Facile Synthesis of Cobalt-Doped Porous Composites with Amorphous Carbon/Zn Shell for High-Performance Microwave Absorption
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
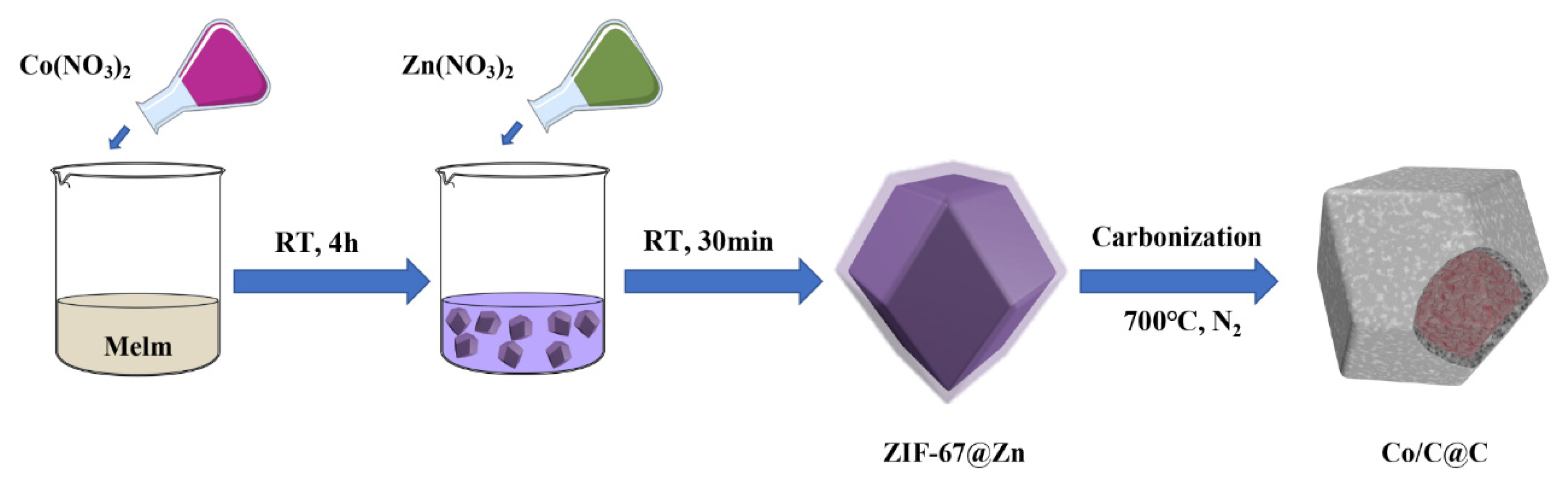
2.2. Preparation
2.3. Electromagnetic Parameter Measurements
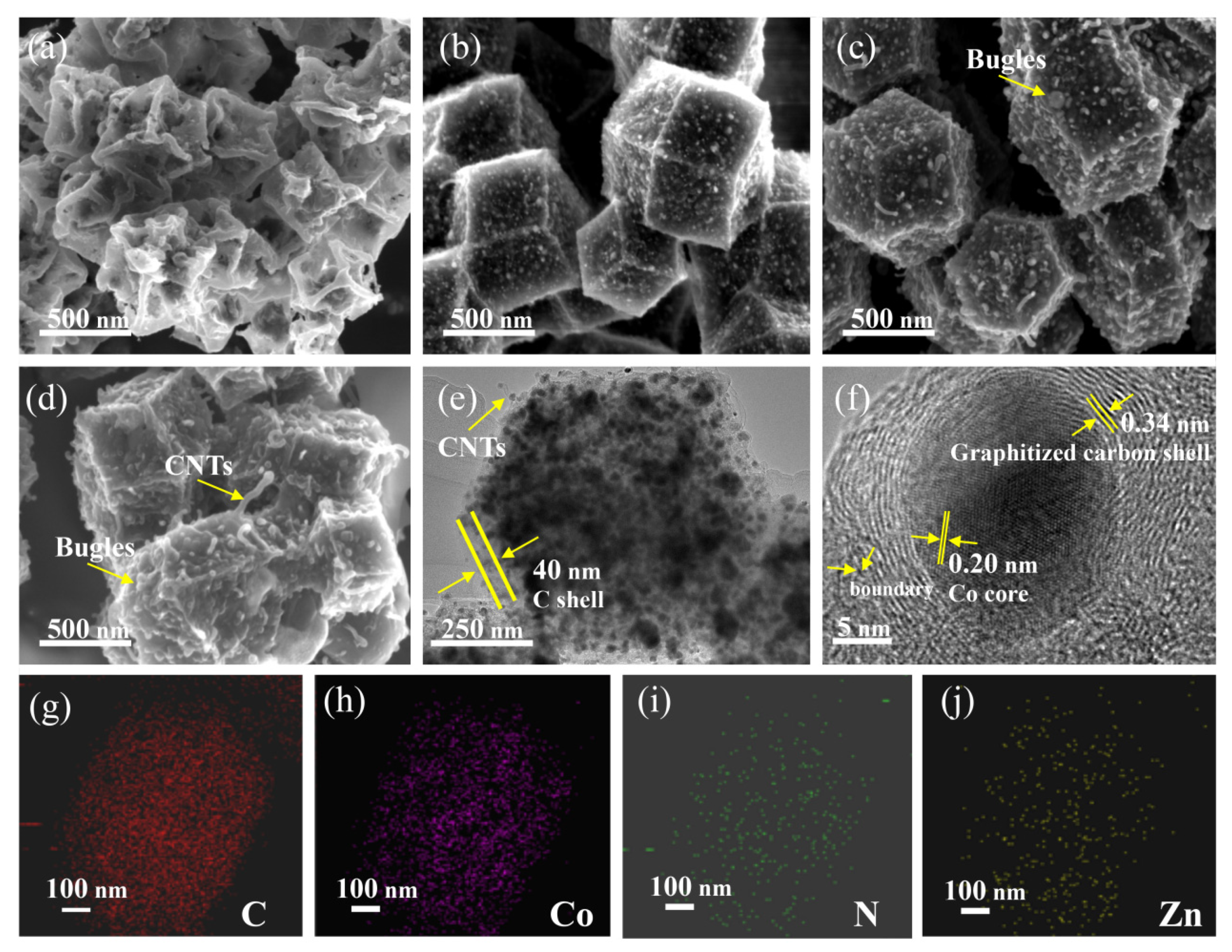
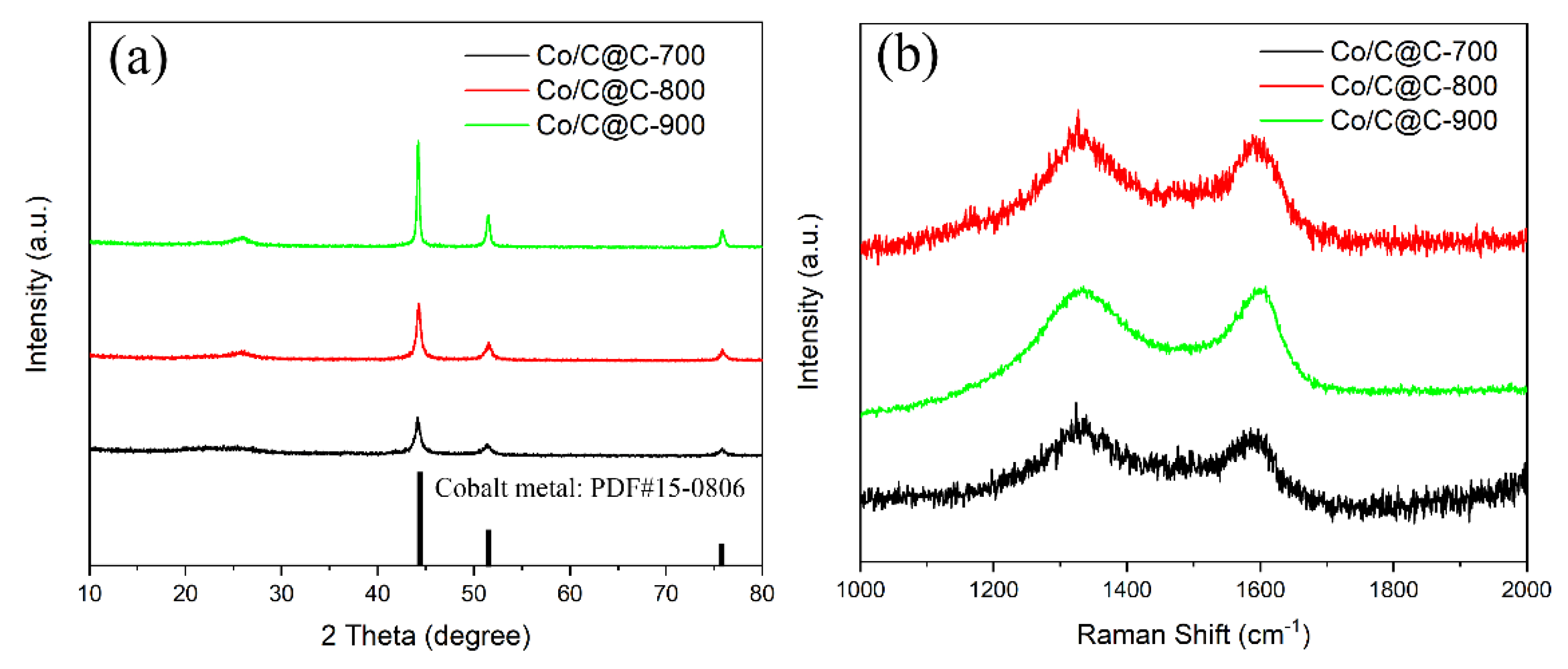
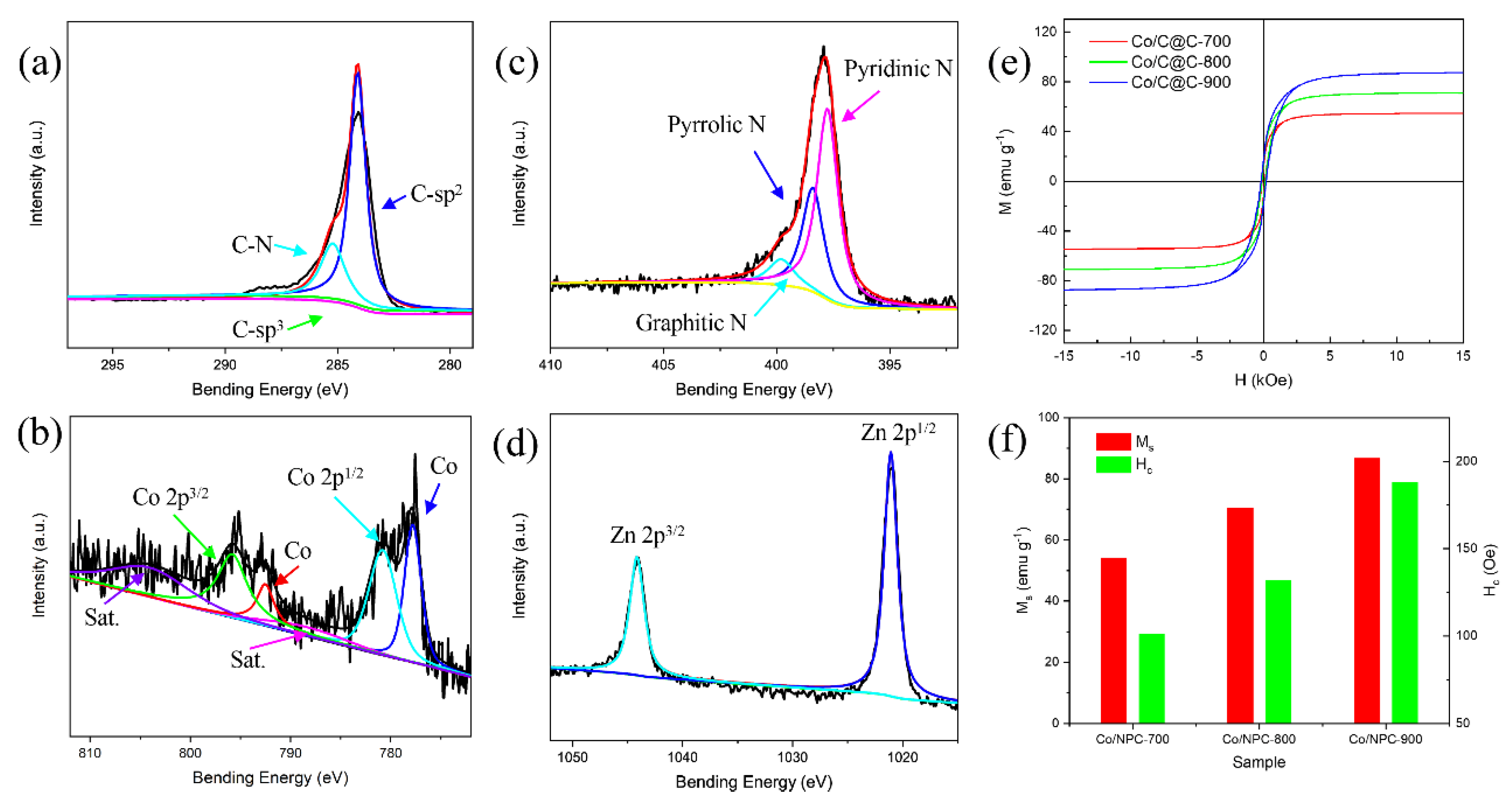
2.4. Characterizations
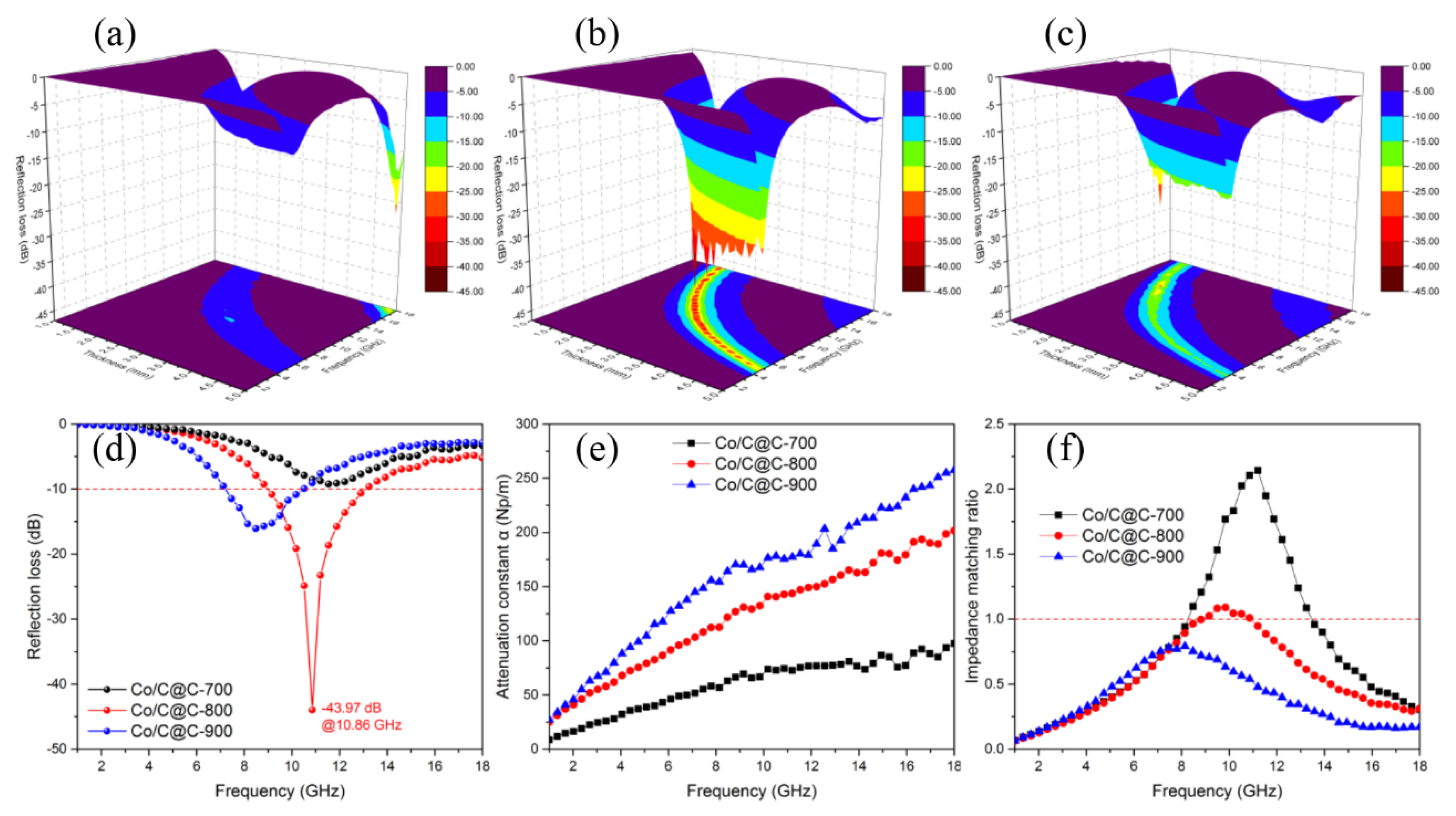
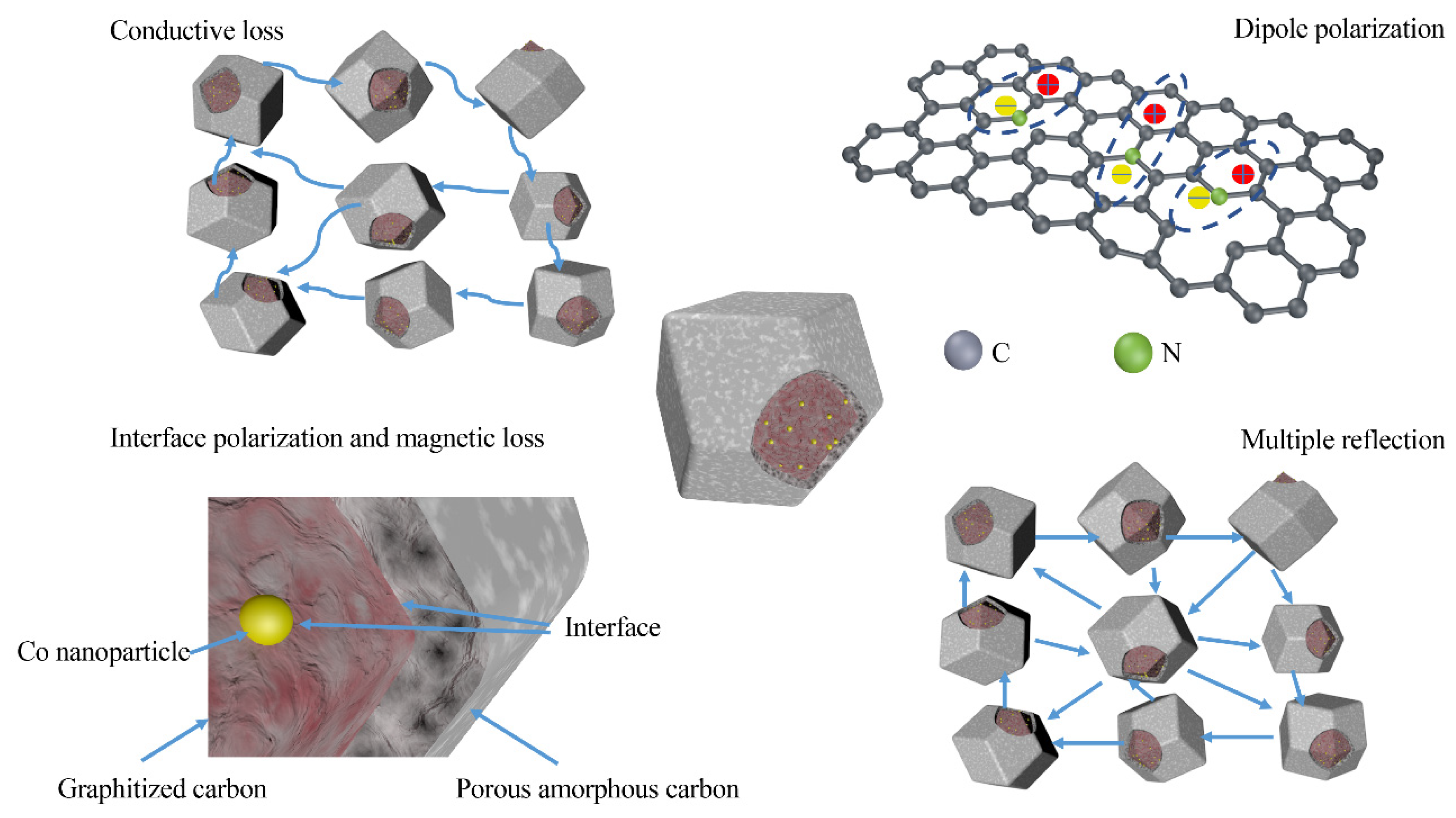
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Gao, X.; Lin, C.; Shi, L.; Li, X.; Wu, G. Metal organic frameworks-derived Fe-Co nanoporous carbon/graphene composite as a high-performance electromagnetic wave absorber. J. Alloys Compd. 2019, 785, 765–773. [Google Scholar] [CrossRef]
- Matzui, L.Y.; Trukhanov, A.V.; Yakovenko, O.S.; Vovchenko, L.L.; Zagorodnii, V.V.; Oliynyk, V.V.; Borovoy, M.O.; Trukhanova, E.L.; Astapovich, K.L.; Karpinsky, D.V.; et al. Functional Magnetic Composites Based on Hexaferrites: Correlation of the Composition, Magnetic and High-Frequency Properties. Nanomaterials 2019, 9, 1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Kim, J.; Kim, K.H. Microwave absorption properties of graphene oxide capsulated carbonyl iron particles. Appl. Surf. Sci. 2019, 475, 1065–1069. [Google Scholar] [CrossRef]
- Shu, R.; Wu, Y.; Li, Z.; Zhang, J.; Wan, Z.; Liu, Y.; Zheng, M. Facile synthesis of cobalt-zinc ferrite microspheres decorated nitrogen-doped multi-walled carbon nanotubes hybrid composites with excellent microwave absorption in the X-band. Compos. Sci. Technol. 2019, 184, 107839. [Google Scholar] [CrossRef]
