Mobility of Cellulose Nanocrystals in Porous Media: Effects of Ionic Strength, Iron Oxides, and Soil Colloids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Porous Media, Soil Colloids, and CNCs
2.2. Column Experiments
2.3. Analysis of CNCs and Soil Colloids
2.4. Theoretical Consideration
2.4.1. Transport Modeling
2.4.2. Calculation of the Maximum Travel Distance
2.4.3. DLVO Interaction Energy Calculations
3. Results and Discussion
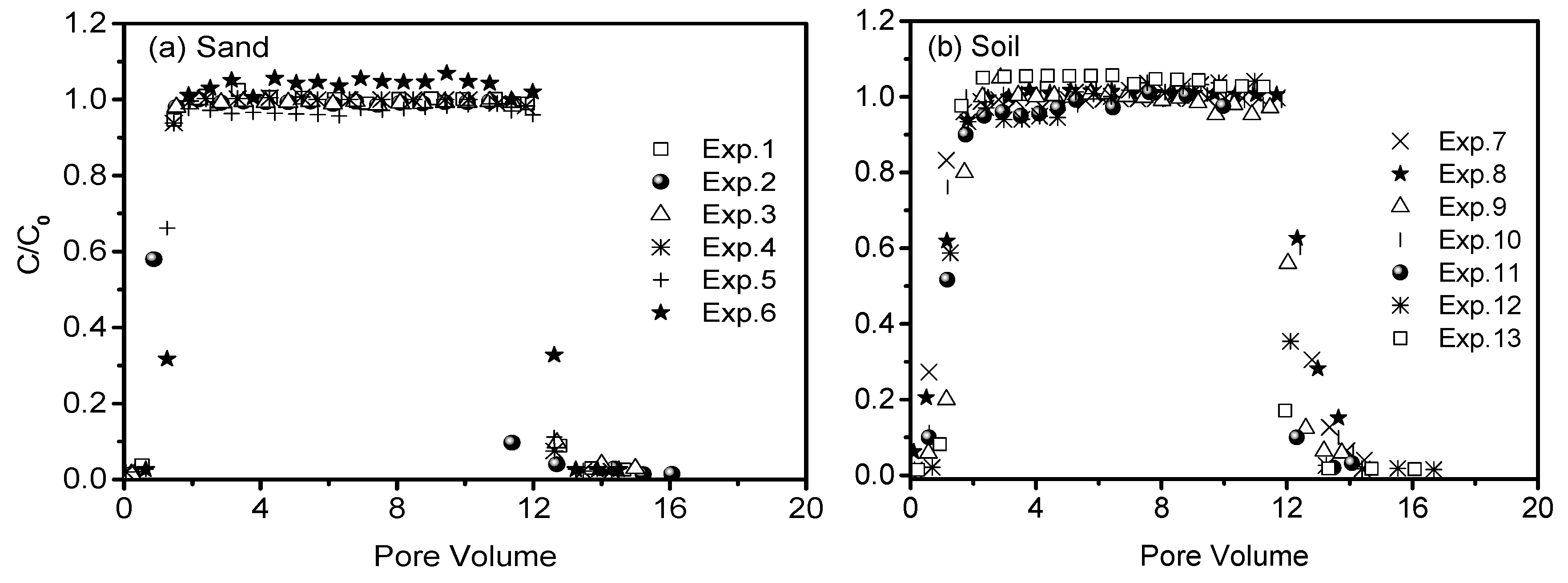
3.1. Transport of Bromide
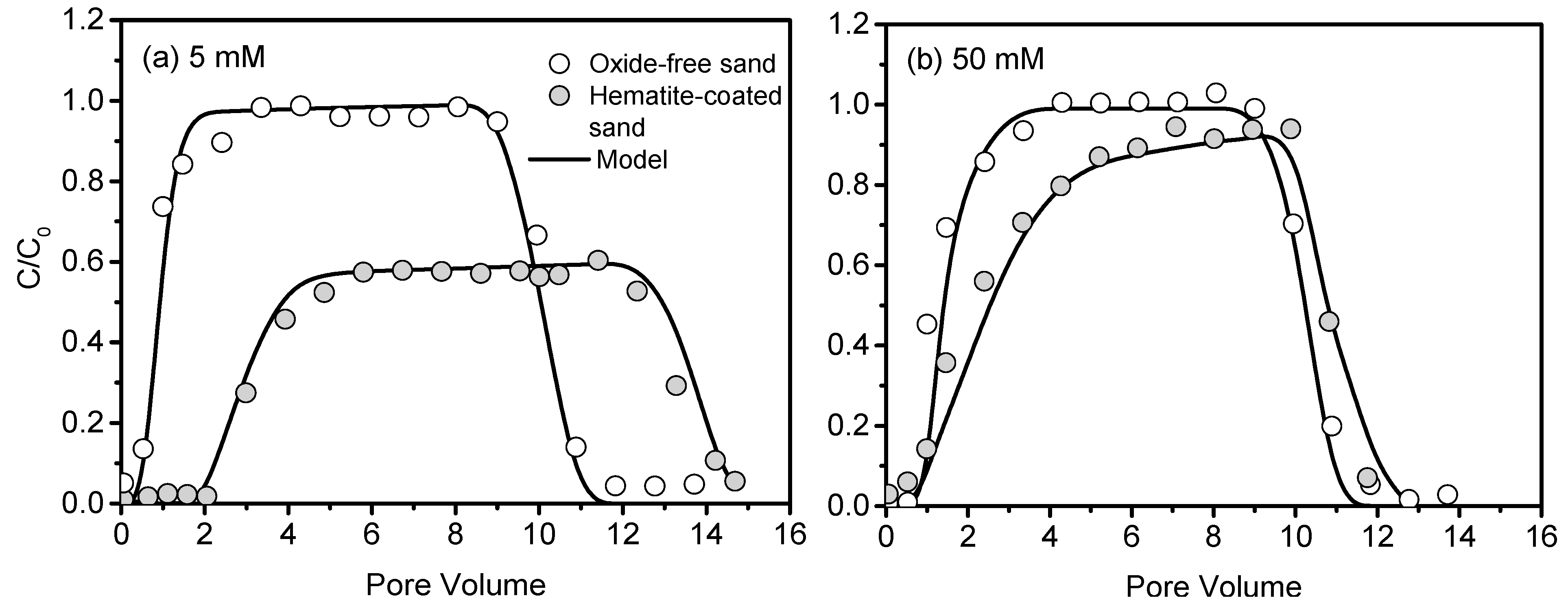
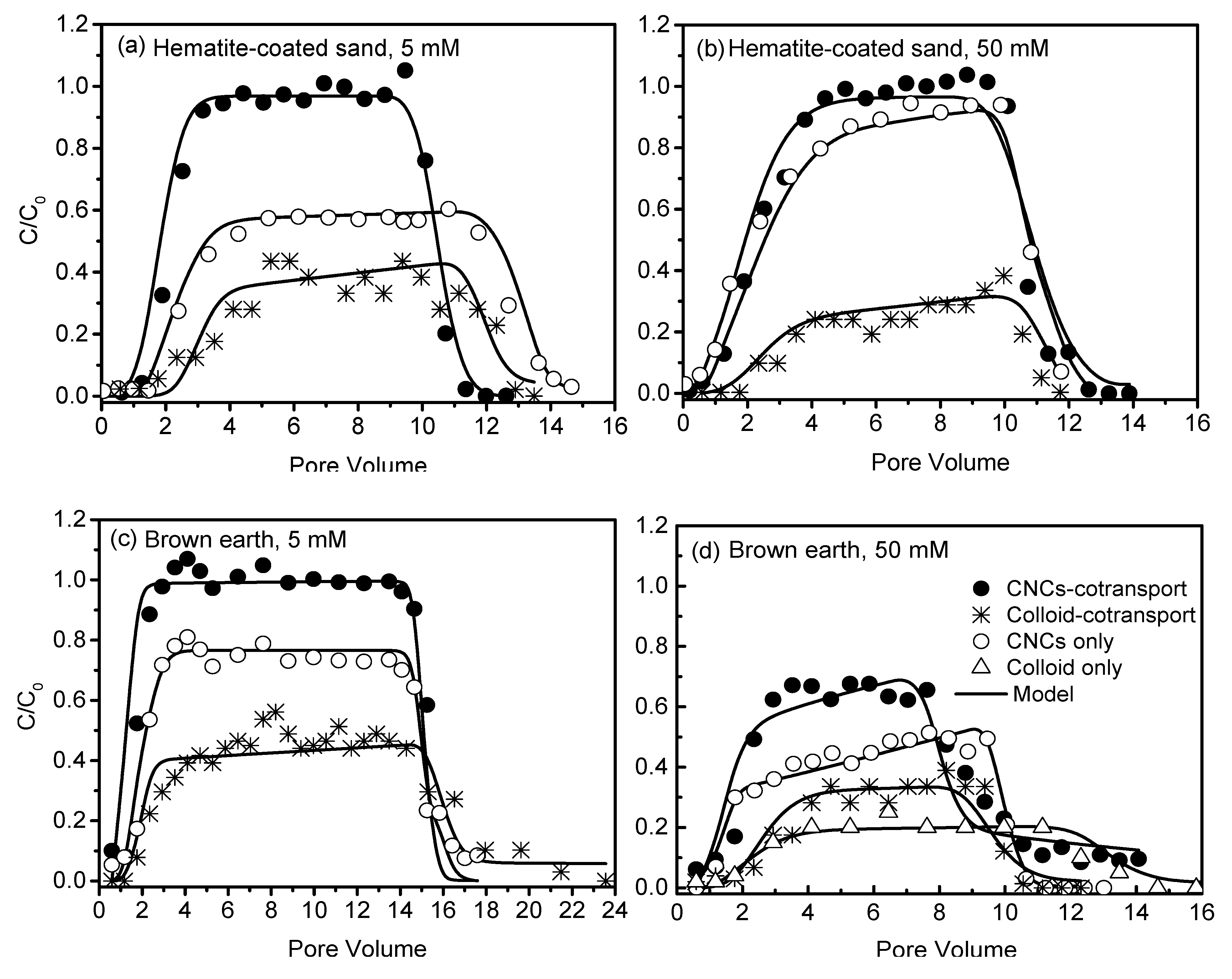
3.2. Transport of CNCs in Hematite-Coated Sand
3.3. Transport of CNCs in Soils
3.4. Effect of Soil Colloids on CNCs Transport
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Jia, W. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.J.; Chen, X.; Jin, Y.; Zhuang, J. Cadmium removal from soil by fulvic acid-aided hydroxyapatite nanofluid. Chemosphere 2019, 215, 227–233. [Google Scholar] [CrossRef]
- Chen, M.; Tao, X.Y.; Wang, D.J.; Xu, Z.B.; Xu, X.Y.; Hu, X.F.; Xu, N.; Cao, X.D. Facilitated transport of cadmium by biochar-Fe3O4 nanocomposites in water-saturated natural soils. Sci. Total Environ. 2019, 684, 265–275. [Google Scholar] [CrossRef]
- Wang, D.J.; Bradford, S.A.; Harvey, R.W.; Gao, B.; Cang, L.; Zhou, D.M. Humic acid facilitates the transport of ARS-labeled hydroxyapatite nanoparticles in iron oxyhydroxide-coated sand. Environ. Sci. Technol. 2012, 46, 2738–2745. [Google Scholar] [CrossRef]
- Zhang, W.; Rattanaudompol, U.S.; Li, H.; Bouchard, D. Effects of humic and fulvic acids on aggregation of aqu/nC (60) nanoparticles. Water Res. 2013, 47, 1793–1802. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Chen, W.; Zhu, S.; Liu, N.; Zhu, L. Immobilization of Lead and Cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ. Pollut. 2010, 158, 514–519. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef]
- Kahru, A.; Dubourguier, H.-C.; Blinova, I.; Ivask, A.; Kasemets, K. Biotests and Biosensors for Ecotoxicology of Metal Oxide Nanoparticles: A Minireview. Sensors 2008, 8, 2153–2170. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Karen, T.; Qasim, C. Engineered nanomaterials in soils and water: How do they behave and could they pose a risk to human health? Nanomedicine 2007, 2, 919–927. [Google Scholar] [CrossRef]
