Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine
Abstract
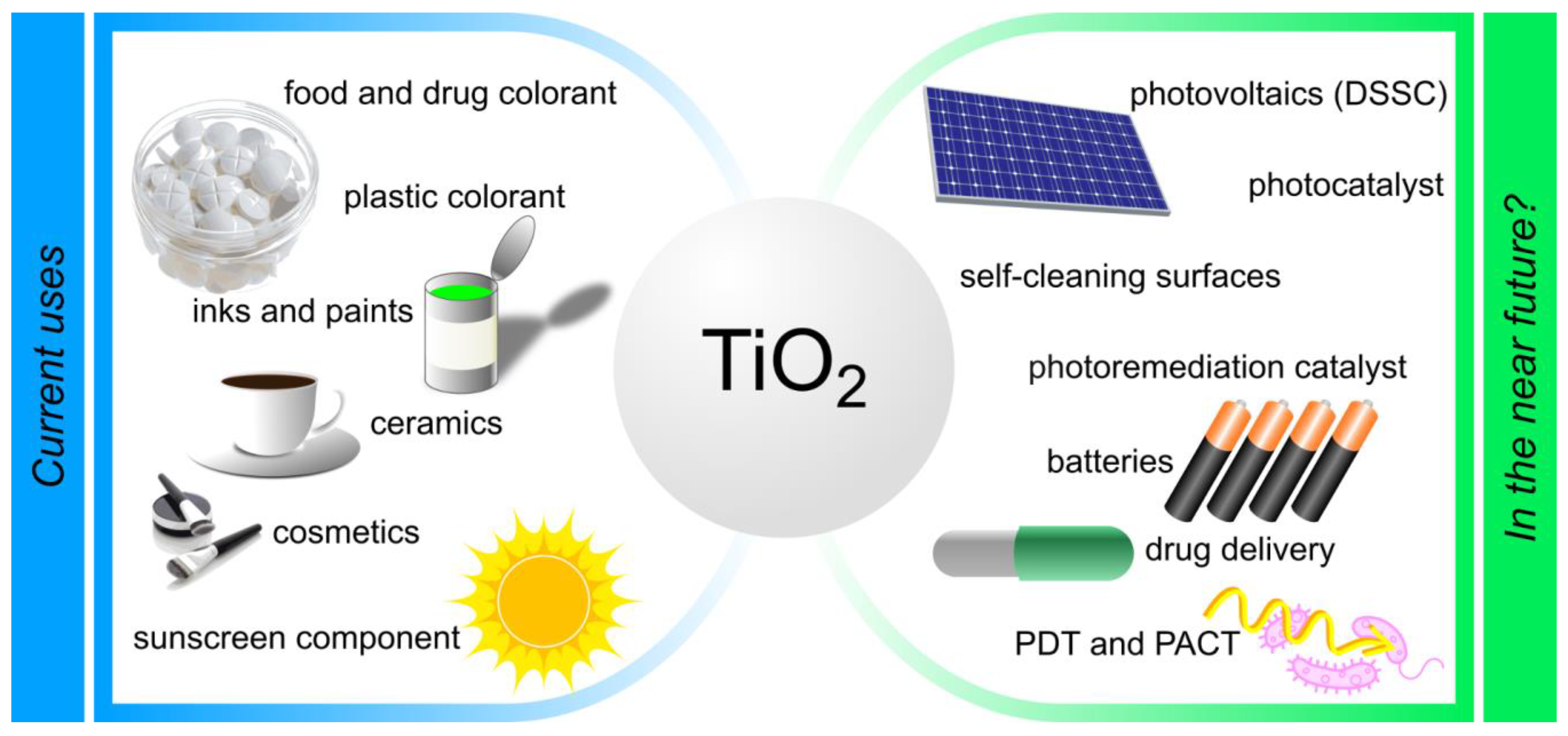
:1. Introduction
2. Pharmacokinetics, Biodistribution, and Biological Fate of Titanium Dioxide
- ○
- The pharmacokinetics of TiO2 NPs depends on many factors, including particle type, surface charge, surface coating, size, dose, and exposure route.
- ○
- Titania does not penetrate the gastrointestinal tract at all or to a minimal extent.
- ○
- Histopathological study indicates that after intravenous administration TiO2 NPs accumulate mainly in the liver, and to some extent in the spleen, lungs and kidneys.
- ○
- Renal excretion is the primary route of TiO2 NPs elimination.
- ○
- The pharmacokinetics and bioavailability of TiO2 NPs require further and intensive research.
3. Toxicity and Biocompatibility—In Vitro and In Vivo Evaluation of the Toxicity of Titanium Dioxide
- ○
- The toxicity of titanium dioxide is low. Various studies consider this material as safe or unsafe, depending on the size and crystal form, which strongly determines TiO2 NPs’ potential toxicity.
- ○
- The in vitro and in vivo studies concerning the skin-related toxicity of TiO2 NPs raise both skin toxicity itself and skin permeation related systemic toxicity. The potential TiO2 NPs related risk on skin after long-term exposure cannot be neglected.
- ○
- The harmful effects of TiO2 inhalation exposure are associated with the so-called TiO2 “overload”, which is rare in everyday life.
- ○
- Some immunomodulation effects related to the stimulation of dendritic cell maturation by TiO2 presented in recent studies cannot be omitted.
- ○
- It seems that TiO2 toxicity can be modified by combining it with photosensitizers.
4. Design of Titanium Dioxide Nanoparticles—Synthesis and Stabilization Procedures, Physicochemical Properties, and Characterization
- ○
- TiO2 occurs naturally in three polymorphic forms: rutile and anatase with a tetragonal structure, and rhombic brookite.
- ○
- Synthetic TiO2 is obtained by sol-gel synthesis, hydrothermal methods, green chemistry, microwave methods, and others.
