Facile One-Step Hydrothermal Fabrication of (Sr0.6Bi0.305)2Bi2O7/SnO2 Heterojunction with Excellent Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalysts
2.3. Characterization
2.4. Determination of Photocatalytic Activity
2.5. Photoelectrochemical Measurement
3. Results and Discussion
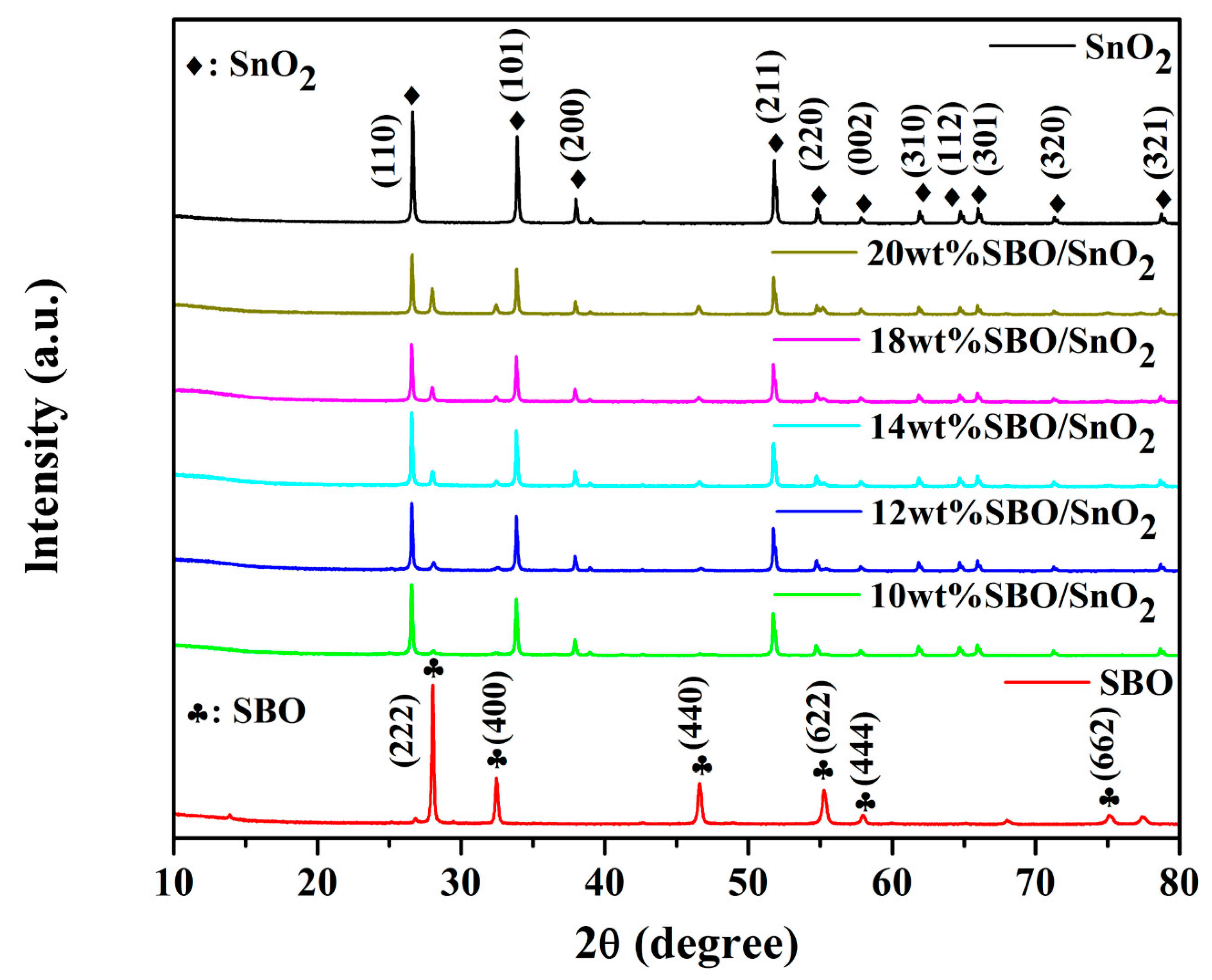
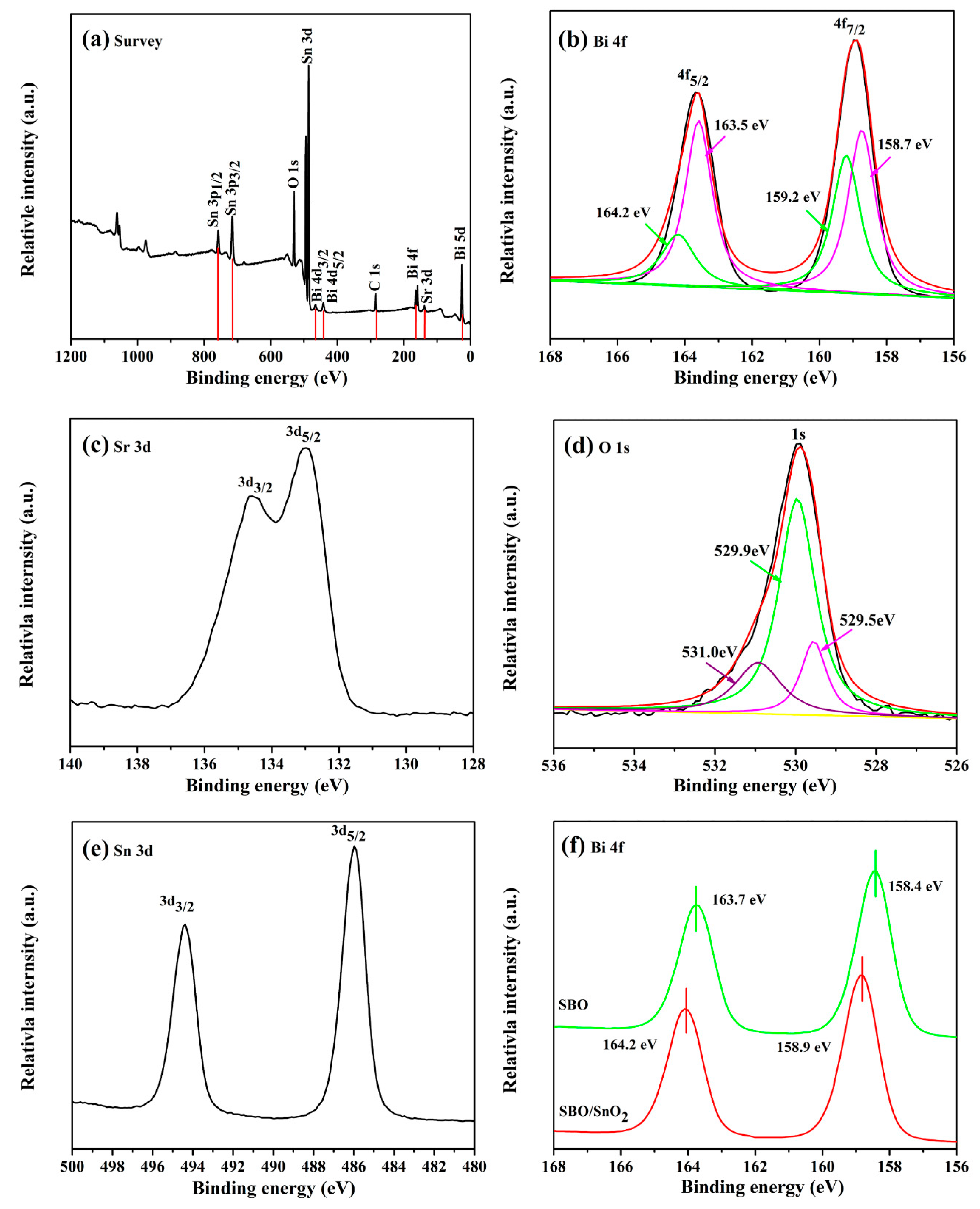
3.1. Phase Structure and Chemical Compositions
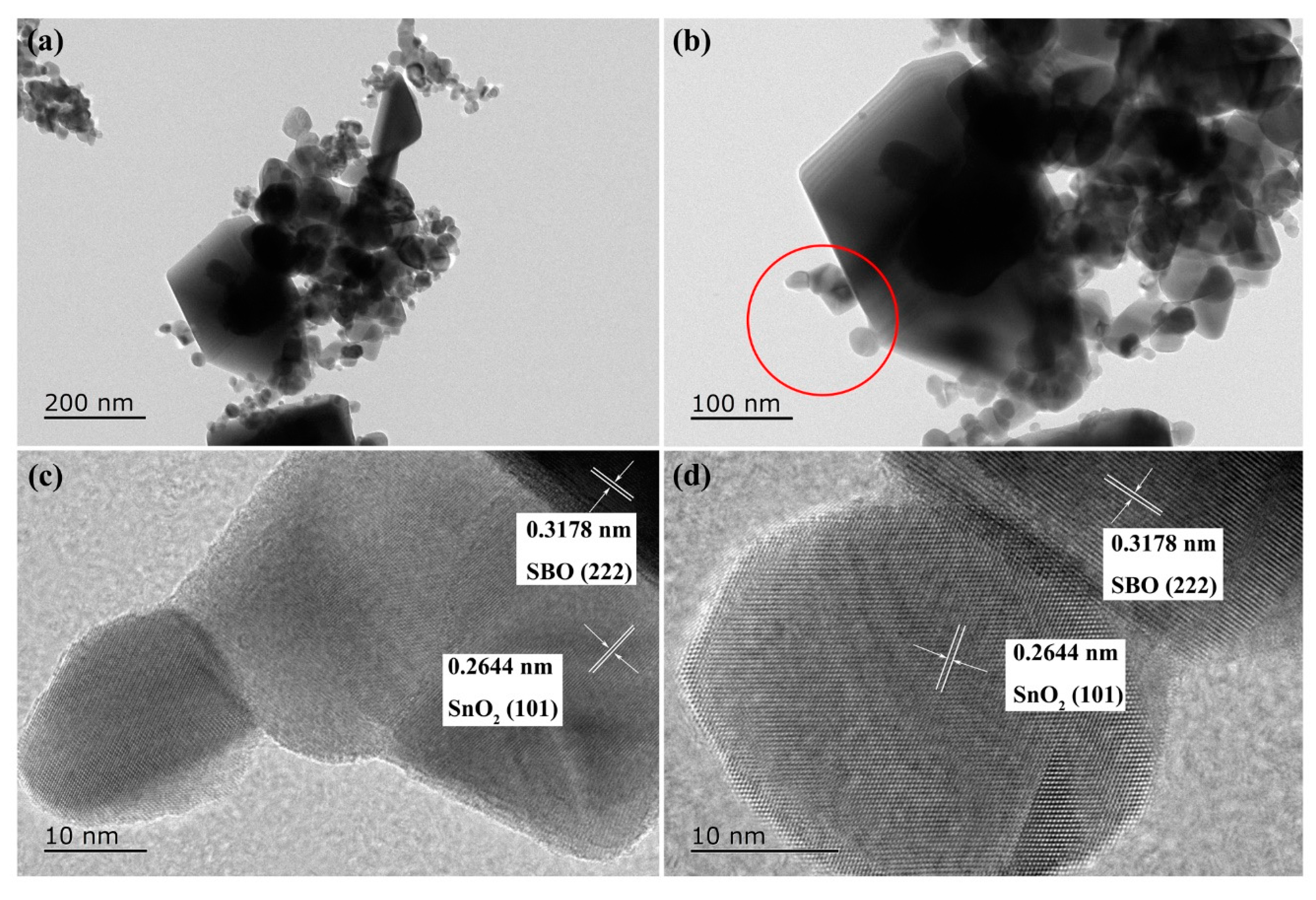
3.2. Morphology Analysis
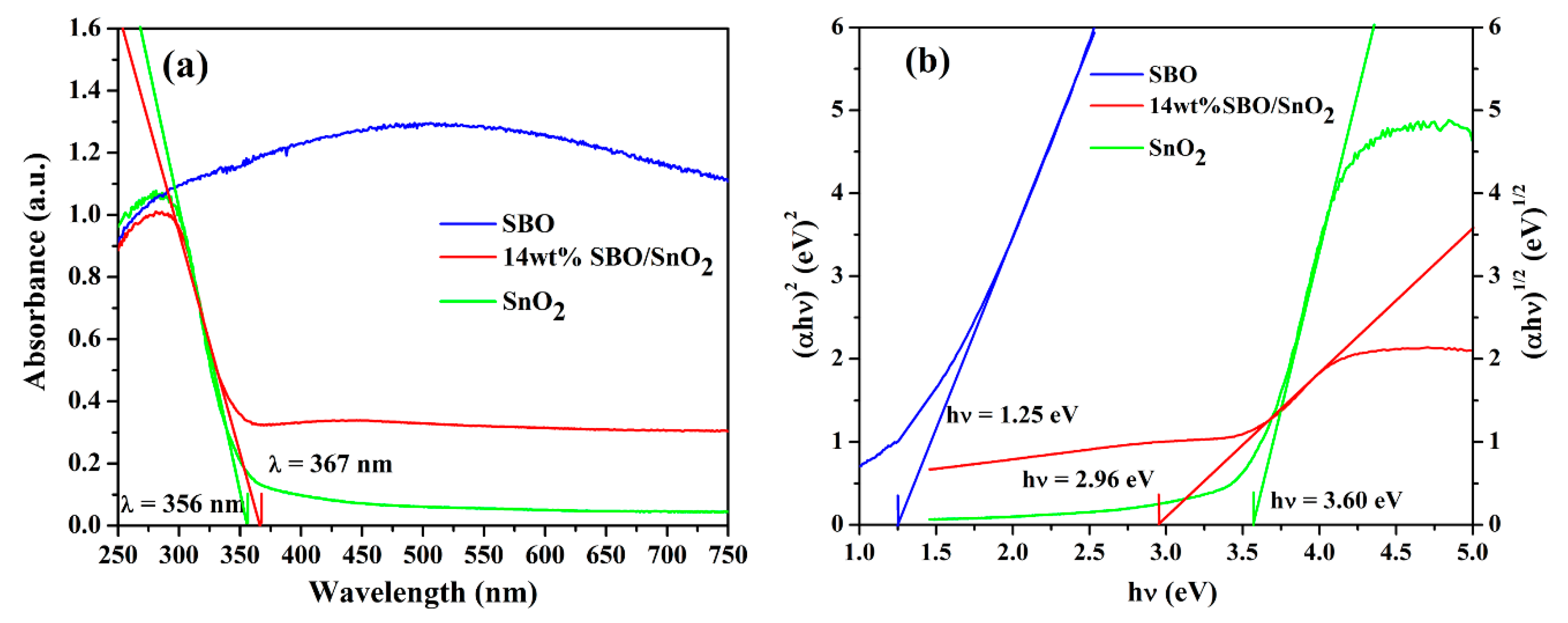
3.3. Optical Absorption Properties of SBO/SnO2 Catalysts
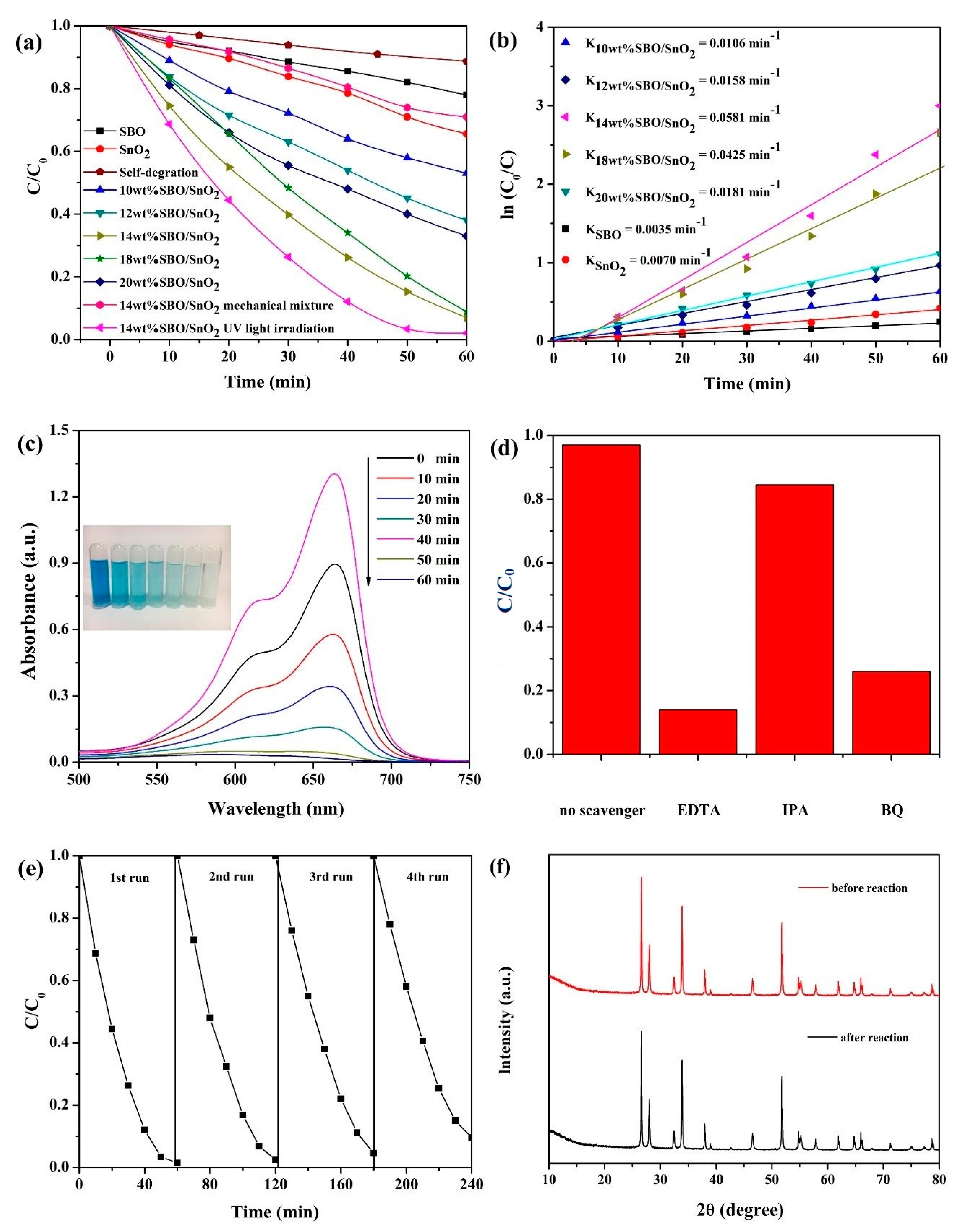
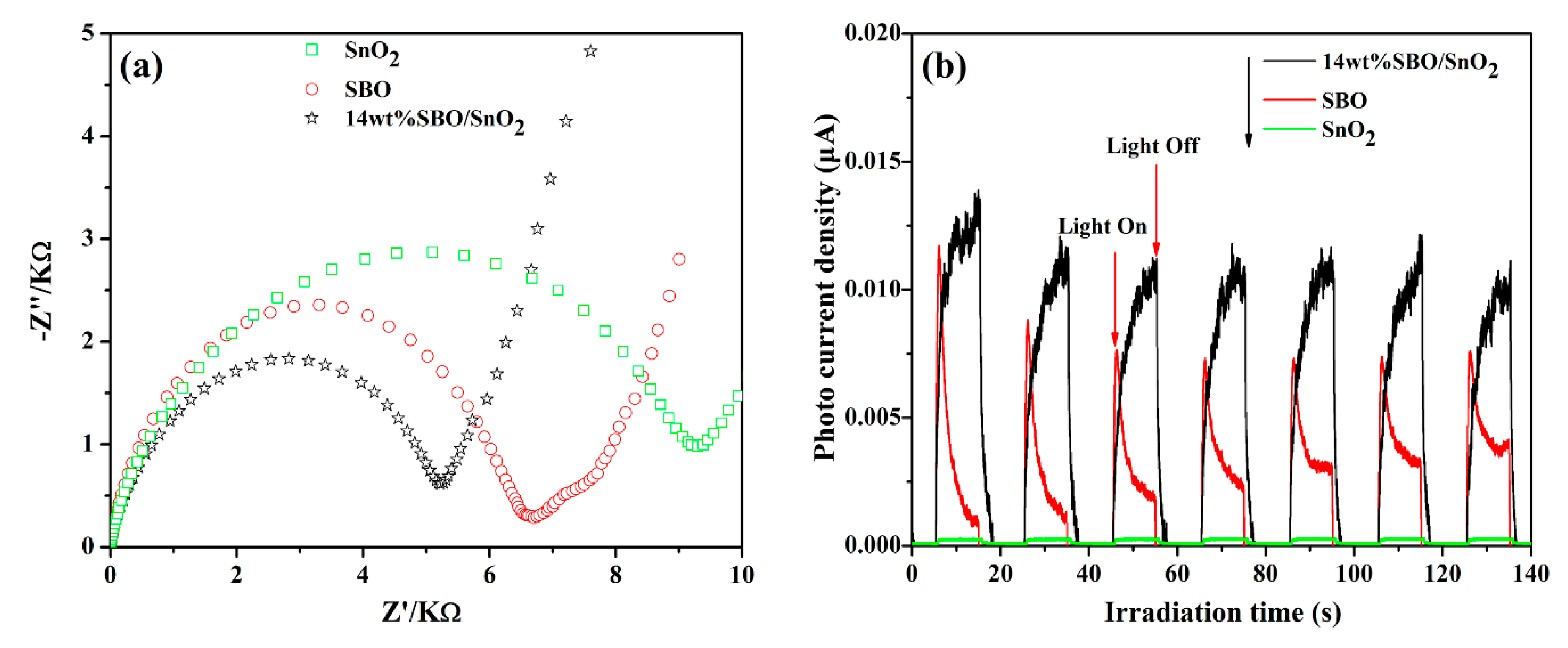
3.4. Photocatalytic Activity and Stability
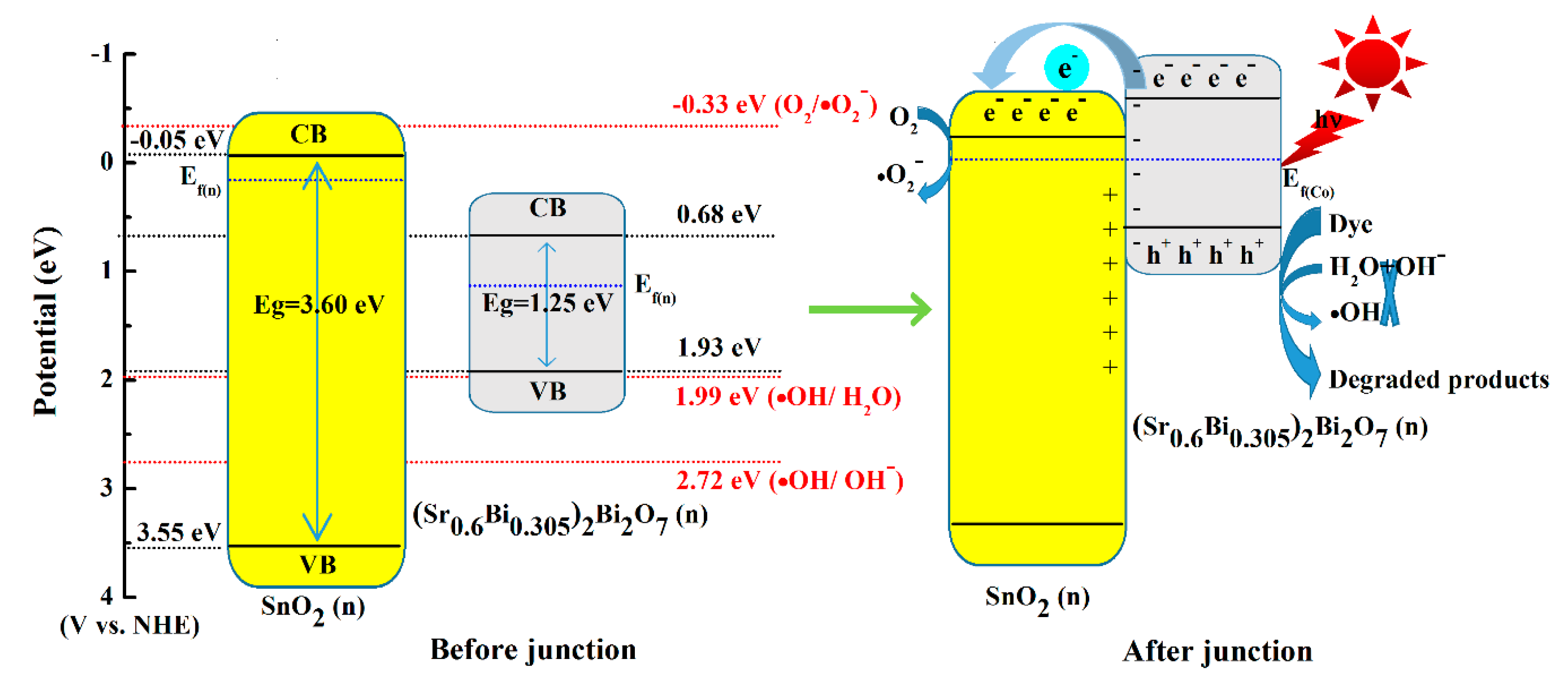
3.5. Possible Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of Pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Kowalska, E.; Yoshiiri, K.; Wei, Z.S.; Zheng, S.Z.; Kastl, E.; Reita, H.; Ohtani, B. Hybrid photocatalysts composed of titania modified with plasmonic nanoparticles and ruthenium complexes for decomposition of organic compounds. Appl. Catal. B Environ. 2015, 178, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Zhao, F.; Liu, Z.; Tong, Y.; Ji, H. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B Environ. 2016, 186, 68–76. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B Environ. 