3390/nano10020246
Zhao S, Lin Z, Wu F, Xiao F, Xu J. Smoothing the Surface and Improving the Electrochemical Properties of NaxMnO2 by a Wet Chemical Method. Nanomaterials. 2020; 10(2):246. https://doi.org/10.3390/nano10020246
Chicago/Turabian StyleZhao, Siliang, Zhiping Lin, Fugen Wu, Feng Xiao, and Jiantie Xu. 2020. "Smoothing the Surface and Improving the Electrochemical Properties of NaxMnO2 by a Wet Chemical Method" Nanomaterials 10, no. 2: 246. https://doi.org/10.3390/nano10020246
APA StyleZhao, S., Lin, Z., Wu, F., Xiao, F., & Xu, J. (2020). Smoothing the Surface and Improving the Electrochemical Properties of NaxMnO2 by a Wet Chemical Method. Nanomaterials, 10(2), 246. https://doi.org/10.3390/nano10020246