- Cowie, H.; Magdolenova, Z.; Saunders, M.; Drlickova, M.; Carreira, S.C.; Kenzaoi, B.H.; Gombau, L.; Guadagnini, R.; Lorenzo, Y.; Walker, L. Suitability of human and mammalian cells of different origin for the assessment of genotoxicity of metal and polymeric engineered nanoparticles. Nanotoxicology 2015, 9, 57–65. [Google Scholar] [CrossRef]
- Fadri, G.; Bernd, N. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.; Miller, R.; Lenihan, H. Accumulation and Toxicity of Copper Oxide Engineered Nanoparticles in a Marine Mussel. Nanomaterials 2014, 4, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Tingaut, P.; Zimmermann, T.; Sèbe, G. Cellulose nanocrystals and microfibrillated cellulose as building blocks for the design of hierarchical functional materials. J. Mater. Chem. 2012, 22, 20105–20111. [Google Scholar] [CrossRef]
- Du, L.Y.; Arnholt, K.; Ripp, S.; Sayler, G.; Wang, S.Q.; Liang, C.H.; Wang, J.K.; Zhuang, J. Biological toxicity of cellulose nanocrystals (CNCs) against the luxCDABE-based bioluminescent bioreporter Escherichia coli 652T7. Ecotoxicology 2015, 24, 2049–2053. [Google Scholar] [CrossRef]
- Lu, Q.L.; Cai, Z.H.; Lin, F.C.; Tang, L.R.; Wang, S.Q.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Carpenter, A.W.; de Lannoy, C.-F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Meng, Y.J.; Wu, Q.; Young, T.M.; Huang, B.; Wang, S.Q.; Li, Y.J. Analyzing three-dimensional structure and geometrical shape of individual cellulose nanocrystal from switchgrass. Polym. Compos. 2017, 38, 2368–2377. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.W.; Li, Q.; Wang, S.Q.; Luo, Y. Estimation of aspect ratio of cellulose nanocrystals by viscosity measurement: Influence of aspect ratio distribution and ionic strength. Polymers 2019, 11, 781. [Google Scholar] [CrossRef] [Green Version]
- Anirudhan, T.S.; Deepa, J.R.; Christa, J. Nanocellulose/nanobentonite composite anchored with multi-carboxyl functional groups as an adsorbent for the effective removal of Cobalt(II) from nuclear industry wastewater samples. J. Colloid Interface Sci. 2016, 467, 307–320. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Kloser, E.; Gray, D.G. Surface Grafting of Cellulose Nanocrystals with Poly (ethylene oxide) in Aqueous Media. Langmuir 2010, 26, 13450–13456. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, S.; Dooley, K.; Wu, Q. A facile approach to fabricate porous nanocomposite gels based on partially hydrolyzed polyacrylamide and cellulose nanocrystals for adsorbing methylene blue at low concentrations. J. Hazard. Mater. 2013, 263, 334–341. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Lei, T.; Negulescu, I.I. Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem. Eng. J. 2014, 251, 17–24. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Sheikhi, A.; Safari, S.; Yang, H.; Tg, V.D.V. Copper removal using electrosterically stabilized nanocrystalline cellulose. ACS Appl. Mater. Int. 2015, 7, 11301. [Google Scholar] [CrossRef]
- Bao, Q.; Lin, Q.; Tian, G.; Wang, G.; Yu, J.; Peng, G. Copper distribution in water-dispersible colloids of swine manure and its transport through quartz sand. J. Hazard. Mater. 2011, 186, 1660–1666. [Google Scholar] [CrossRef]
- Zhong, L.; Fu, S.; Peng, X.; Zhan, H.; Sun, R. Colloidal stability of negatively charged cellulose nanocrystalline in aqueous systems. Carbohyd. Polym. 2012, 90, 644–649. [Google Scholar] [CrossRef]
- Singh, K.; Arora, J.; Jai Mangal Sinha, T.; Srivastava, S. Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technol. Environ. Policy 2014, 16, 1179–1191. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, J.; Dong, Y.; Klumpp, E.; Šimůnek, J.; Bradford, S.A. Evidence for the critical role of nanoscale surface roughness on the retention and release of silver nanoparticles in porous media. Environ. Pollut. 2019, 258, 113803. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.Y.; Jin, Y.; Zhuang, J.; Li, T.T.; Xing, B.S. Role and importance of surface heterogeneities in transport of particles in saturated porous media. Crit. Rev. Environ. Sci. Technol. 2020, 50, 244–329. [Google Scholar] [CrossRef]
- Torkzaban, S.; Bradford, S.A.; van Genuchten, M.T.; Walker, S.L. Colloid transport in unsaturated porous media: The role of water content and ionic strength on particle straining. J. Contam. Hydrol. 2008, 96, 113–127. [Google Scholar] [CrossRef]