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Yi, Z.G.; Ye, J.H.; Kikugawa, N.; Kako, T.; Ouyang, S.X.; Stuart-Williams, H.; Yang, H.; Cao, J.Y.; Luo, W.J.; Li, Z.S.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under Uv/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based photocatalysts for hydrogen production. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, B.; Yu, H.; Waterhouse, G.I.; Zhou, C.; Zhao, Y.; Tahir, M.; Wu, L.Z.; Tung, C.H.; Zhang, T. Integrating metallic nanoparticles of Au and Pt with MoS2–CdS hybrids for high-efficient photocatalytic hydrogen generation via plasmon-induced electron and energy transfer. Adv. Energy Mater. 2016, 6, 1501241. [Google Scholar]
- Zhao, X.; Liu, H.; Qu, J. Photoelectrocatalytic degradation of organic contaminants at Bi2O3/TiO2 nanotube array electrode. Appl. Surf. Sci. 2011, 257, 4621–4624. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-Light-Induced Degradation of Rhodamine B by Nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H.; Am, J. A Novel Aqueous Process for Preparation of crystal form-controlled and highly crystalline BiVO4 powder from layer ed vanadates at room temperature and its photocatalytic properties. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Huang, F.; Zheng, C.; Wang, W. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Li, W.; Jia, X.; Li, P.; Zhang, B.; Zhang, H.; Geng, W.; Zhang, Q. Hollow mesoporous SiO2-BiOBr nanophotocatalyst: Synthesis, characterization and application in photodegradation of organic dyes under visible-light irradiation. ACS Sustain. Chem. Eng. 2015, 3, 1101–1110. [Google Scholar] [CrossRef]
- Wu, J.; Huang, F.; Lü, X.; Chen, P.; Wan, D.; Xu, F. Improved visible-light photocatalysis of nano-Bi2Sn2O7 with dispersed s-bands. J. Mater. Chem. 2011, 21, 3872–3876. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Ho, W.; Cao, J.; Shen, Z.; Lee, S.C. Fabrication of Bi2O2C O3/g-C3N4 heterojunctions for efficiently photocatalytic NO in air removal: Insitu self-sacrificial synthesis characterizations and mechanistic study. Appl. Catal. B Environ. 2016, 199, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Kako, T.; Zou, Z.; Katagiri, M.; Ye, J. Decomposition of organic compound over NaBiO3 under visible light irradiation. Chem. Mater. 2007, 19, 198–202. [Google Scholar] [CrossRef]
- Takei, T.; Haramoto, R.; Dong, Q.; Kumada, N.; Yonesaki, Y.; Kinomura, N.; Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Photocatalytic activities of various pentavalent bismuthates under visible light irradiation. J. Solid State Chem. 2011, 184, 2017–2022. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Liu, G.; Shen, Z.; Xia, D.; Wong, P.K.; Yu, J.C. Monoclinic dibismuth tetraoxide: A new visible-light-driven photocatalyst for environmental remediation. Appl. Catal. B Environ. 