- ○
- The TiO2 particles can be modified by the addition of various surfactants or dopants or by post-synthetic modifications, such as doping, surface functionalization, or binding with organic molecules.
- ○
- Titania NPs, when dispersed tend to form agglomerates. The TiO2 NPs functionalized on their surface can form stable, non-aggregating formulations in aqueous solutions.
5. Photodynamic Activity of Neat TiO2 Nanoparticles and in Drug Delivery Systems
- ○
- The applications of neat titania NPs in photodynamic therapy are limited by the necessity to use UV light of very low tissue penetration, and harmful impact on the human body.
- ○
- Neat TiO2 NPs and in combination with various molecules, antibodies, or polymers revealed interesting photocytotoxicity against cancer cells and microbes, thus unveiling potential for PDT.
- ○
- The SiO2 shell influences the activity of TiO2 NPs in photodynamic therapy. Only the optimal SiO2-layer thickness guarantees optimal preservation of the photodynamic properties of TiO2 NPs as well as the improvement of their biocompatibility.
- ○
- TiO2 and its composites with chitosan, poly(N-vinylpyrrolidone) can broaden the current PDT applications towards the area of wound healing management.
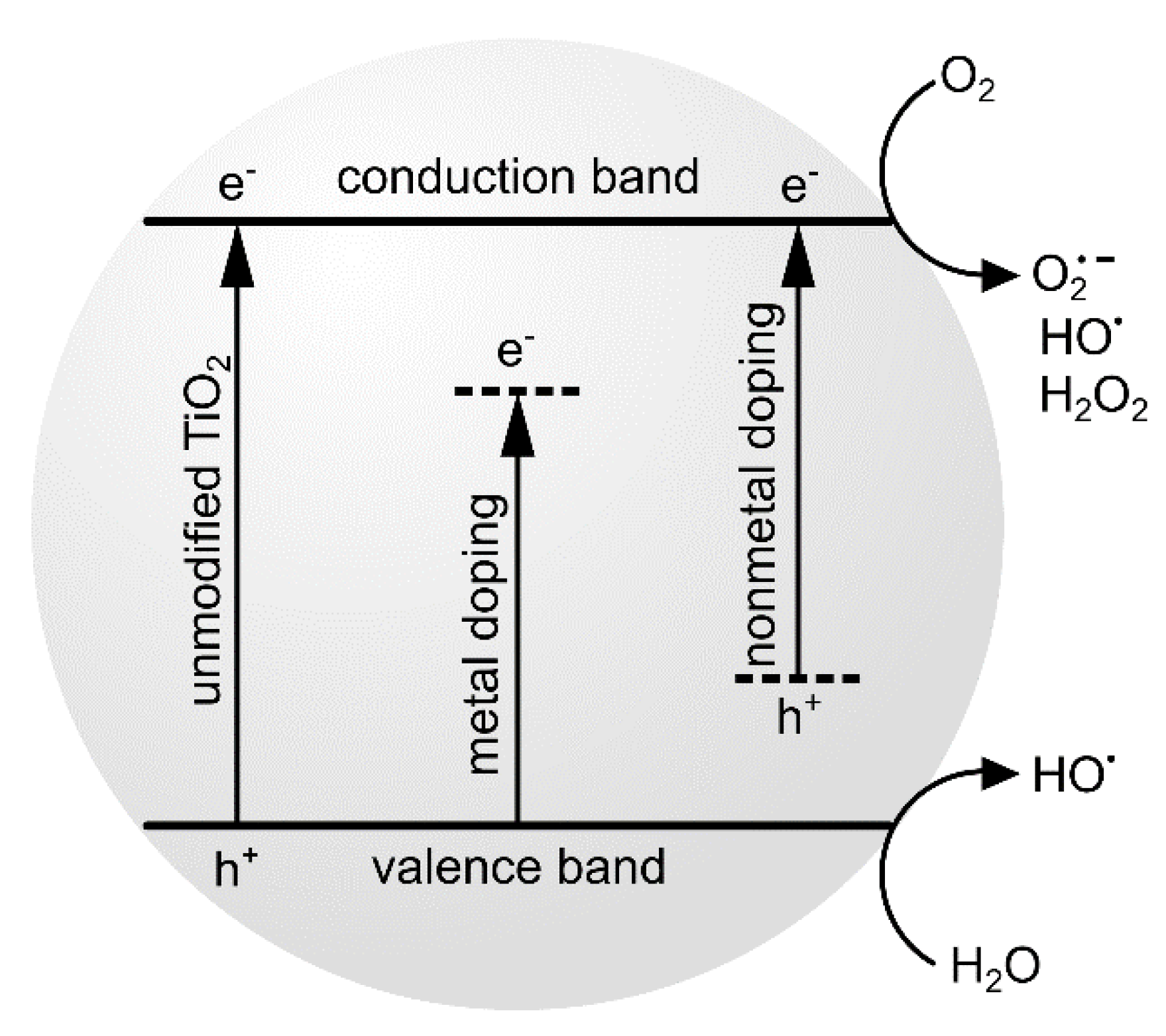
6. Doping of TiO2 Nanoparticles with Inorganic Compounds and Carbon-Based Nanomaterials
- ○
- Doping or modification of TiO2 NPs “turns on” their excitation possibilities by visible light and increases their activity in photodynamic activity study.
- ○
- The combinations of TiO2 with inorganic dopants and carbon-based nanomaterials modifying its photochemical properties seem to be an alternative not only to neat TiO2, but also to conventional photosensitizers in PDT.
- ○
- The combinations of TiO2 with inorganic dopants and carbon-based nanomaterials were studied towards antimicrobial and anticancer PDT.
7. Modifications of TiO2 Nanoparticles with Photosensitizers Aiming to Improve Their Optical and Biological Properties
7.1. TiO2 Nanoparticles Combined with Phthalocyanines
7.2. TiO2 Nanoparticles Combined with Porphyrins, Chlorins and Methylene Blue
- ○
- The combination of photosensitizers with TiO2 nanoparticles can be beneficial for the effectiveness of PDT and can reduce the side effects of chemotherapy.
