Efficient and Stable CdSe/CdS/ZnS Quantum Rods-in-Matrix Assembly for White LED Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CdSe/CdS QRs and CdSe/CdS/ZnS QRAs
2.3. Fabrication of WLEDs with QRs and QRAs
2.4. Characterizations
3. Results and Discussion
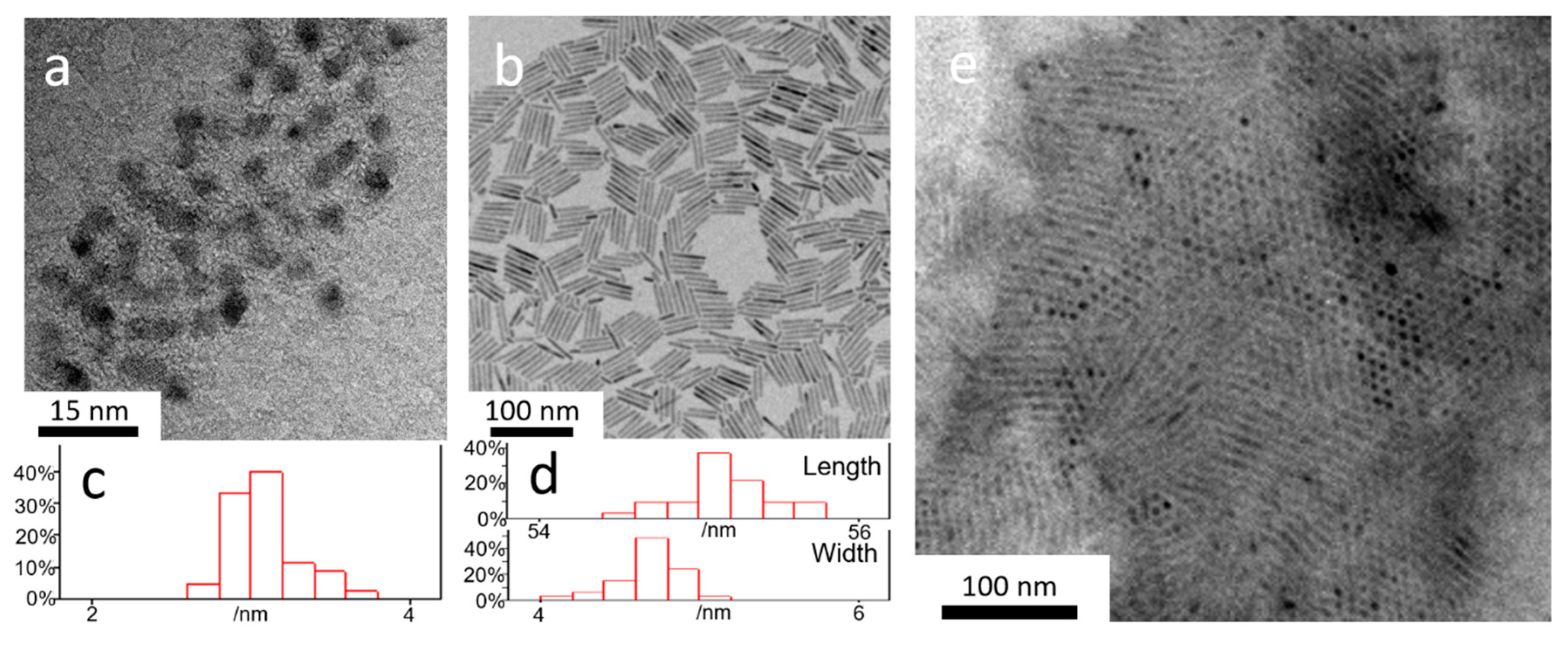
3.1. Growth and Structure Characterization of CdSe/CdS/ZnS QRAs
3.2. Optical Characterizations of CdSe/CdS/ZnS QRAs
3.3. Photostability and LED Application of CdSe/CdS/ZnS QRAs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Talapin, D.V.; Koeppe, R.; Götzinger, S.; Kornowski, A.; Lupton, J.M.; Rogach, A.L.; Benson, O.; Feldmann, J.; Weller, H. Highly emissive colloidal CdSe/CdS heterostructures of mixed dimensionality. Nano Lett. 2003, 3, 1677–1681. [Google Scholar] [CrossRef]
- Eshet, H.; Grunwald, M.; Rabani, E. The electronic structure of CdSe/CdS core/shell seeded nanorods: Type-I or quasi-type-II? Nano Lett. 2013, 13, 5880–5885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costi, R.; Saunders, A.E.; Banin, U. Colloidal hybrid nanostructures: A new type of functional materials. Angew. Chem. Int. Ed. 2010, 49, 4878–4897. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Nelson, J.H.; Shevchenko, E.V.; Aloni, S.; Sadtler, B.; Alivisatos, A.P. Seeded growth of highly luminescent CdSe/CdS nanoheterostructures with rod and tetrapod morphologies. Nano Lett. 2007, 7, 2951–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadar, I.; Hitin, G.B.; Sitt, A.; Faust, A.; Banin, U. Polarization Properties of Semiconductor Nanorod Heterostructures: From Single Particles to the Ensemble. J. Phys. Chem. Lett. 2013, 4, 502–507. [Google Scholar] [CrossRef]
- Yarema, M.; Xing, Y.; Lechner, R.T.; Ludescher, L.; Dordevic, N.; Lin, W.M.M.; Yarema, O.; Wood, V. Mapping the Atomistic Structure of Graded Core/Shell Colloidal Nanocrystals. Sci. Rep. 2017, 7, 11718. [Google Scholar] [CrossRef]
- Wu, K.; Lian, T. Quantum confined colloidal nanorod heterostructures for solar-to-fuel conversion. Chem. Soc. Rev. 2016, 45, 3781–3810. [Google Scholar] [CrossRef]
- Planelles, J.; Rajadell, F.; Climente, J.I. Electronic Origin of Linearly Polarized Emission in CdSe/CdS Dot-in-Rod Heterostructures. J. Phys. Chem. C 2016, 120, 27724–27730. [Google Scholar] [CrossRef] [Green Version]
- Dadabayev, R.; Shabairou, N.; Zalevsky, Z.; Malka, D. A visible light RGB wavelength demultiplexer based on silicon-nitride multicore PCF. Opt. Laser Technol. 2019, 111, 411–416. [Google Scholar] [CrossRef]
- Dadabayev, R.; Malka, D. A visible light RGB wavelength demultiplexer based on polycarbonate multicore polymer optical fiber. Opt. Laser Technol. 2019, 116, 239–245. [Google Scholar] [CrossRef]
- Shoresh, T.; Katanov, N.; Malka, D. 1 × 4 MMI visible light wavelength demultiplexer based on a GaN slot-waveguide structure. Photonic. Nanostruct. 2018, 30, 45–49. [Google Scholar] [CrossRef]
