Nano-Sized Fe(III) Oxide Particles Starting from an Innovative and Eco-Friendly Synthesis Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ferrihydrite Nanoparticles by Ion Exchange Process
2.3. Production of Hematite Nanoparticles from Ferrihydrite as Precursor
2.4. Characterization of the Nanoparticles
3. Results and Discussion
3.1. Production and Characterization of Ferrihydrite NPs
3.2. Production and Characterization of Hematite NPs
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cornell, M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Blake, R.L.; Hessevick, R.E.; Zoltai, T.; Finger, L.W. Refinement of the hematite structure. Am. Mineral. 1966, 51, 123–129. [Google Scholar]
- Lu, H.; Meng, X.K. Morin Temperature and Néel Temperature of Hematite Nanocrystals. J. Phys. Chem. C 2010, 114. [Google Scholar] [CrossRef]
- Qin, W.; Yang, C.; Yi, R.; Gao, G. Hydrothermal Synthesis and Characterization of Single-Crystalline a-Fe2O3 Nanocubes. J. Nanomater. 2011. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Deng, L.; Caballero-Guzman, A.; Nowack, B. Are engineered nano iron oxide particles safe? An environmental risk assessment by probabilistic exposure, effects and risk modelling. Nanotoxicology 2016, 10, 1545–1554. [Google Scholar] [CrossRef]
- Figuerola, A.; Corato, R.; Manna, L.; Pellegrino, T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol. Res. 2010, 62, 126–143. [Google Scholar] [CrossRef]
- Sivula, K.; Zboril, R.; Le Formal, F.; Robert, R.; Weidenkaff, A.; Tucek, J.; Frydrych, J.; Grätzel, M. Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J. Am. Chem. Soc. 2010, 132, 7436–7444. [Google Scholar] [CrossRef]
- Schwaminger, S.; Surya, R.; Filser, S.; Wimmer, A.; Weigl, F.; Fraga-García, P.; Berensmeiern, S. Formation of iron oxide nanoparticles for the photooxidation of water: Alteration of finite size effects from ferrihydrite to hematite. Sci. Rep. 2017, 7, 12609. [Google Scholar] [CrossRef] [Green Version]
- Nidhin, M.; Indumathy, R.; Sreeram, K.; Unni Nair, B. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull. Mater. Sci. 2008, 31, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Xinglong, G.; Guoxiu, W.; Jinsoo, P.; Hao, L.; Juan, Y. Monodisperse hematite porous nanospheres: Synthesis, characterization, and applications for gas sensors. Nanotechnology 2008, 19, 125606. [Google Scholar] [CrossRef]