- Wang, D.J.; Bradford, S.A.; Harvey, R.W.; Hao, X.Z.; Zhou, D.M. Transport of ARS-labeled hydroxyapatite nanoparticles in saturated granular media is influenced by surface charge variability even in the presence of humic acid. J. Hazard. Mater. 2012, 229–230, 170–176. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, Y.; Bobcombe, Y.; Jones, K.L.; Liu, J.; Wiesner, M.R. Deposition of silver nanoparticles in geochemically heterogeneous porous media: Predict-ing affinity from surface composition analysis. Environ. Sci. Technol. 2011, 45, 5209–5215. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, J.; Jiang, Q.; Zhao, L. Water-soluble Fe3O4 nanoparticles with high solubility for removal of heavy-metal ions from waste water. Dalton Trans. 2012, 41, 4544–4551. [Google Scholar] [CrossRef]
- Yan, J.; Lazouskaya, V.; Jin, Y. Soil colloid release affected by dissolved organic matter and redox conditions. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Ma, L.Q.; Dong, X.; Harris, W.G.; Bonzongo, J.; Han, F. Ionic strength reduction and flow interruption enhanced colloid-facilitated Hg transport in contaminated soils. J. Hazard. Mater. 2014, 264, 286–292. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, D.; Cang, L.; Hao, X.; Chu, L. Transport and re-entrainment of soil colloids in saturated packed column: Effects of pH and ionic strength. J. Soil Sediment. 2011, 11, 491–503. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Sletten, R.S.; Bailey, R.P.; Bennett, T. Sorption and filtration of metals using iron-oxide-coated sand. Water Res. 1996, 30, 2609–2620. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Barmettler, K.; Grolimund, D.; Yan, Y.D.; Borkovec, M.; Sticher, H. Experimental determination of colloid deposition rates and collision efficiencies in natural porous media. Water Resour. Res. 1997, 33, 1129–1137. [Google Scholar] [CrossRef]
- Bradford, S.A.; Simunek, J.; Bettahar, M.; Van Genuchten, M.T.; Yates, S.R. Modeling colloid attachment, straining, and exclusion in saturated porous media. Environ. Sci. Technol. 2003, 37, 2242–2250. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Siwek, B.; Zembala, M.; Belouschek, P. Kinetics of localized adsorption of colloid particles. Adv. Colloid Interface 1994, 48, 151–280. [Google Scholar] [CrossRef]