2015, 176–177, 444–453. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Yan, T.; Ji, P.; Zhao, X.; Yuan, K.; Wei, D.; Du, B. Fabrication of heterostructured Bi2O2CO3/Bi2O4 photocatalyst and efficient photodegradation of organic contaminants under visible-light. J. Hazard. Mater. 2017, 333, 169–178. [Google Scholar] [CrossRef]
- Kumada, N.; Hosoda, M.; Kinomura, N. Preparation of alkaline earth bismuth pyrochlores containing Bi5+ by low temperature hydrothermal reaction. J. Solid State Chem. 1993, 106, 476–484. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; An, H.; Zhong, Y.; Wang, D.; Tang, C.; Hu, C. (Sr0.6Bi0.305)2 Bi2O7 as a new visible-light-responsive photocatalyst: An experimental and theoretical study. Mater. Res. Bull. 2019, 118, 110484. [Google Scholar] [CrossRef]
- Liang, N.; Wang, M.; Jin, L.; Huang, S.; Chen, W.; Xu, M.; He, Q.; Zai, J.; Fang, N.; Qian, X. Highly efficient Ag2O/Bi2O2CO3 pn heterojunction photocatalysts with improved visible-light responsive activity. ACS Appl. Mater. Interfaces 2014, 6, 11698–11705. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yu, J.; Zhang, Y.; Cui, Z.; Sun, Y.; Hou, B. Fabrication of InVO4/AgVO3 heterojunctions with enhanced photocatalytic antifouling efficiency under visible-light. Appl. Catal. B Environ. 2018, 220, 57–66. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Xu, M.; Han, L.; Dong, S. Facile fabrication of highly efficient g-C3N4/Ag2O heterostructured photocatalysts with enhanced visible-Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2013, 5, 12533–12540. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; An, H.; Hao, W.; Wei, J.; Dai, Y.; Ma, C. Preparation of 2D/2D g-C3N4 nanosheet@ZnIn2S4 nanoleaf heterojunctions with well-designed high-speed charge transfer nanochannels towards high-efficiency photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 220, 542–552. [Google Scholar] [CrossRef]
- Yang, G.; Yan, Z.; Xiao, T. Preparation and characterization of SnO2/ZnO/TiO2 composite semiconductor with enhanced photocatalytic activity. Appl. Surf. Sci. 2012, 258, 8704–8712. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Müller, M.M.; Kleebe, H.; Ziegler, J.; Jaegermann, W. Stability, durability and regeneration ability of a novel Ag-based photocatalyst Ag2Nb4O11. Inorg. Chem. 2012, 54, 7764–7773. [Google Scholar]
- Wen, X.; Niu, C.; Zhang, L.; Zeng, G. Fabrication of SnO2 nanopaticles/BiOI n–p heterostructure for wider spectrum visible-light photocatalytic degradation of antibiotic oxytetracycline hydrochloride. ACS Sustain. Chem. Eng. 2017, 5, 5134–5147. [Google Scholar] [CrossRef]