- Nezhad, H.Y.; Thakur, V.K. Effect of morphological changes due to increasing carbon nanoparticles content on the quasi-static mechanical response of epoxy resin. Polymers 2018, 10, 1106. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.L.; Yin, X.W.; Fan, X.M.; Tang, Z.M.; Hou, Z.X.; Li, M.H.; Li, X.L.; Zhang, L.T.; Cheng, L.F. Constructing a tunable heterogeneous interface in bimetallic metal-organic frameworks derived porous carbon for excellent microwave absorption performance. Carbon 2019, 148, 421–429. [Google Scholar] [CrossRef]
- Zhao, H.B.; Cheng, J.B.; Wang, Y.Z. Biomass-derived Co@crystalline carbon@carbon aerogel composite with enhanced thermal stability and strong microwave absorption performance. J. Alloys Compd. 2018, 736, 71–79. [Google Scholar] [CrossRef]
- Saini, L.; Patra, M.K.; Dhaka, M.K.; Jani, R.K.; Gupta, G.K.; Dixit, A.; Vadera, S.R. Ni/graphitic carbon core-shell nanostructure-based light weight elastomeric composites for Ku-band microwave absorption applications. Crystengcomm 2018, 20, 4630–4640. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Poźoga, I.; Thakur, V.K. Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Yin, X.; Li, M.; Li, X.; Li, X.; Dang, X.; Zhang, L.; Cheng, L. Ultralight Cellular Foam from Cellulose Nanofiber/Carbon Nanotube Self-Assemblies for Ultrabroad-Band Microwave Absorption. ACS Appl. Mater. Interfaces 2019, 11, 22628–22636. [Google Scholar] [CrossRef]