- Carbone, L.; Nobile, C.; De Giorgi, M.; Sala, F.D.; Morello, G.; Pompa, P.; Hytch, M.; Snoeck, E.; Fiore, A.; Franchini, I.R.; et al. Synthesis and micrometer-scale assembly of colloidal CdSe/CdS nanorods prepared by a seeded growth approach. Nano Lett. 2007, 7, 2942–2950. [Google Scholar] [CrossRef]
- Kaur, S.; Murali, G.; Manda, R.; Chae, Y.C.; Yun, M.; Lee, J.H.; Lee, S.H. Functional Film with Electric-Field-Aided Aligned Assembly of Quantum Rods for Potential Application in Liquid Crystal Display. Adv. Opt. Mater. 2018, 6, 1800235. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, M.; Wu, W.; Yang, X.; Sun, X.W.; Zhang, J.; Wang, H.-C.; Liu, R.-S.; Han, C.-Y.; Yang, H.; et al. Recent advances in quantum dot-based light-emitting devices: Challenges and possible solutions. Mater. Today 2019, 24, 69–93. [Google Scholar] [CrossRef]
- Chan, E.M.; Mathies, R.A.; Alivisatos, A.P. Size-controlled growth of CdSe nanocrystals in microfluidic reactors. Nano Lett. 2003, 3, 199–201. [Google Scholar] [CrossRef]
- Xing, W.; Zhang, X.; Geng, C.; Xie, Y.; Deng, Y.; Li, P.; Li, H.; Xu, S.; Bi, W. Multi-quantum-well quantum dots with stable dual emission. Nanoscale 2019, 11, 8475–8484. [Google Scholar] [CrossRef]
- Yang, W.; Zhong, P.; Mei, S.; Chen, Q.; Zhang, W.; Zhu, J.; Guo, R.; He, G. Photometric Optimization of Color Temperature Tunable Quantum Dots Converted White LEDs for Excellent Color Rendition. IEEE Photonics J. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Jang, E.; Jun, S.; Jang, H.; Lim, J.; Kim, B.; Kim, Y. White-light-emitting diodes with quantum dot color converters for display backlights. Adv. Mater. 2010, 22, 3076–3080. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Yang, W.; Manna, L.; Wang, L.; Alivisatos, A.P. Linearly polarized emission from colloidal semiconductor quantum rods. Science 2001, 292, 2060–2063. [Google Scholar] [CrossRef]
- Borys, N.J.; Walter, M.J.; Huang, J.; Talapin, D.V.; Lupton, J.M. The role of particle morphology in interfacial energy transfer in CdSe/CdS heterostructure nanocrystals. Science 2010, 330, 1371–1374. [Google Scholar] [CrossRef]
- Mashford, B.S.; Stevenson, M.; Popovic, Z.; Hamilton, C.; Zhou, Z.; Breen, C.; Steckel, J.; Bulovic, V.; Bawendi, M.; Coe-Sullivan, S.; et al. High-efficiency quantum-dot light-emitting devices with enhanced charge injection. Nat. Photonics 2013, 7, 407–412. [Google Scholar] [CrossRef]
- Xie, Y.; Geng, C.; Liu, X.; Xu, S.; Xing, W.; Zhang, X.; Zhang, Z.H.; Zhang, Y.; Bi, W. Synthesis of highly stable quantum-dot silicone nanocomposites via in situ zinc-terminated polysiloxane passivation. Nanoscale 2017, 9, 16836–16842. [Google Scholar] [CrossRef] [PubMed]
- Panfil, Y.E.; Oded, M.; Banin, U. Colloidal Quantum Nanostructures: Emerging Materials for Display Applications. Angew. Chem. Int. Ed. 2018, 57, 4274–4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halivni, S.; Shemesh, S.; Waiskopf, N.; Vinetsky, Y.; Magdassi, S.; Banin, U. Inkjet printed fluorescent nanorod layers exhibit superior optical performance over quantum dots. Nanoscale 2015, 7, 19193–19200. [Google Scholar] [CrossRef] [PubMed]