- ○
- Among organic dyes, most often utilized for combining with TiO2 are porphyrins and phthalocyanines, which were numerously applied as photosensitizers for PDT.
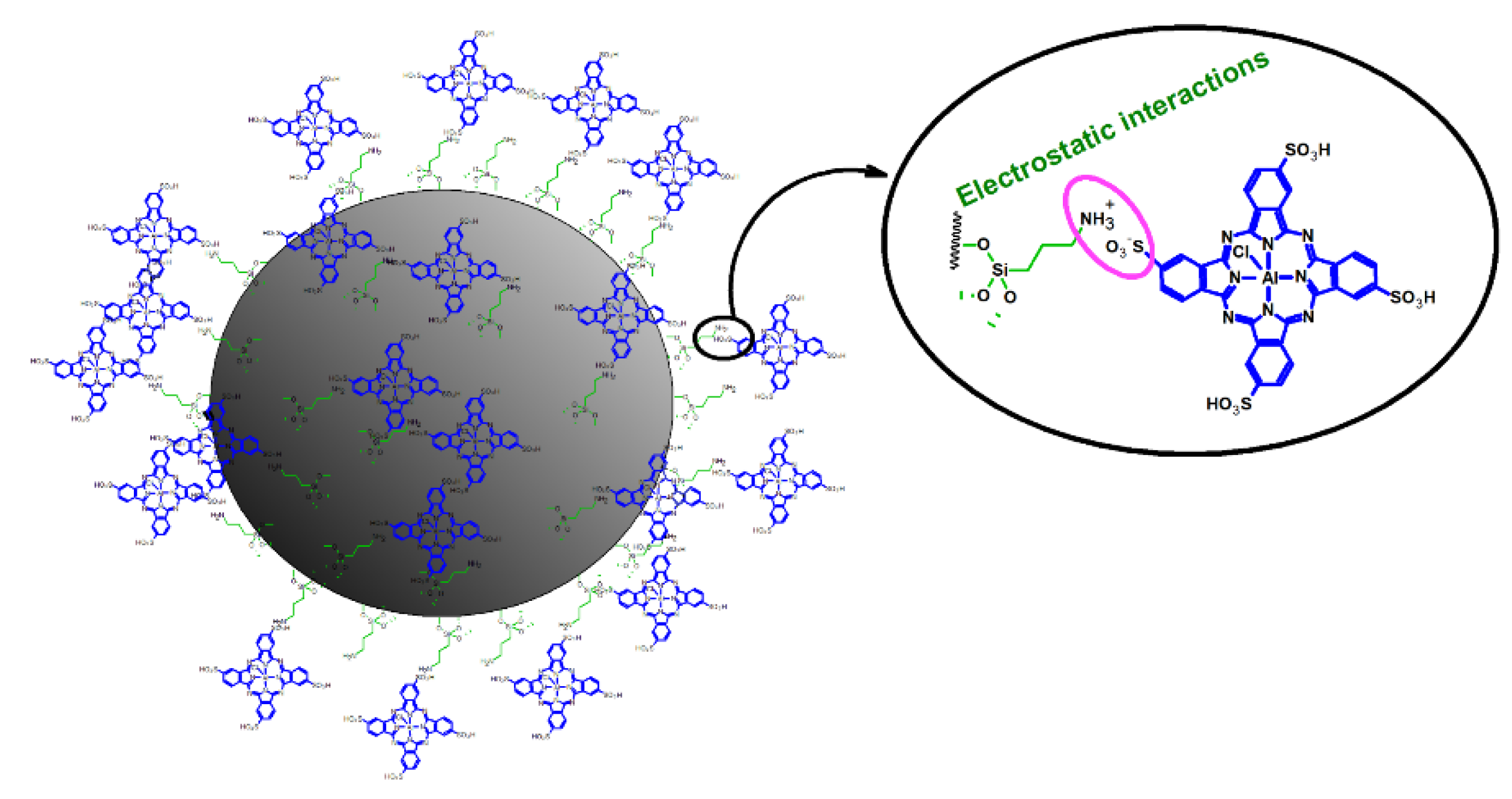
- ○
- The nitrogen-doping of TiO2 NPs combined with phthalocyanines can significantly increase the efficacy of photodynamic activity, as it greatly enhances the formation of singlet oxygen and superoxide anion radicals, whereas it suppresses the generation of hydroxyl radicals.
- ○
- Phthalocyanines anchored onto TiO2 NPs and labeled with 131I were assessed for PDT diagnosis of selected cancers.
- ○
- Hybrid materials composed of phthalocyanines, porphyrazines, or chlorines bound to TiO2 were studied in terms of their effectiveness in antimicrobial PDT against bacteria, fungi, and parasites.
- ○
- The combination of fluorinated porphyrins and TiO2 NPs after exposure to visible light revealed 7 logs reduction in colony-forming units of Staphylococcus aureus. The addition of KI transformed the hybrid materials into effective antimicrobial photosensitizers able to efficiently inactivate Gram-negative bacteria.
- ○
- The application of PDT with the use of porphyrins and their derivatives as well as various NPs was extended to rheumatoid arthritis, atherosclerosis, macular degeneration, and diabetes mellitus.
- ○
- The combination of methylene blue with TiO2 NPs irradiated with light sources simultaneously (405 and 625 nm) reduced the number of S. aureus cells by up to 90%. Almost identical results were obtained using a combination of photosensitizers against C. albicans.