- Gao, X.H.; Wu, X.Y.; Qiu, J. High Electromagnetic Waves Absorbing Performance of a Multilayer-Like Structure Absorber Containing Activated Carbon Hollow Porous Fibers-Carbon Nanotubes and Fe3O4 Nanoparticles. Adv. Electron. Mater. 2018, 4, 1700565. [Google Scholar] [CrossRef]
- Song, Z.M.; Liu, X.F.; Sun, X.; Li, Y.; Nie, X.Y.; Tang, W.K.; Yu, R.H.; Shui, J.L. Alginate-templated synthesis of CoFe/carbon fiber composite and the effect of hierarchically porous structure on electromagnetic wave absorption performance. Carbon 2019, 151, 36–45. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.J.; Wang, Z.J.; Li, Y.B.; He, X.D.; Yuan, Y. Microwave absorption enhancement of porous C@CoFe2O4 nanocomposites derived from eggshell membrane. Carbon 2019, 143, 507–516. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Sun, C.; Thakur, V.K. Graphitic Carbon Nitride Doped Copper–Manganese Alloy as High–Performance Electrode Material in Supercapacitor for Energy Storage. Nanomaterials 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, S.; Wang, J.; Luo, Q.; Sun, M.; Jiang, L.; Han, Q.; Liu, J.; Bi, H. Starfish-like C/CoNiO2 heterostructure derived from ZIF-67 with tunable microwave absorption properties. Chem. Eng. J. 2019, 373, 122–130. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Liu, W.; Zhang, X.; Gao, Q.; Li, Y.; Du, Y. A novel Co/TiO2 nanocomposite derived from a metal–organic framework: Synthesis and efficient microwave absorption. J. Mater. Chem. C 2016, 4, 1860–1870. [Google Scholar] [CrossRef]