- Sitt, A.; Hadar, I.; Banin, U. Band-gap engineering, optoelectronic properties and applications of colloidal heterostructured semiconductor nanorods. Nano Today 2013, 8, 494–513. [Google Scholar] [CrossRef]
- Shen, H.; Bai, X.; Wang, A.; Wang, H.; Qian, L.; Yang, Y.; Titov, A.; Hyvonen, J.; Zheng, Y.; Li, L.S. High-Efficient Deep-Blue Light-Emitting Diodes by Using High Quality ZnxCd1-xS/ZnS Core/Shell Quantum Dots. Adv. Funct. Mater. 2014, 24, 2367–2373. [Google Scholar] [CrossRef]
- Pal, B.N.; Ghosh, Y.; Brovelli, S.; Laocharoensuk, R.; Klimov, V.I.; Hollingsworth, J.A.; Htoon, H. ‘Giant’ CdSe/CdS core/shell nanocrystal quantum dots as efficient electroluminescent materials: Strong influence of shell thickness on light-emitting diode performance. Nano Lett. 2012, 12, 331–336. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yoon, D.-E.; Jang, J.; Lee, D.; Choi, G.-M.; Chang, J.H.; Lee, J.Y.; Lee, D.C.; Bae, B.-S. Quantum dot/siloxane composite film exceptionally stable against oxidation under heat and moisture. J. Am. Chem. Soc. 2016, 138, 16478–16485. [Google Scholar] [CrossRef]
- Cai, X.; Martin, J.E.; Shea-Rohwer, L.E.; Gong, K.; Kelley, D.F. Thermal quenching mechanisms in II–VI semiconductor nanocrystals. J. Phys. Chem. C 2013, 117, 7902–7913. [Google Scholar] [CrossRef]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, H.-S.; Fukumura, D.; Jain, R.K. Compact high-quality CdSe–CdS core–shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 2013, 12, 445. [Google Scholar] [CrossRef] [Green Version]
- Chern, M.; Nguyen, T.T.; Mahler, A.H.; Dennis, A.M. Shell thickness effects on quantum dot brightness and energy transfer. Nanoscale 2017, 9, 16446–16458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahler, B.; Spinicelli, P.; Buil, S.; Quelin, X.; Hermier, J.P.; Dubertret, B. Towards non-blinking colloidal quantum dots. Nat. Mater. 2008, 7, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Polovitsyn, A.; Dang, Z.; Movilla, J.L.; Martin-Garcia, B.; Khan, A.H.; Bertrand, G.H.V.; Brescia, R.; Moreels, I. Synthesis of Air-Stable CdSe/ZnS Core-Shell Nanoplatelets with Tunable Emission Wavelength. Chem. Mater. 2017, 29, 5671–5680. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Mohs, A.M.; Nie, S. Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat. Nanotechnol. 2009, 4, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Manna, L.; Scher, E.C.; Li, L.-S.; Alivisatos, A.P. Epitaxial growth and photochemical annealing of graded CdS/ZnS shells on colloidal CdSe nanorods. J. Am. Chem. Soc. 2002, 124, 7136–7145. [Google Scholar] [CrossRef]
- Adel, P.; Wolf, A.; Kodanek, T.; Dorfs, D. Segmented CdSe@CdS/ZnS Nanorods Synthesized via a Partial Ion Exchange Sequence. Chem. Mater. 2014, 26, 3121–3127. [Google Scholar] [CrossRef]