8. TiO2 Nanoparticles As a Vehicle for Chemotherapeutics
- ○
- TiO2 in combination with anticancer agents offers a platform for more efficient delivery of chemotherapeutics. Thanks to either release mechanism used: pH-dependent, irradiation-triggered, or simple delivery, drug release in tumor cells is much higher than in healthy cells. As a result, the amount of drug used in the treatment can be significantly lower, while the pharmacological effect is maintained and fewer potential adverse effects occur. This can still be improved by not only combining different therapies, as shown by utilization of PDT and classical chemotherapy, but also by assessment of a mixture of anticancer drugs and other anticancer therapies.
- ○
- Most research concerns the combination of TiO2 NPs with doxorubicin, and the results are encouraging.
- ○
- The decreased cytotoxicity of doxorubicin-loaded on upconverting nanoparticles containing TiO2 was observed in many studies.
- ○
- Mixed chemotherapy and PDT were studied after encapsulation of doxorubicin into “capsules” composed of Au-TiO2 NPs. The diffusion of the drug around the tumor was noted as the result of the acidic environment of the tissue core.
9. Other Applications of TiO2 Nanoparticles in Medicine
- ○
- TiO2 NPs were evaluated for use in pharmacy, especially in pharmaceutical chemistry and technology, as well as medicine, including growing areas related to dentistry and surgery.
- ○
- In dentistry, the photochemical activity of TiO2 was utilized for the improvement of tooth personal care and teeth whitening.
- ○
- Eggshell-TiO2 composite was found useful for occluding opened dentine tubules, allowing for efficient dentine occlusion.
- ○
- TiO2 scaffolds were applied for the preparation of implants for surgery in bone tissue engineering.
- ○
- In pharmaceutical sciences, TiO2 was applied as a pharmaceutical excipient in the manufacture of tablets, as well as a catalytic system able to eliminate dangerous chemical and pharmaceutical pollutants
10. Summary
Acknowledgments
Conflicts of Interest
References
- Horikoshi, S.; Serpone, N. Introduction to nanoparticles. Microw. Nanopart. Synth. Fundam. Appl. 2013, 1–24. [Google Scholar] [CrossRef]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar] [CrossRef]
- ISO/TS 80004-2:2015(en). Nanotechnologies—Vocabulary—Part 2: Nano-objects. Available online: https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-2:ed-1:v1:en (accessed on 5 December 2019).
- Caep, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Huang, J.; Chen, C.; Liu, G.; Jiang, Y.; Zhao, Y.; Jiang, Z. Photokilling cancer cells using highly cell-specific antibody–TiO2 bioconjugates and electroporation. Bioelectrochemistry 2007, 71, 217–222. [Google Scholar] [CrossRef]
- Ni, W.; Li, M.; Cui, J.; Xing, Z.; Li, Z.; Wu, X.; Song, E.; Gong, M.; Zhou, W. 808 nm light triggered black TiO2 nanoparticles for killing of bladder cancer cells. Mater. Sci. Eng. C 2017, 81, 252–260. [Google Scholar] [CrossRef]
- Carlander, U.; Li, D.; Jolliet, O.; Emond, C.; Johanson, G. Toward a general physiologically-based pharmacokinetic model for intravenously injected nanoparticles. Int. J. Nanomed. 2016, 11, 625. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Monteiro-Riviere, N.A.; Riviere, J.E. Pharmacokinetics of metallic nanoparticles: Pharmacokinetics of metallic nanoparticles. WIREs NanoMed. Nanobiotechnol. 2015, 7, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.; Mas del Molino, E.; Fernández-Rosas, E.; Fernández, A.; Vázquez-Campos, S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2014, 228, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Bachler, G.; von Goetz, N.; Hungerbuhler, K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology 2015, 9, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Landsiedel, R.; Ma-Hock, L.; Wiench, K.; Wohlleben, W.; van Ravenzwaay, B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch. Toxicol. 2008, 82, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Oomen, A.G.; Krystek, P.; Jacobsen, N.R.; Wallin, H.; Laurentie, M.; Verharen, H.W.; Brandon, E.F.; de Jong, W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, G.; Wang, C.; Sun, J.; Zhong, G. Tissue distribution and excretion of intravenously administered titanium dioxide nanoparticles. Toxicol. Lett. 2011, 205, 55–61. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Xue, C.; Zhou, S.; Lan, F.; Bi, L.; Xu, H.; Yang, X.; Zeng, F.-D. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009, 191, 1–8. [Google Scholar] [CrossRef]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; Palma, G.D.; Bovenzi, M.; et al. Titanium Dioxide Nanoparticle Penetration into the Skin and Effects on HaCaT Cells. Int. J. Environ. Res. Public Health 2015, 12, 9282. [Google Scholar] [CrossRef]
- Yin, J.-J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes—Generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Trochimowicz, H.J.; Reinhardt, C.F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol. Appl. Pharmacol. 1985, 79, 179–192. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; Vermeulen, J.P.; van Engelen, L.B.; de Jong, B.; Verhagen, L.M.; de la Fonteyne-Blankestijn, L.J.; Hoonakker, M.E.; de Jong, W.H. The crystal structure of titanium dioxide nanoparticles influences immune activity in vitro and in vivo. Part. Fibre Toxicol. 2018, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, D.; Haak, S.; Sisirak, V.; Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013, 13, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226. [Google Scholar] [PubMed]