- Heng, L.Y.; Zhang, Z.L.; Chen, X.Q.; Wang, S.; Wu, Z.; Xie, Z.Y.; Tang, Z.X.; Zou, Y.H. Fe/nanoporous carbon hybrid derived from metal-organic framework for highly effective microwave absorption. Appl. Organomet. Chem. 2019, 33, e4991. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, J.; Jin, H.; Yan, T.; Yi, G.; Su, X.; Dai, W.; Wang, X. MOF-derived rambutan-like nanoporous carbon/nanotubes/Co composites with efficient microwave absorption property. Mater. Lett. 2019, 244, 138–141. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. Crystengcomm 2013, 15, 1794–1801. [Google Scholar] [CrossRef]
- Wu, Q.L.; Jin, H.H.; Chen, W.; Huo, S.Q.; Chen, X.; Su, X.G.; Wang, H.; Wang, J. Graphitized nitrogen-doped porous carbon composites derived from ZIF-8 as efficient microwave absorption materials. Mater. Res. Express 2018, 5, 065602. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.C.; Liu, Y. CO3O4/ZnO nanoheterostructure derived from core-shell ZIF-8@ZIF-67 for supercapacitors. Rsc. Adv. 2016, 6, 52137–52142. [Google Scholar] [CrossRef]
- Kikuchi, H.; Sumida, C. Incident Power Influence on Magnetoimpedance Element with Domain-Wall Resonance. IEEE Trans. Magn. 2018, 54, 4001605. [Google Scholar] [CrossRef]
- Park, S.H.; Sohn, Y.H.; Shin, S.U.; Hong, S.W.; Cho, G.H. An Accurate and Practical Core Loss Analysis for Compact High Step-Up Converters. IEEE Trans. Power Electr. 2019, 34, 8368–8376. [Google Scholar] [CrossRef]
- Abdalla, I.; Salim, A.; Zhu, M.; Yu, J.; Li, Z.; Ding, B. Light and Flexible Composite Nanofibrous Membranes for High-Efficiency Electromagnetic Absorption in a Broad Frequency. ACS Appl. Mater. Interfaces 2018, 10, 44561–44569. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, L.; Wu, H.; Feng, X.; Wang, J.; Rao, H.; Wang, Y.; Dai, J.; Tang, Y. Two-Step Solvothermal Synthesis of (Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4)@C-MWCNTs Hybrid with Enhanced Low Frequency Microwave Absorbing Performance. Nanomaterials 2019, 9, 1601. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, Y.; Wang, Q.; Wu, X.; Zhang, W.; Zong, M.; Zhang, L. Facile synthesis of a novel flower-like BiFeO3 microspheres/graphene with superior electromagnetic wave absorption performances. Ceram. Int. 2019, 45, 3325–3332. [Google Scholar] [CrossRef]
- Jian, S.L.; Hsiao, L.Y.; Yeh, M.H.; Ho, K.C. Designing a carbon nanotubes-interconnected ZIF-derived cobalt sulfide hybrid nanocage for supercapacitors. J. Mater. Chem. A 2019, 7, 1479–1490. [Google Scholar] [CrossRef]
- Chu, C.S.; Rao, S.; Ma, Z.F.; Han, H.L. Copper and cobalt nanoparticles doped nitrogen-containing carbon frameworks derived from CuO-encapsulated ZIF-67 as high-efficiency catalyst for hydrogenation of 4-nitrophenol. Appl. Catal. B Environ. 2019, 256, 117792. [Google Scholar] [CrossRef]
- Si, Y.; Lv, Z.; Lu, L.; Liu, M.; Wen, Y.; Chen, Y.; Jin, H.; Liu, J.; Song, W. Revealing important role of graphitic carbon nitride surface catalytic activity in photocatalytic hydrogen evolution by using different carbon co-catalysts. Appl. Surf. Sci. 2019, 491, 236–244. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Guo, H.; Xia, W.; Jiang, C.; Gao, B.; Wu, C.; Wang, T.; He, J. N-Doped porous carbon nanosheets decorated with graphitized carbon layer encapsulated Co9S8 nanoparticles: An efficient bifunctional electrocatalyst for the OER and ORR. Nanoscale 2019, 11, 901–907. [Google Scholar] [CrossRef]