- Fang, J.; Shan, X.; Wen, B.; Lin, J.; Owens, G. Stability of titania nanoparticles in soil suspensions and transport in saturated homogeneous soil columns. Environ. Pollut. 2009, 157, 1101–1109. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Agarwal, G.K. Extended DLVO interactions between spherical particles and rough surfaces. J. Colloid Interface Sci. 2006, 298, 50–58. [Google Scholar] [CrossRef]
- Xu, S.; Qi, J.; Chen, X.J.; Lazouskaya, V.; Jin, Y.; Zhuang, J. Coupled effect of extended DLVO and capillary interactions on the retention and transport of colloids through unsaturated porous media. Sci. Total Environ. 2016, 573, 564–572. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Prieve, D.C. Adsorption and desorption of particles and their chromatographic separation. AIChE J. 1976, 22, 276–283. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Elimelech, M. Surface element integration: A novel technique for evaluation of DLVO interaction between a particle and a flat plate. J. Colloid Interface Sci. 1997, 193, 273–285. [Google Scholar] [CrossRef]
- Hamaker, H.C. The London-van der Waals attraction between spherical particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Hogg, R.; Healy, T.W.; Fuerstenau, D.W. Mutual coagulation of colloidal dispersions. Trans. Faraday Soc. 1966, 62, 1638–1651. [Google Scholar] [CrossRef]
- Oliveira, R. Understanding adhesion: A means for preventing fouling. Exp. Therm. Fluid Sci. 1997, 14, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Wiesner, M.R. Deposition of aggregated nanoparticles-A theoretical and experimental study on the effect of aggregation state on the affinity between nanoparticles and a collector surface. Environ. Sci. Technol. 2012, 46, 13270–13277. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Bradford, S.A.; Wu, L.; Kim, H. Interaction energies for hollow and solid cylinders: Role of aspect ratio and particle orientation. Colloid Surf. Asp. 2019, 580, 123781. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Bradford, S.A.; Wu, L.; Chen, H.; Shi, X.; Wu, J. Transport, retention, and size perturbation of graphene oxide in saturated porous media: Effects of input concentration and grain size. Water Res. 2015, 68, 24–33. [Google Scholar] [CrossRef]
- Zhuang, J.; Jin, Y. Interactions between viruses and goethite during saturated flow: Effects of solution pH, carbonate, and phosphate. J. Contam. Hydrol. 2008, 98, 15–21. [Google Scholar] [CrossRef]