- Madhubala, V.; Pugazhendhi, A.; Thirunavukarasu, K. Cytotoxic and immunomodulatory effects of the low concentration of titanium dioxide nanoparticles (TiO2 NPs) on human cell lines—An in vitro study. Process Biochem. 2019, 86, 186–195. [Google Scholar] [CrossRef]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Selke, M.; Wang, X. Protective effect of TiO2 nanowhiskers on Tetra Sulphonatophenyl Porphyrin (TSPP) complexes induced oxidative stress during photodynamic therapy. Photodiagnosis Photodyn. Ther. 2016, 13, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef] [Green Version]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Muniandy, S.S.; Kaus, N.H.M.; Jiang, Z.-T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef] [Green Version]
- Falk, G.S.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Rodrigues Neto, J.B.; Moreno, R. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanopart. Res. 2018, 20, 23. [Google Scholar] [CrossRef]
- Macyk, W.; Szaciłowski, K.; Stochel, G.; Buchalska, M.; Kuncewicz, J.; Łabuz, P. Titanium (IV) complexes as direct TiO2 photosensitizers. Coord. Chem. Rev. 2010, 254, 2687–2701. [Google Scholar] [CrossRef]
- Yuan, R.; Zhou, B.; Hua, D.; Shi, C.; Ma, L. Effect of metal-ion doping on the characteristics and photocatalytic activity of TiO2 nanotubes for the removal of toluene from water. Water Sci. Technol. 2014, 69, 1697–1704. [Google Scholar] [CrossRef]
- Gupta, N.; Pal, B. Photocatalytic activity of transition metal and metal ions impregnated TiO2 nanostructures for iodide oxidation to iodine formation. J. Mol. Catal. A Chem. 2013, 371, 48–55. [Google Scholar] [CrossRef]
- Savinkina, E.; Obolenskaya, L.; Kuzmicheva, G. Efficiency of sensitizing nano-titania with organic dyes and peroxo complexes. Appl. Nanosci. 2015, 5, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Kondratyeva, I.; Orzeł, Ł.; Kobasa, I.; Doroshenko, A.; Macyk, W. Photosensitization of titanium dioxide with 4′-dimethylaminoflavonol. Mater. Sci. Semicond. Process. 2016, 42, 62–65. [Google Scholar] [CrossRef]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules 2014, 20, 88–110. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Zhang, S.; Wu, H.; Lou, X. A novel folic acid-conjugated TiO2-SiO2 photosensitizer for cancer targeting in photodynamic therapy. Colloids Surf. B Biointerfaces 2015, 125, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Zaleska, A. Doped-TiO2: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Guiot, C.; Spalla, O. Stabilization of TiO2 Nanoparticles in Complex Medium through a pH Adjustment Protocol. Environ. Sci. Technol. 2013, 47, 1057–1064. [Google Scholar] [CrossRef]
- Xu, F. Review of analytical studies on TiO2 nanoparticles and particle aggregation, coagulation, flocculation, sedimentation, stabilization. Chemosphere 2018, 212, 662–677. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwińska-Ciesielczyk, K.; Goscianska, J.; Dobrowolska, A.; Gabała, E.; Czaczyk, K.; Jesionowski, T. Hydrothermal-assisted synthesis of highly crystalline titania-copper oxide binary systems with enhanced antibacterial properties. Mater. Sci. Eng. C 2019, 104, 109839. [Google Scholar] [CrossRef]
- Lagopati, N.; Kitsiou, P.V.; Kontos, A.I.; Venieratos, P.; Kotsopoulou, E.; Kontos, A.G.; Dionysiou, D.D.; Pispas, S.; Tsilibary, E.C.; Falaras, P. Photo-induced treatment of breast epithelial cancer cells using nanostructured titanium dioxide solution. J. Photochem. Photobiol. A Chem. 2010, 214, 215–223. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Tie, X.; Qiu, B.; Wu, A.; Zheng, Z. Induction of cytotoxicity by photoexcitation of TiO2 can prolong survival in glioma-bearing mice. Mol. Biol. Rep. 2011, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, S.; Lou, X. Controlling silica coating thickness on TiO2 nanoparticles for effective photodynamic therapy. Colloids Surf. B Biointerfaces 2013, 107, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugapriya, K.; Kang, H.W. Engineering pharmaceutical nanocarriers for photodynamic therapy on wound healing: Review. Mater. Sci. Eng. C 2019, 105, 110110. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lv, L.; Fan, H.; Ma, J.; Li, Y.; Wan, Y.; Zhao, X.S. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 2010, 348, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kayani, Z.N.; Riaz, S.; Naseem, S. Magnetic and antibacterial studies of sol-gel dip coated Ce doped TiO2 thin films: Influence of Ce contents. Ceram. Int. 2020, 46, 381–390. [Google Scholar] [CrossRef]
- Shah, Z.; Nazir, S.; Mazhar, K.; Abbasi, R.; Samokhvalov, I.M. PEGylated doped- and undoped-TiO2 nanoparticles for photodynamic Therapy of cancers. Photodiagn. Photodyn. Ther. 2019, 27, 173–183. [Google Scholar] [CrossRef]
- Zeni, P.F.; Santos, D.P.D.; Canevarolo, R.R.; Yunes, J.A.; Padilha, F.F.; de Albuquerque, J.R.L.C.; Egues, S.M.; Hernández-Macedo, M.L. Photocatalytic and Cytotoxic Effects of Nitrogen-Doped TiO2 Nanoparticles on Melanoma Cells. J. Nanosci. Nanotechnol. 2018, 18, 3722–3728. [Google Scholar] [CrossRef]