- Mccreary, A.; An, Q.; Forster, A.M.; Liu, K.W.; He, S.Y.; Macosko, C.W.; Stein, A. Walker ARH. Raman imaging of surface and sub-surface graphene oxide in fiber reinforced polymer nanocomposites. Carbon 2019, 143, 793–801. [Google Scholar] [CrossRef]
- Shah, S.A.; Shen, X.; Xie, M.; Zhu, G.; Ji, Z.; Zhou, H.; Xu, K.; Yue, X.; Yuan, A.; Zhu, J.; et al. Nickel@Nitrogen-Doped Carbon@MoS2 Nanosheets: An Efficient Electrocatalyst for Hydrogen Evolution Reaction. Small 2019, 15, e1804545. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Choi, S.W.; Lee, K.B. Effect of carbonization temperature on the physical properties and CO2 adsorption behavior of petroleum coke-derived porous carbon. Fuel 2019, 248, 85–92. [Google Scholar] [CrossRef]
- Morita, R.; Gotoh, K.; Kubota, K.; Komaba, S.; Hashi, K.; Shimizu, T.; Ishida, H. Correlation of carbonization condition with metallic property of sodium clusters formed in hard carbon studied using Na-23 nuclear magnetic resonance. Carbon 2019, 145, 712–715. [Google Scholar] [CrossRef]
- Vecera, P.; Chacón-Torres, J.C.; Pichler, T.; Reich, S.; Soni, H.R.; Görling, A.; Edelthalhammer, K.; Peterlik, H.; Hauke, F.; Hirsch, A. The First Precise Determination of Graphene Functionalisation by in situ Raman Spectroscopy. arXiv 2017, arXiv:1703.02498. [Google Scholar]
- Jin, H.; Zhou, H.; Li, W. In situ derived Fe/N/S-codoped carbon nanotubes from ZIF-8 crystals as efficient electrocatalysts for the oxygen reduction reaction and zinc–air batteries. J. Mater. Chem. A 2018, 6, 20093–20099. [Google Scholar] [CrossRef]