- Hermanek, M.; Zboril, R.; Medrik, I.; Pechousek, J.; Gregor, C. Catalytic Efficiency of Iron(III) Oxides in Decomposition of Hydrogen Peroxide: Competition between the Surface Area and Crystallinity of Nanoparticles. J. Am. Chem. Soc. 2007, 129, 10929–10936. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Jana, T.; De, K.; Das, S.; Dey, K.; Chatterjee, K. Morphology dependent magnetic properties of a-Fe2O3 nanostructures. Mater. Res. Express 2014, 1, 046104. [Google Scholar] [CrossRef]
- Chaudharia, S.; Srinivasan, M. 1D hollow a-Fe2O3 electrospun nanofibers as high-performance anode material for lithium ion batteries. J. Mater. Chem. 2012, 22, 23049–23056. [Google Scholar] [CrossRef]
- Dewei, W.; Qihua, W.; Tingmei, W. Controlled synthesis of mesoporous hematite nanostructures and their application as electrochemical capacitor electrodes. Nanotechnology 2011, 22, 135604. [Google Scholar] [CrossRef]
- Subarna, M.; Soumen, D.; Kalyan, M.; Subhadra, C. Synthesis of a α-Fe2O3 nanocrystal in its different morphological attributes: Growth mechanism, optical and magnetic properties. Nanotechnology 2007, 18, 275608. [Google Scholar] [CrossRef]
- Katsuki, H.; Choi, E.; Lee, W.; Hwang, K.; Cho, W.; Huan, W.; Komarneni, S. Ultrafast microwave-hydrothermal synthesis of hexagonal plates of hematite. Mater. Chem. Phys. 2018, 205, 210–216. [Google Scholar] [CrossRef]
- Soltis, J.A.; Feinberg, J.M.; Gilbert, B.; Lee Penn, R. Phase transformation and particle-mediated growth in the formation of hematite from 2-line ferrihydrite. Cryst. Growth Des. 2016, 16, 922–932. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Jim Hendry, M.; Essilfie-Dughan, J. Transformation of two-line ferrihydrite to goethite and hematite as a function of pH and temperature. Environ. Sci. Technol. 2011, 45, 268–275. [Google Scholar] [CrossRef]
- Burrows, N.D.; Hale, C.R.; Lee Penn, R. Effect of pH on the kinetics of crystal growth by oriented aggregation. Cryst. Growth Des. 2013, 13, 3396–3403. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Dekkers, M.; Barrón, V.; Torrent, J.; Roberts, A. Control of Earth-like magnetic fields on the transformation of ferrihydrite to hematite and goethite. Sci. Rep. 2016, 6, 30395. [Google Scholar] [CrossRef] [Green Version]
- Schwertmann, U.; Friedl, J.; Stanjek, H. From Fe(III) ions to Ferrihydrite and then to Hematite. J. Colloid Interface Sci. 1999, 209, 215–223. [Google Scholar] [CrossRef]