- Shang, H.; Han, D.; Ma, M.; Li, S.; Xue, W.; Zhang, A. Enhancement of the photokilling effect of TiO2 in photodynamic therapy by conjugating with reduced graphene oxide and its mechanism exploration. J. Photochem. Photobiol. B Biol. 2017, 177, 112–123. [Google Scholar] [CrossRef]
- Ismail, A.F.M.; Ali, M.M.; Ismail, L.F.M. Photodynamic therapy mediated antiproliferative activity of some metal-doped ZnO nanoparticles in human liver adenocarcinoma HepG2 cells under UV irradiation. J. Photochem. Photobiol. B Biol. 2014, 138, 99–108. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ramesh, B.; Seifalian, A.M. Fluorescence nanoparticles “quantum dots” as drug delivery system and their toxicity: A review. J. Drug Target. 2011, 19, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Jia, L. Nanoparticles Improve Biological Functions of Phthalocyanine Photosensitizers Used for Photodynamic Therapy. Curr. Drug Metab. 2012, 13, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Biroli, A.O.; Tessore, F.; Caramori, S.; Pizzotti, M. β-Substituted ZnII porphyrins as dyes for DSSC: A possible approach to photovoltaic windows. Coord. Chem. Rev. 2018, 358, 153–177. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Wang, X. Biomedical applications of nano-titania in theranostics and photodynamic therapy. Biomater. Sci. 2016, 4, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of TiO2 by halogenated porphyrin derivatives for visible light biomedical and environmental photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Pan, X.; Xie, J.; Li, Z.; Chen, M.; Wang, M.; Wang, P.-N.; Chen, L.; Mi, L. Enhancement of the photokilling effect of aluminum phthalocyanine in photodynamic therapy by conjugating with nitrogen-doped TiO2 nanoparticles. Colloids Surf. B Biointerfaces 2015, 130, 292–298. [Google Scholar] [CrossRef]
- Pan, X.; Liang, X.; Yao, L.; Wang, X.; Jing, Y.; Ma, J.; Fei, Y.; Chen, L.; Mi, L. Study of the Photodynamic Activity of N-Doped TiO2 Nanoparticles Conjugated with Aluminum Phthalocyanine. Nanomaterials 2017, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Yurt, F.; Ocakoglu, K.; Ince, M.; Colak, S.G.; Er, O.; Soylu, H.M.; Gunduz, C.; Biray Avci, C.; Caliskan Kurt, C. Photodynamic therapy and nuclear imaging activities of zinc phthalocyanine-integrated TiO2 nanoparticles in breast and cervical tumors. Chem. Biol. Drug Des. 2018, 91, 789–796. [Google Scholar] [CrossRef]
- Tunçel, A.; Öztürk, İ.; Ince, M.; Ocakoglu, K.; Hoşgör-Limoncu, M.; Yurt, F. Antimicrobial photodynamic therapy against Staphylococcus aureus using zinc phthalocyanine and zinc phthalocyanine-integrated TiO2 nanoparticles. J. Porphyr. Phthalocyanines 2019, 23, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, I.; Tunçel, A.; Ince, M.; Ocakoglu, K.; Hoşgör-Limoncu, M.; Yurt, F. Antibacterial properties of subphthalocyanine and subphthalocyanine-TiO2 nanoparticles on Staphylococcus aureus and Escherichia coli. J. Porphyr. Phthalocyanines 2018, 22, 1099–1105. [Google Scholar] [CrossRef]
- Mantareva, V.; Eneva, I.; Kussovski, V.; Borisova, E.; Angelov, I. Antimicrobial photodisinfection with Zn(II) phthalocyanine adsorbed on TiO2 upon UVA and red irradiation. In Proceedings of the 18th International School on Quantum Electronics: Laser Physics and Applications; International Society for Optics and Photonics, Sozopol, Bulgaria, 8 January 2015; Volume 9447, p. 94470. [Google Scholar]
- Lopez, T.; Ortiz, E.; Alvarez, M.; Navarrete, J.; Odriozola, J.A.; Martinez-Ortega, F.; Páez-Mozo, E.A.; Escobar, P.; Espinoza, K.A.; Rivero, I.A. Study of the stabilization of zinc phthalocyanine in sol-gel TiO2 for photodynamic therapy applications. NanoMed. Nanotechnol. Biol. Med. 2010, 6, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Perillo, P.M.; Getz, F.C. Dye Sensitized TiO2 Nanopore Thin Films with Antimicrobial Activity Against Methicillin Resistant Staphylococcus Aureus Under Visible Light. World J. Appl. Chem. 2016, 1, 9–15. [Google Scholar]
- Zhao, C.; Rehman, F.U.; Yang, Y.; Li, X.; Zhang, D.; Jiang, H.; Selke, M.; Wang, X.; Liu, C. Bio-imaging and Photodynamic Therapy with Tetra Sulphonatophenyl Porphyrin (TSPP)-TiO2 Nanowhiskers: New Approaches in Rheumatoid Arthritis Theranostics. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, F.; Zhao, C.; Jiang, H.; Selke, M.; Wang, X.D. Photoactivated TiO2 Nanowhiskers and Tetra Sulphonatophenyl Porphyrin Normoglycemic Effect on Diabetes Mellitus During Photodynamic Therapy. J. Nanosci. Nanotechnol. 2016, 16, 12691–12694. [Google Scholar] [CrossRef]
- Youssef, Z.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Moussaron, A.; Baros, F.; Toufaily, J.; Hamieh, T.; Roques-Carmes, T.; Frochot, C. Titania and silica nanoparticles coupled to Chlorin e6 for anti-cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2018, 22, 115–126. [Google Scholar] [CrossRef]
- Tuchina, E.S.; Tuchin, V.V. TiO2 nanoparticle enhanced photodynamic inhibition of pathogens. Laser Phys. Lett. 2010, 7, 607. [Google Scholar] [CrossRef]
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.A.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in nuclear medicine practice. Onco Targets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef] [Green Version]