- Alshehri, N.; Al-Marzouki, F.; Alshehrie, A.; Hafez, M. Synthesis, characterization and band alignment characteristics of NiO/SnO2 bulk heterojunction nano architecture for promising photocatalysis applications. J. Alloys Compd. 2018, 757, 161–168. [Google Scholar] [CrossRef]
- Inderan, V.; Lim, S.Y.; Ong, T.S.; Bastien, S.; Braidy, N.; Lee, H.L. Synthesis and characterizations of SnO2 nanorods via low temperature hydrothermal method. Superlattices Microstruct. 2015, 88, 396–402. [Google Scholar] [CrossRef]
- Hu, C.H.; Zhuang, J.; Zhong, L.S.; Zhong, Y.; Wang, D.H.; Zhou, H.Y. Significantly enhanced photocatalytic activity of visible light responsive AgBr/Bi2Sn2O7 heterostructured composites. Appl. Surf. Sci. 2017, 426, 1173–1181. [Google Scholar] [CrossRef]
- Xia, D.; Wang, W.; Yin, R.; Jiang, Z.; An, T.; Li, G.; Zhao, H.; Wong, P.K. Enhanced photocatalytic inactivation of Escherichia coli by a novel Z-scheme g-C3N4/m-Bi2O4 hybrid photocatalyst under visible light: The role of reactive oxygen species. Appl. Catal. B Environ. 2017, 214, 23–33. [Google Scholar] [CrossRef]
- Tian, J.; Hao, P.; Wei, N.; Cui, H.; Liu, H. 3D Bi2MoO6 Nanosheet/TiO2 Nanobelt Heterostructure: Enhanced Photocatalytic Activities and Photoelectochemistry Performance. ACS. Catal. 2015, 5, 4530–4536. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, X.; Zhao, J.; Yang, P. Assembled fabrication of α-Fe2O3/BiOCl heterojunctions with enhanced photocatalytic performance. Appl. Surf. Sci. 2018, 430, 585–594. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, C.H.; Zhuang, J.; Zhong, Y.; Wang, D.H.; Zhou, H.Y. AgBr/MgBi2O6 heterostructured composites with highly efficient visible-light-driven photocatalytic activity. J. Phys. Chem. Solids 2018, 117, 94–100. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Yin, J.; Huang, S.; Jian, Z.; Wang, Z.; Zhang, Y. Fabrication of heterojunction SnO2/BiVO4 composites having enhanced visible light photocatalystic activity. Mater. Sci. Semicond. Process. 2015, 34, 198–204. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zhu, Y. Visible photocatalytic activity enhancement of ZnWO4 by graphene hybridization. ACS Catal. 2012, 2, 2769–2778. [Google Scholar] [CrossRef]
- Shi, F.; Yang, C.G.; Niu, D.W. SrTiO3 nanocubes decorated with Ag/AgCl nanoparticles as photocatalysts with enhanced visible-light photocatalytic activity towards the degradation of dyes, phenol and bisphenol A. Environ Sci. 2017, 4, 585–595. [Google Scholar]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. AM Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Zhang, S.W.; Li, J.X.; Zeng, M.Y.; Zhao, G.X.; Xu, J.Z.; Hu, W.P. In situ synthesis of water-soluble magnetic graphitic carbon nitride photocatalyst and its synergistic catalytic performance. ACS Appl. Mater. Interface 2013, 5, 12735–12743. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.L.; Xu, C.X.; Chen, S.F.; Fu, X.L. Ag3PO4/ZnO: An efficient visible-light-sensitized composite application in photocatalytic degradation of Rhodamine B with its application in photocatalytic degradation of Rhodamine B. Mater. Res. Bull. 2013, 48, 106–113. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Wang, X.; An, H.; Zhong, Y.; Wang, D.; Tang, C.; Hu, C. Facile One-Step Hydrothermal Fabrication of (Sr0.6Bi0.305)2Bi2O7/SnO2 Heterojunction with Excellent Photocatalytic Activity. Nanomaterials 2020, 10, 321. https://doi.org/10.3390/nano10020321
Zhu D, Wang X, An H, Zhong Y, Wang D, Tang C, Hu C. Facile One-Step Hydrothermal Fabrication of (Sr0.6Bi0.305)2Bi2O7/SnO2 Heterojunction with Excellent Photocatalytic Activity. Nanomaterials. 2020; 10(2):321. https://doi.org/10.3390/nano10020321
Chicago/Turabian StyleZhu, Di, Xinling Wang, Huiting An, Yan Zhong, Dianhui Wang, Chengying Tang, and Chaohao Hu. 2020. "Facile One-Step Hydrothermal Fabrication of (Sr0.6Bi0.305)2Bi2O7/SnO2 Heterojunction with Excellent Photocatalytic Activity" Nanomaterials 10, no. 2: 321. https://doi.org/10.3390/nano10020321
APA StyleZhu, D., Wang, X., An, H., Zhong, Y., Wang, D., Tang, C., & Hu, C. (2020). Facile One-Step Hydrothermal Fabrication of (Sr0.6Bi0.305)2Bi2O7/SnO2 Heterojunction with Excellent Photocatalytic Activity. Nanomaterials, 10(2), 321. https://doi.org/10.3390/nano10020321