- Gulino, A.; Dapporto, P.; Rossi, P.; Anastasi, G.; Fragalà, I. Viable route for the synthesis of the anhydrous Co (hfac)2 adduct with monoglyme: A useful precursor for thin films of CoO. J. Mater. Chem. 2004, 14, 2549–2553. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Zhou, S.; Li, X.; Li, L.; Yu, Z.; Gu, L. ZIF-8/ZIF-67-Derived Co-Nx-Embedded 1D Porous Carbon Nanofibers with Graphitic Carbon-Encased Co Nanoparticles as an Efficient Bifunctional Electrocatalyst. Small 2018, 14, e1800423. [Google Scholar] [CrossRef]
- Du, J.; Zhou, T.C.; Lian, L.X.; Liu, Y.; Du, Y.B. Two-step sintering of M-type strontium ferrite with high coercivity. Ceram. Int. 2019, 45, 6978–6984. [Google Scholar] [CrossRef]
- Huber, C.; Sepehri-Amin, H.; Goertler, M.; Groenefeld, M.; Teliban, I.; Hono, K.; Suess, D. Coercivity enhancement of selective laser sintered NdFeB magnets by grain boundary infiltration. Acta Mater. 2019, 172, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; You, W.; Zhao, Y.; Li, X.; Shao, Z.; Che, R. Multi-scale magnetic coupling of Fe@SiO2@C-Ni yolk@triple-shell microspheres for broadband microwave absorption. Nanoscale 2019, 11, 17270–17276. [Google Scholar] [CrossRef]
- Huang, T.; Wu, Z.C.; Yu, Q.; Tan, D.G.; Li, L. Preparation of hierarchically porous carbon/magnetic particle composites with broad microwave absorption bandwidth. Chem. Eng. J. 2019, 359, 69–78. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wang, T.; Su, X.; Yang, S.; Chen, W.; Wang, J.; Sun, J.; Peng, J. High-performance microwave absorption epoxy composites filled with hollow nickel nanoparticles modified graphene via chemical etching method. Compos. Sci. Technol. 2019, 176, 54–63. [Google Scholar] [CrossRef]