- Chernyshova, I.; Hochella, M.; Madden, A. Size-dependent structural transformations of hematite nanoparticles. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Stolyar, S.; Yaroslavtsev, R.; Bayukov, O.; Balaev, D.; Krasikov, A.; Iskhakov, R.; Vorotynov, A.; Ladygina, V.; Purtov, K.; Volochaev, M. Preparation, structure and magnetic properties of synthetic ferrihydrite nanoparticles. J. Phys. Conf. Ser. 2018, 994, 012003. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Li, K. One-step synthesis of mesoporous two-line ferrihydrite for effective elimination of arsenic contaminants from natural water. Dalton Trans. 2011, 40, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- Rout, K.; Mohapatra, M.; Anand, S. 2-line ferrihydrite: Synthesis, characterization and its adsorption behavior for removal of Pb(II), Cd(II), Cu(II) and Zn(II) from aqueous solutions. Dalton Trans. 2012, 41, 3302–3312. [Google Scholar] [CrossRef]
- Huffman, G.; Ganguly, B.; Zhao, J.; Rao, K.R.; Shah, N.; Feng, Z.; Huggins, F.E.; Mehdi Taghiei, M.; Lu, F. Structure and dispersion of iron-based catalysts for direct coal liquefaction. Energy Fuels 1993, 7, 285–296. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ukita, M.; Sakuma, R.; Nakanishi, M.; Fujii, T.; Imanishi, N.; Takada, J. Bio-inspired 2-line ferrihydrite as a high-capacity and high-rate-capability anode material for lithium-ion batteries. J. Power Sources 2016, 328, 503–509. [Google Scholar] [CrossRef]
- Pariona, N.; Martinez, A.; Hdz-Garci, H.; Cruz, L.; Hernandez-Valdes, A. Effects of hematite and ferrihydrite nanoparticles on germination and growth of maize seedlings. Saudi J. Biol. Sci. 2017, 24, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Liu, Q.; Roberts, A.P.; Barrón, V.; Torrent, J.; Zhang, Q. A new model for transformation of ferrihydrite to hematite in soils and sediments. Geology 2018, 46, 987–990. [Google Scholar] [CrossRef] [Green Version]
- Jambor, J.; Dutrizac, J. Occurrence and Constitution of Natural and Synthetic Ferrihydrite, a Widespread Iron Oxyhydroxide. Chem. Rev. 1998, 98, 2549–2586. [Google Scholar] [CrossRef]
- Cornell, R.; Giovanoli, R.; Schneider, W. Review of the hydrolysis of iron(III) and the crystallization of amorphous iron(III) hydroxide. J. Chem. Technol. Biotechnol. 2007, 46, 115–134. [Google Scholar] [CrossRef]
- Taglieri, G.; Felice, B.; Daniele, V.; Volpe, R.; Mondelli, C. Analysis of the carbonatation process of nanosized Ca(OH)2 particles synthesized by exchange ion process. J. Nanoeng. Nanosyst. 2016, 230, 25–31. [Google Scholar] [CrossRef]