- Bradford, S.A.; Torkzaban, S. Colloid Interaction Energies for Physically and Chemically Heterogeneous Porous Media. Langmuir 2013, 29, 3668–3676. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lazouskaya, V.; Zhang, H.Y.; Li, B.G.; Jin, Y.; Huang, Y. Influence of surface chemical heterogeneity on attachment and detachment of microparticles. Colloids Surf. Asp. 2013, 433, 14–29. [Google Scholar] [CrossRef]
- Zhuang, J.; Jin, Y.; Flury, M. Comparison of Hanford colloids and kaolinite transport in porous media. Vadose Zone J. 2004, 3, 395–402. [Google Scholar] [CrossRef] [Green Version]



| Exp. # | Porous Media | CNCs (mg L−1) | Colloid (mg L−1) | IS (mM) | D (cm2 h−1) | ρb (g cm−3) | θ (%) | Vp (cm h−1) | Smax (mg L−1) | kdep (h−1) | kdet (h−1) | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Oxide-free sand | 200 | 0 | 5 | 0.14 | 1.72 | 36.24 | 14.80 | 0.05 | 0.14 | 4 × 10−4 | 0.98 |
| 2 | 200 | 0 | 50 | 0.15 | 1.72 | 36.21 | 14.75 | 0.07 | 0.15 | 2 × 10−3 | 0.95 | |
| 3 | Hematite-coated sand | 200 | 0 | 5 | 0.36 | 1.72 | 36.40 | 14.75 | 3.61 | 0.92 | 5 × 10−5 | 0.97 |
| 4 | 200 | 0 | 50 | 0.24 | 1.72 | 36.43 | 14.75 | 0.40 | 0.31 | 5 × 10−5 | 0.91 | |
| 5 | 200 | 50 | 5 | 0.13 | 1.49 | 43.82 | 11.40 | 0.12 | 0.03 | 5 × 10−6 | 0.98 | |
| 6 | 200 | 50 | 50 | 0.23 | 1.49 | 43.84 | 13.81 | 0.09 | 0.03 | 5 × 10−3 | 0.97 | |
| 7 | Red earth | 200 | 0 | 5 | 0.23 | 1.15 | 57.40 | 9.32 | 0.17 | 0.03 | 4 × 10−3 | 0.87 |
| 8 | 200 | 0 | 50 | 0.51 | 1.15 | 57.42 | 12.19 | 15.94 | 5.55 | 4 × 10−2 | 0.63 | |
| 9 | Brown earth | 200 | 0 | 5 | 0.15 | 1.37 | 48.24 | 11.44 | 0.08 | 0.05 | 2 × 10−3 | 0.98 |
| 10 | 200 | 0 | 50 | 0.44 | 1.33 | 46.61 | 9.34 | 3.56 | 2.87 | 4 × 10−3 | 0.85 | |
| 11 | 200 | 50 | 5 | 0.15 | 1.33 | 47.03 | 12.02 | 0.04 | 0.17 | 2 × 10−3 | 0.89 | |
| 12 | 200 | 50 | 50 | 0.50 | 1.33 | 47.01 | 12.06 | 1.57 | 1.10 | 3 × 10−1 | 0.89 | |
| 13 | 0 | 50 | 50 | 0.32 | 1.33 | 46.64 | 11.44 | - | - | - | - |
| Exp.# | Porous Media | CNCs (mg L−1) | Soil Colloids (mg L−1) | Is (mM) | CNCs | Soil Colloids | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meff (%) | Mret (%) | Mtot (%) | Lmax (cm) | Meff (%) | Lmax (cm) | |||||
| 1 | Oxide-free sand | 200 | 0 | 5 | 99.78 | 0.68 | 100.46 | 7245 | - | - |