- Makhseed, S.; Machacek, M.; Alfadly, W.; Tuhl, A.; Vinodh, M.; Novakova, V.; Kubat, P.; Rudolf, E.; Zimcik, P. Water-soluble non-aggregating zinc phthalocyanine and in vitro study for photodynamic therapy. Chem. Commun. 2013, 49, 11149. [Google Scholar] [CrossRef]
- Yurt, F.; Ince, M.; Colak, S.G.; Ocakoglu, K.; Er, O.; Soylu, H.M.; Gunduz, C.; Avci, C.B.; Kurt, C.C. Investigation of in vitro PDT activities of zinc phthalocyanine immobilised TiO2 nanoparticles. Int. J. Pharm. 2017, 524, 467–474. [Google Scholar] [CrossRef]
- Erdural, B.K.; Yurum, A.; Bakir, U.; Karakas, G. Antimicrobial properties of titanium nanoparticles. In Functionalized Nanoscale Materials, Devices and Systems; Springer: Dordrecht, The Netherlands, 2008; pp. 409–414. [Google Scholar]
- Shirai, R.; Miura, T.; Yoshida, A.; Yoshino, F.; Ito, T.; Yoshinari, M.; Yajima, Y. Antimicrobial effect of titanium dioxide after ultraviolet irradiation against periodontal pathogen. Dent. Mater. J. 2016, 35, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, T.; Narita, K.; Ono, A.; Wada, K.; Tanaka, T.; Kumagai, G.; Yamauchi, R.; Nakane, A.; Ishibashi, Y. Bactericidal and antimicrobial effects of pure titanium and titanium alloy treated with short-term, low-energy UV irradiation. Bone Jt. Res. 2017, 6, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Marin, J.J.; Romero, M.R.; Blazquez, A.G.; Herraez, E.; Keck, E.; Briz, O. Importance and limitations of chemotherapy among the available treatments for gastrointestinal tumours. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2009, 9, 162–184. [Google Scholar] [CrossRef]
- Zimmermann, S.; Dziadziuszko, R.; Peters, S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat. Rev. 2014, 40, 716–722. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853. [Google Scholar] [CrossRef]
- Lai, Y.-K.; Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Chen, Z.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Al-Deyab, S.S. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. NanoMed. 2016, 11, 4819–4834. [Google Scholar] [CrossRef] [Green Version]
- Raja, G.; Cao, S.; Kim, D.-H.; Kim, T.-J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater. Sci. Eng. C 2020, 107, 110303. [Google Scholar] [CrossRef]
- Flak, D.; Yate, L.; Nowaczyk, G.; Jurga, S. Hybrid ZnPc@TiO2 nanostructures for targeted photodynamic therapy, bioimaging and doxorubicin delivery. Mater. Sci. Eng. C 2017, 78, 1072–1085. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Tong, R.; An, N.; Qu, F. Near-infrared light-mediated DOX-UCNPs@mHTiO2 nanocomposite for chemo/photodynamic therapy and imaging. Colloids Surf. B Biointerfaces 2017, 154, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Zhang, C. Synthesis of Diamond-Shaped Mesoporous Titania Nanobricks as pH-Responsive Drug Delivery Vehicles for Cancer Therapy. ChemistrySelect 2019, 4, 8225–8228. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Lu, X.; Tian, H.; Jiang, H.; Lv, G.; Guo, D.; Wu, C.; Chen, B. The incorporation of daunorubicin in cancer cells through the use of titanium dioxide whiskers. Biomaterials 2009, 30, 4708–4715. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, X.; Gao, Z.; Song, Y.-Y.; Schmuki, P. Visible-Light-Triggered Drug Release from TiO2 Nanotube Arrays: A Controllable Antibacterial Platform. Angew. Chem. Int. Ed. 2016, 55, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Pan, Y.; Tian, Y.; Wang, X.; Ren, W.; Wang, S.; Lu, G.; Wu, A. Doxorubicin-loaded NaYF4:Yb/Tm-TiO2 inorganic photosensitizers for NIR-triggered photodynamic therapy and enhanced chemotherapy in drug-resistant breast cancers. Biomaterials 2015, 57, 93–106. [Google Scholar] [CrossRef]
- Tong, R.; Lin, H.; Chen, Y.; An, N.; Wang, G.; Pan, X.; Qu, F. Near-infrared mediated chemo/photodynamic synergistic therapy with DOX-UCNPs@mSiO2/TiO2-TC nanocomposite. Mater. Sci. Eng. C 2017, 78, 998–1005. [Google Scholar] [CrossRef]
- Akram, M.W.; Raziq, F.; Fakhar-e-Alam, M.; Aziz, M.H.; Alimgeer, K.S.; Atif, M.; Amir, M.; Hanif, A.; Aslam Farooq, W. Tailoring of Au-TiO2 nanoparticles conjugated with doxorubicin for their synergistic response and photodynamic therapy applications. J. Photochem. Photobiol. A Chem. 2019, 384, 112040. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Sazgarnia, A.; Seifi, M.; Hadizadeh, F.; Rajabzadeh, G.; Mohajeri, S.A. TiO2-based Mitoxantrone Imprinted Poly (Methacrylic acid-co-polycaprolctone diacrylate) Nanoparticles as a Drug Delivery System. Curr. Pharm. Des. 2017, 23, 2685–2694. [Google Scholar] [CrossRef]
- Kurzmann, C.; Verheyen, J.; Coto, M.; Kumar, R.V.; Divitini, G.; Shokoohi-Tabrizi, H.A.; Verheyen, P.; De Moor, R.J.G.; Moritz, A.; Agis, H. In vitro evaluation of experimental light activated gels for tooth bleaching. Photochem. Photobiol. Sci. 2019, 18, 1009–1019. [Google Scholar] [CrossRef]
- Onwubu, S.C.; Mdluli, P.S.; Singh, S.; Tlapana, T. A novel application of nano eggshell/titanium dioxide composite on occluding dentine tubules: An in vitro study. Braz. Oral Res. 2019, 33. [Google Scholar] [CrossRef]