- Taglieri, G.; Daniele, V.; Macera, L. Synthesizing Alkaline Earth Metal Hydroxides Nanoparticles through an Innovative, Single-Step and Eco-Friendly Method. Solid State Phenom. 2019, 286, 3–14. [Google Scholar] [CrossRef]
- Taglieri, G.; Felice, B.; Daniele, V.; Ferrante, F. Mg(OH)2 nanoparticles produced at room temperature by an innovative, facile and scalable synthesis route. J. Nanoparticles Res. 2015, 17, 411–424. [Google Scholar] [CrossRef]
- Taglieri, G.; Daniele, V.; Mondelli, C. MgO nanoparticles synthesized starting from an innovative one-step process. J. Am. Ceram. Soc. 2018, 101, 1780–1789. [Google Scholar] [CrossRef]
- Bish, D.; Post, J. Modern Powder Diffraction, 1st ed.; Mineralogical Society of America: Washington, DC, USA, 1989. [Google Scholar]
- Tüysüz, H.; Salabaş, E.; Weidenthaler, C.; Schüth, F. Synthesis and Magnetic Investigation of Ordered Mesoporous 2-Line Ferrihydrite. Am. Chem. Soc. 2008, 130, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Blanton, T. International Centre for Diffraction Data; ICDD 00-065-0727; Private Communication: Newtown Square, PA, USA, 2014. [Google Scholar]
- Hausner, D.; Bhandari, N.; Pierre-Louis, A.; Kubicki, J.; Strongin, D. Ferrihydrite reactivity toward carbon dioxide. J. Colloid Interface Sci. 2009, 337, 492–500. [Google Scholar] [CrossRef]
- Rahman, M.; Khan, S.; Jamal, A.; Faisal, A.; Aisiri, A. Iron Oxide Nanoparticles. Nanomaterials 2011. [Google Scholar] [CrossRef]
- Zhu, B.S.; Jia, Y.; Jin, Z.; Sun, B.; Luo, T.; Kong, L.; Liu, J.H. A facile precipitation synthesis of mesoporous 2-line ferrihydrite with good removal properties. RSC Adv. 2015, 103, 84389. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Miller, D.; Biesinger, M.; Mc Intyre, N. Interactions of CO2 and CO at fractional atmosphere pressures with iron and iron oxide surfaces: One possible mechanism for surface contamination. Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]
- D’Archivio, A.; Maggi, M.; Odoardi, A.; Santucci, S.; Passacantando, M. Adsorption of triazine herbicides from aqueous solution by functionalized multiwall carbon nanotubes grown on silicon substrate. Nanotechnology 2018, 29, 065701. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, M.; Limosani, F.; Passacantando, M.; D’Orazio, F.; Nardone, M.; Cacciotti, I.; Arduini, F.; Gautron, E.; De Crescenzi, M. Influence of Iron Catalyst in the Carbon Spheres Synthesis for Energy and Electrochemical Applications. Adv. Mater. Interfaces 2018, 5, 1800070. [Google Scholar] [CrossRef]