- Deka, S.; Quarta, A.; Lupo, M.G.; Falqui, A.; Boninelli, S.; Giannini, C.; Morello, G.; De Giorgi, M.; Lanzani, G.; Spinella, C.; et al. CdSe/CdS/ZnS Double Shell Nanorods with High Photoluminescence Efficiency and Their Exploitation As Biolabeling Probes. J. Am. Chem. Soc. 2009, 131, 2948–2958. [Google Scholar] [CrossRef]
- Sitt, A.; Della Sala, F.; Menagen, G.; Banin, U. Multiexciton Engineering in Seeded Core/Shell Nanorods: Transfer from Type-I to Quasi-type-II Regimes. Nano Lett. 2009, 9, 3470–3476. [Google Scholar] [CrossRef]
- Wang, X.; Qu, L.; Zhang, J.; Peng, X.; Xiao, M. Surface-related emission in highly luminescent CdSe quantum dots. Nano Lett. 2003, 3, 1103–1106. [Google Scholar] [CrossRef]
- Pidluzhna, А.; Ivaniuk, K.; Stakhira, P.; Hotra, Z.; Chapran, M.; Ulanski, J.; Tynkevych, O.; Khalavka, Y.; Baryshnikov, G.V.; Minaev, B. Multi-channel electroluminescence of CdTe/CdS core-shell quantum dots implemented into a QLED device. Dyes Pigment. 2019, 162, 647–653. [Google Scholar] [CrossRef]
- Efros, A.L.; Rosen, M.; Kuno, M.; Nirmal, M.; Norris, D.J.; Bawendi, M. Band-edge exciton in quantum dots of semiconductors with a degenerate valence band: Dark and bright exciton states. Phys. Rev. B 1996, 54, 4843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, W.G.; Xu, S.; Geng, C.; Zhao, F.; Jiang, X.F. Quantum Dots Enabled LCD Displays and Solid-State Lighting. SPIE 2016, 9924, 992403. [Google Scholar]







| Materials | Suppliers | Purity |
|---|---|---|
| CdO | Alfa Aesar | 99.5% |
| Se | Alfa Aesar | 99.5% |
| Cd(St)2 | Debo | 98% |
| ODPA | EPSILON | 98% |
| HPA | IRRITANT | 98% |
| ODE | TCI | >90% |
| Zn(DDTC)2 | TCI | >99% |
| HDA | TCI | >95% |
| TOP | TCI | 85% |
| TOPO | Macklin | 98% |
| Ethanol | Tianjin Damao | AR |
| Dimethylbenzene | Tianjin Damao | AR |
| Silicone-6662 | Dow Corning | N/A |
| Materials | Peak Wavelength | FWHM | Efficiency as Power | Stokes Shift | Air- and Photostability | Thermal Quenching |
|---|---|---|---|---|---|---|
| KSF | ~630 nm | ~15 nm | 60–70% | Large | Moderate | Moderate |
| QRs | ~630 nm * | <50 nm * | <50% | Large | Unstable | High |
| QRAs | ~630 nm * | <50 nm * | >80% | Large | Moderate | Moderate |
| Giant QDs | ~630 nm * | <50 nm * | 60–80% | Moderate | Moderate | Moderate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xing, W.; Liu, Y.; Zhang, X.; Xie, Y.; Shen, C.; Liu, J.G.; Geng, C.; Xu, S. Efficient and Stable CdSe/CdS/ZnS Quantum Rods-in-Matrix Assembly for White LED Application. Nanomaterials 2020, 10, 317. https://doi.org/10.3390/nano10020317
Chen Y, Xing W, Liu Y, Zhang X, Xie Y, Shen C, Liu JG, Geng C, Xu S. Efficient and Stable CdSe/CdS/ZnS Quantum Rods-in-Matrix Assembly for White LED Application. Nanomaterials. 2020; 10(2):317. https://doi.org/10.3390/nano10020317
Chicago/Turabian StyleChen, Yujuan, Weishuo Xing, Yixuan Liu, Xinsu Zhang, Yangyang Xie, Chongyu Shen, Jay Guoxu Liu, Chong Geng, and Shu Xu. 2020. "Efficient and Stable CdSe/CdS/ZnS Quantum Rods-in-Matrix Assembly for White LED Application" Nanomaterials 10, no. 2: 317. https://doi.org/10.3390/nano10020317