| 2 | 200 | 0 | 50 | 99.56 | 1.64 | 101.20 | 5573 | - | - | |
| 3 | Hematite-coated sand | 200 | 0 | 5 | 51.58 | 42.80 | 94.38 | 129 | - | - |
| 4 | 200 | 0 | 50 | 85.56 | 13.19 | 98.75 | 622 | - | - | |
| 5 | 200 | 50 | 5 | 93.67 | 7.87 | 101.54 | 3623 | 38.37 | 79.09 | |
| 6 | 200 | 50 | 50 | 91.44 | 10.40 | 101.84 | 7245 | 19.47 | 60.38 | |
| 7 | Red earth | 200 | 0 | 5 | 96.31 | 1.67 | 97.98 | 2415 | - | - |
| 8 | 200 | 0 | 50 | 4.37 | 94.17 | 98.54 | 24 | - | - | |
| 9 | Brown earth | 200 | 0 | 5 | 80.39 | 18.20 | 98.59 | 325 | - | - |
| 10 | 200 | 0 | 50 | 37.94 | 52.54 | 90.48 | 102 | - | - | |
| 11 | 200 | 50 | 5 | 98.79 | 3.14 | 101.93 | 7245 | 47.94 | 98.71 | |
| 12 | 200 | 50 | 50 | 69.19 | 15.20 | 84.39 | 181 | 30.76 | 65.27 | |
| 13 | 0 | 50 | 50 | - | - | - | - | 19.02 | 45.00 | |
| Is (mM) | dh (nm) | ε (mV) | |
|---|---|---|---|
| CNCs | 5 | 276.53 ± 22.82 | −39.26 ± 9.04 |
| 50 | 429.33 ± 76.11 | −28.03 ± 2.28 | |
| Oxide−free sand | 5 | − | −47.20 ± 3.75 |
| 50 | − | −37.91 ± 4.72 | |
| Hematite−coated sand | 5 | − | 3.87 ± 1.04 |
| 50 | − | 0.47 ± 0.10 | |
| Soil colloids | 5 | − | −40.07 ± 5.21 |
| 50 | − | −30.86 ± 1.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Shen, C.; Zhang, X.; Chen, X.; Radosevich, M.; Wang, S.; Zhuang, J. Mobility of Cellulose Nanocrystals in Porous Media: Effects of Ionic Strength, Iron Oxides, and Soil Colloids. Nanomaterials 2020, 10, 348. https://doi.org/10.3390/nano10020348
Xu S, Shen C, Zhang X, Chen X, Radosevich M, Wang S, Zhuang J. Mobility of Cellulose Nanocrystals in Porous Media: Effects of Ionic Strength, Iron Oxides, and Soil Colloids. Nanomaterials. 2020; 10(2):348. https://doi.org/10.3390/nano10020348
Chicago/Turabian StyleXu, Shuang, Chongyang Shen, Xueyong Zhang, Xijuan Chen, Mark Radosevich, Siqun Wang, and Jie Zhuang. 2020. "Mobility of Cellulose Nanocrystals in Porous Media: Effects of Ionic Strength, Iron Oxides, and Soil Colloids" Nanomaterials 10, no. 2: 348. https://doi.org/10.3390/nano10020348
APA StyleXu, S., Shen, C., Zhang, X., Chen, X., Radosevich, M., Wang, S., & Zhuang, J. (2020). Mobility of Cellulose Nanocrystals in Porous Media: Effects of Ionic Strength, Iron Oxides, and Soil Colloids. Nanomaterials, 10(2), 348. https://doi.org/10.3390/nano10020348