- Preisinger, M.; Krispin, M.; Rudolf, T.; Horn, S.; Strongin, D. Electronic structure of nanoscale iron oxide particles measured by scanning tunneling and photoelectron spectroscopies. Phys. Rev. B 2005, 71, 165409. [Google Scholar] [CrossRef] [Green Version]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice, 1st ed.; Geological Institute of Hungary: Budapest, Hungary, 2011. [Google Scholar]
- Zhang, X.; Chen, Y.; Zhao, N.; Liuac, H.; Wei, Y. Citrate modified ferrihydrite microstructures: Facile synthesis, strong adsorption and excellent Fenton like catalytic properties. RSC Adv. 2014, 41, 21575–22158. [Google Scholar] [CrossRef]
- Hiemstra, T. Formation, stability, and solubility of metal oxide nanoparticles: Surface entropy, enthalpy, and free energy of ferrihydrite. Geochimica et Cosmochimica Acta 2015, 158, 179–198. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Stafford, K.; Sing, W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1429–1715. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.; Pelletier, M.; Michot, L.; Stradner, A.; Schurtenbergerc, P.; Kretzschmar, R. Characterization of the pores in hydrous ferric oxide aggregates formed by freezing and thawing. J. Colloid Interface Sci. 2004, 271, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Křížek, M.; Pechoušek, J.; Tuček, J.; Šafářová, K.; Medřík, I.; Machala, L. Iron Oxide Nanoparticle Powders with High Surface Area. AIP Conf. Proc. 2012, 1489, 88. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.; Martis, V.; Parkin, I. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef] [Green Version]
- Villalobos, M.; Antelo, J. A unified surface structural model for ferrihydrite: Proton charge, electrolyte binding, and arsenate adsorption. Rev. Int. Contam. Ambient. 2011, 27, 139–151. [Google Scholar]
- Pariona, N.; Camacho, K.; Ramos-González, R.; Martinez, A.; Herrera-Trejo, M.; Baggio-Saitovitch, E. Magnetic and structural properties of ferrihydrite/hematite nanocomposites. J. Magn. Magn. Mater. 2016, 406, 221–227. [Google Scholar] [CrossRef]
- Rani, C.; Tiwari, S. Estimation of particle magnetic moment distribution for antiferromagnetic ferrihydrite nanoparticles. J. Magn. Magn. Mater. 2015, 385, 272–276. [Google Scholar] [CrossRef]