- Chen, H.; Hong, R.; Liu, Q.; Li, S.; Huang, F.; Lu, Y.; Wang, L.; Li, K.; Zhang, H. CNFs@carbonaceous Co/CoO composite derived from CNFs penetrated through ZIF-67 for high-efficient electromagnetic wave absorption material. J. Alloys Compd. 2018, 752, 115–122. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Y.; Wang, M.; Liu, X.; Zong, M. Design and microwave absorption properties of thistle-like CoNi enveloped in dielectric Ag decorated graphene composites. J. Colloid Interface Sci. 2019, 534, 110–121. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Jin, H.; Zhang, B.; Huo, S.; Yang, S.; Su, X.; Wang, J. Facile Synthesis of Cobalt-Doped Porous Composites with Amorphous Carbon/Zn Shell for High-Performance Microwave Absorption. Nanomaterials 2020, 10, 330. https://doi.org/10.3390/nano10020330
Wu Q, Jin H, Zhang B, Huo S, Yang S, Su X, Wang J. Facile Synthesis of Cobalt-Doped Porous Composites with Amorphous Carbon/Zn Shell for High-Performance Microwave Absorption. Nanomaterials. 2020; 10(2):330. https://doi.org/10.3390/nano10020330
Chicago/Turabian StyleWu, Qilei, Huihui Jin, Bin Zhang, Siqi Huo, Shuang Yang, Xiaogang Su, and Jun Wang. 2020. "Facile Synthesis of Cobalt-Doped Porous Composites with Amorphous Carbon/Zn Shell for High-Performance Microwave Absorption" Nanomaterials 10, no. 2: 330. https://doi.org/10.3390/nano10020330