- Pannalal, S.; Crowe, S.; Cioppa, M.; Symons, D.T.; Sturm, A.; Fowle, D.A. Room-temperature magnetic properties of ferrihydrite: A potential magnetic remanence carrier. Earth Planet. Sci. Lett. 2005, 236, 856–870. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; Mc Intyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Barrón, V.; Andrés-Vergés, M.; Serna, C.J.; Veintemillas-Verdaguer, S.; Morales, M.P.; Lázaro, F.J. Detailed magnetic monitoring of the enhanced magnetism of ferrihydrite along its progressive transformation into hematite. J. Geophys. Res. Solid Earth 2016, 121, 4118–4129. [Google Scholar] [CrossRef]

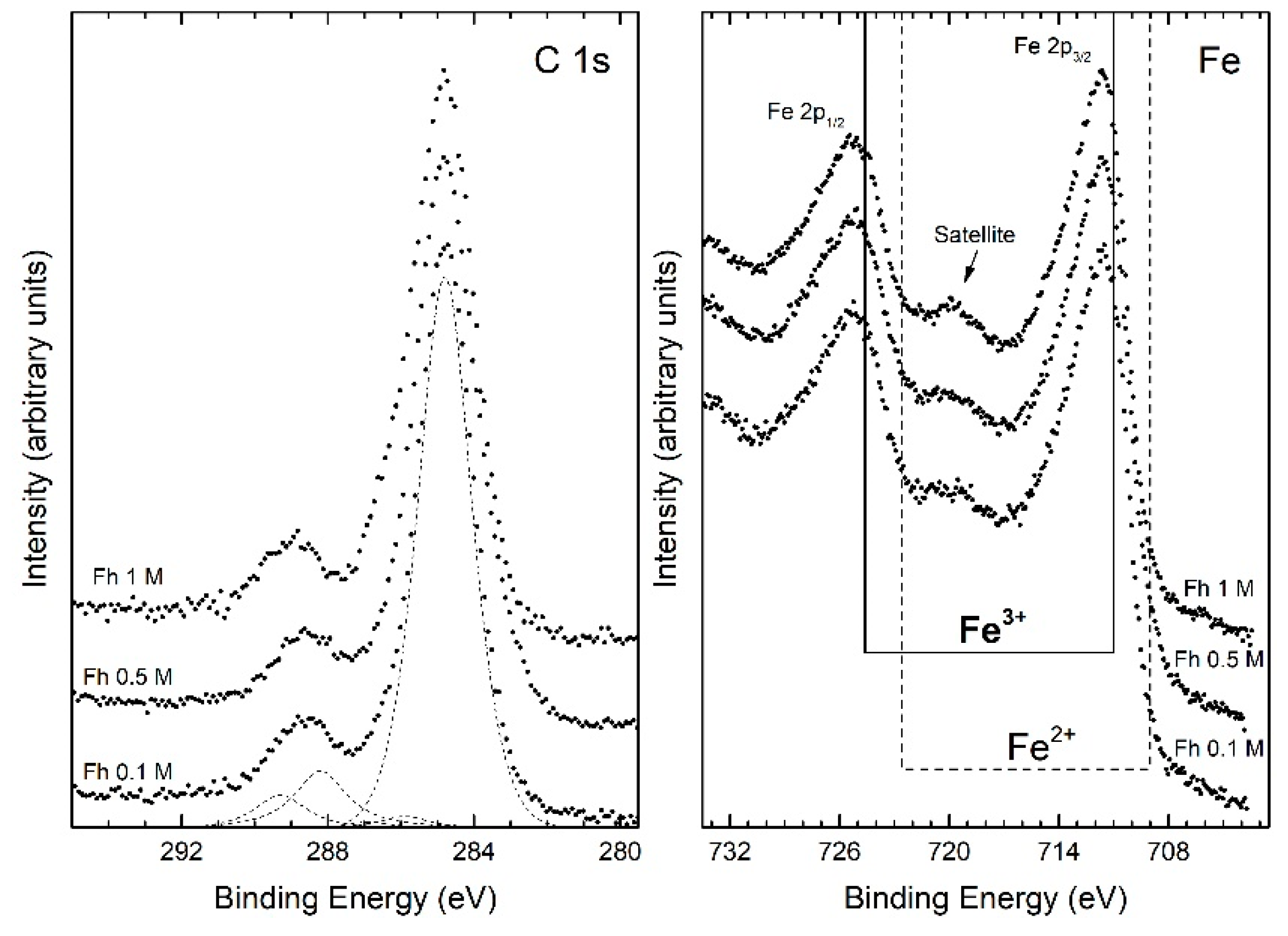


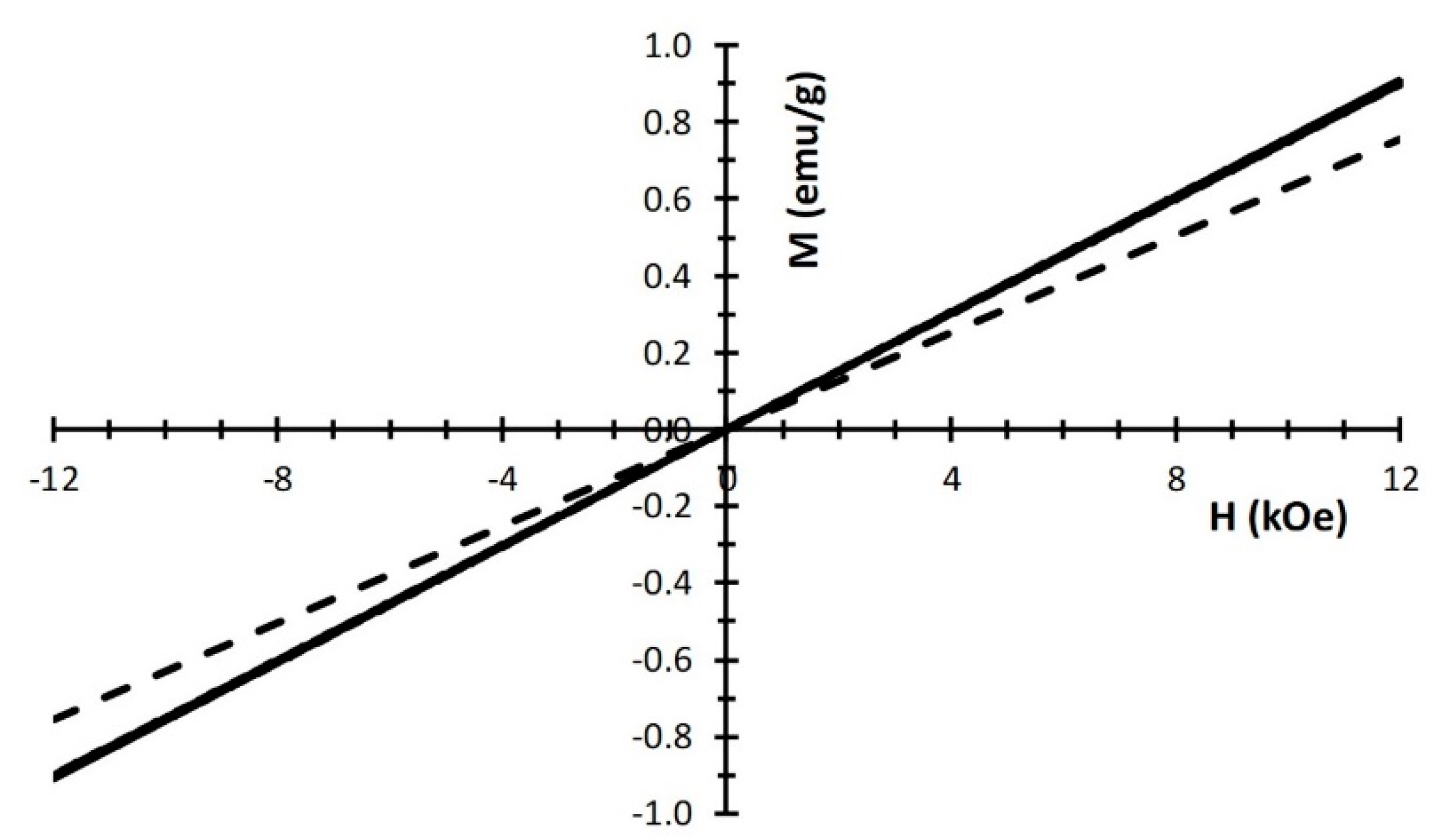





| Fh0.1M | Fh0.5M | Fh1M | |||||||
|---|---|---|---|---|---|---|---|---|---|
| t (minutes) | CC (mg/L) | Y (%) | pH | CC (mg/L) | Y (%) | pH | CC (mg/L) | Y (%) | pH |
| 0 | 10,635 | - | 3 | 53,175 | - | 3 | 106,350 | - | 3 |
| 0.5 | 323 | 97.0 | 5 | 352 | 99.3 | 5 | 502 | 99.5 | 5 |
| 1 | 98 | 99.1 | 6 | 107 | 99.8 | 6 | 190 | 99.8 | 6 |
| 3 | 27 | 99.7 | 7 | 40 | 99.9 | 7 | 55 | 99.9 | 7 |
| 5 | 23 | 99.8 | 7 | 32 | 99.9 | 7 | 39 | 99.9 | 7 |
| 15 | 23 | 99.8 | 7 | 23 | 99.9 | 7 | 29 | 99.9 | 7 |
| 30 | 19 | 99.8 | 7 | 23 | 99.9 | 7 | 26 | 99.9 | 7 |
| 60 | 19 | 99.8 | 7 | 20 | 99.9 | 7 | 22 | 99.9 | 7 |
| Sample | BJH Pore Diameter (nm) | BJH Pore Volume (cc/g) | BET Surface Area (m2/g) |
|---|---|---|---|
| Fh0.1M | 3.4 | 0.253 | 283 |
| Fh0.5M | 3.0 | 0.236 | 305 |
| Fh1M | 1.5 | 0.238 | 421 |
| Fh1M fast | 1.5 | 0.144 | 327 |
| Sample | BJH Pore Diameter (nm) | BJH Pore Volume (cc/g) | BET Surface Area (m2/g) |
|---|---|---|---|
| HRT2 | 2.9 | 0.261 | 263 |
| HRT7 | 3.5 | 0.200 | 154 |
| H500 | 6.5 | 0.221 | 57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macera, L.; Taglieri, G.; Daniele, V.; Passacantando, M.; D’Orazio, F. Nano-Sized Fe(III) Oxide Particles Starting from an Innovative and Eco-Friendly Synthesis Method. Nanomaterials 2020, 10, 323. https://doi.org/10.3390/nano10020323
Macera L, Taglieri G, Daniele V, Passacantando M, D’Orazio F. Nano-Sized Fe(III) Oxide Particles Starting from an Innovative and Eco-Friendly Synthesis Method. Nanomaterials. 2020; 10(2):323. https://doi.org/10.3390/nano10020323
Chicago/Turabian StyleMacera, Ludovico, Giuliana Taglieri, Valeria Daniele, Maurizio Passacantando, and Franco D’Orazio. 2020. "Nano-Sized Fe(III) Oxide Particles Starting from an Innovative and Eco-Friendly Synthesis Method" Nanomaterials 10, no. 2: 323. https://doi.org/10.3390/nano10020323