- Shaikhaliyev, A.I.; Polisan, A.A.; Ivanov, S.Y.; Parkhomenko, Y.N.; Malinkovich, M.D.; Yarygin, K.N.; Arazashvili, L.D. Effect of the Surface of Medical Titanium Endoprostheses on the Efficiency of Fibrointegration. J. Synch. Investig. 2019, 13, 644–651. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Ran, Z.; Wang, L.; Fang, Y.; Ma, C.; Li, S. Photocatalytic Degradation of Atenolol by TiO2 Irradiated with an Ultraviolet Light Emitting Diode: Performance, Kinetics, and Mechanism Insights. Catalysts 2019, 9, 876. [Google Scholar] [CrossRef] [Green Version]
- Cuppini, M.; Leitune, V.C.B.; de Souza, M.; Alves, A.K.; Samuel, S.M.W.; Collares, F.M. In vitro evaluation of visible light-activated titanium dioxide photocatalysis for in-office dental bleaching. Dent. Mater. J. 2019, 38, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodagar, A.; Akhoundi, M.S.A.; Bahador, A.; Jalali, Y.F.; Behzadi, Z.; Elhaminejad, F.; Mirhashemi, A.H. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dent. Press J. Orthod. 2017, 22, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Singh, G.; Singh, A.; Tandon, P.; Nagar, A. A comparison of shear bond strength of orthodontic brackets bonded with four different orthodontic adhesives. J. Orthod. Sci. 2014, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Xu, J.; Sun, Z.; Zheng, F.; Liu, C.; Wang, C.; Hu, X.; Xia, L.; Liu, Z.; Xia, R. Decreased Porphyromonas gingivalis adhesion and improved biocompatibility on tetracycline-loaded TiO2 & nbsp;nanotubes: An in vitro study. Int. J. NanoMed. 2018, 13, 6769–6777. [Google Scholar]
- Huang, L.; Jing, S.; Zhuo, O.; Meng, X.; Wang, X. Surface Hydrophilicity and Antifungal Properties of TiO2 Films Coated on a Co-Cr Substrate. BioMed Res. Int. 2017, 2017, 2054723. [Google Scholar] [CrossRef]
- Gillam, D.G. (Ed.) Dentine Hypersensitivity: Advances in Diagnosis, Management, and Treatment; Springer: Berlin, Germany; New York, NY, USA, 2015; ISBN 978-3-319-14576-1. [Google Scholar]
- Sereda, G.; Rashwan, K.; Karels, B.; Fritza, A. Novel Materials for Desensitizing and Remineralizing Dentifrices. Advanced Materials: TechConnect Briefs 2016, 1, 135–138. [Google Scholar]
- Cuervo-Osorio, G.; Jiménez-Valencia, A.M.; Mosquera-Agualimpia, C.; Escobar-Sierra, D.M. Manufacture of titanium dioxide scaffolds for medical applications. Revista Facultad de Ingeniería 2018, 27, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Chang, R.; Webster, T. Atomic Layer Deposition Coating of TiO2 Nano-Thin Films on Magnesium-Zinc Alloys to Enhance Cytocompatibility for Bioresorbable Vascular Stents. Int. J. NanoMed. 2019, 14, 9955–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hautala, J.; Kääriäinen, T.; Hoppu, P.; Kemell, M.; Heinämäki, J.; Cameron, D.; George, S.; Juppo, A.M. Atomic layer deposition—A novel method for the ultrathin coating of minitablets. Int. J. Pharm. 2017, 531, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, F.; Khan, S.; Shah, S.M.H.; Rahim, H.; Hussain, Z.; Sohail, M.; Ullah, R.; Alsaid, M.S.; Shahat, A.A. A new strategy for taste masking of azithromycin antibiotic: Development, characterization, and evaluation of azithromycin titanium nanohybrid for masking of bitter taste using physisorption and panel testing studies. Drug Des. Dev. Ther. 2018, 12, 3855–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendel, P.M.; Rytwo, G. Degradation kinetics of caffeine in water by UV/H2O2 and UV/TiO2. Desalin. Water Treat. 2020, 173, 231–242. [Google Scholar] [CrossRef]
- Majumdar, A.; Pal, A. Recent advancements in visible-light-assisted photocatalytic removal of aqueous pharmaceutical pollutants. Clean Technol. Environ. Policy 2019, 22, 11–42. [Google Scholar] [CrossRef]
- Mestre, A.S.; Carvalho, A.P. Photocatalytic Degradation of Pharmaceuticals Carbamazepine, Diclofenac, and Sulfamethoxazole by Semiconductor and Carbon Materials: A Review. Molecules 2019, 24, 3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Geenen, F.A.M.G.; Franssen, M.C.R.; Miikkulainen, V.; Ritala, M.; Zuilhof, H.; Kostiainen, R.; Nielen, M.W.F. TiO2 Photocatalyzed Oxidation of Drugs Studied by Laser Ablation Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Koltsakidou, A.; Terzopoulou, Z.; Kyzas, G.; Bikiaris, D.; Lambropoulou, D. Biobased Poly(ethylene furanoate) Polyester/TiO2 Supported Nanocomposites as Effective Photocatalysts for Anti-inflammatory/Analgesic Drugs. Molecules 2019, 24, 564. [Google Scholar] [CrossRef] [Green Version]
- Osathaphan, K.; Chucherdwatanasak, B.; Rachdawong, P.; Sharma, V.K. Photocatalytic oxidation of cyanide in aqueous titanium dioxide suspensions: Effect of ethylenediaminetetraacetate. Sol. Energy 2008, 82, 1031–1036. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, L.; Ferronato, C.; Yang, X.; Salvador, A.; Zeng, C.; Chovelon, J.-M. Photocatalytic degradation of atenolol in aqueous titanium dioxide suspensions: Kinetics, intermediates and degradation pathways. J. Photochem. Photobiol. A Chem. 2013, 254, 35–44. [Google Scholar] [CrossRef]
- Wang, Z.; Srivastava, V.; Wang, S.; Sun, H.; Thangaraj, S.K.; Jänis, J.; Sillanpää, M. UVC-assisted photocatalytic degradation of carbamazepine by Nd-doped Sb2O3/TiO2 photocatalyst. J. Colloid Interface Sci. 2020, 562, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Píšťková, V.; Tasbihi, M.; Vávrová, M.; Štangar, U.L. Photocatalytic degradation of β-blockers by using immobilized titania/silica on glass slides. J. Photochem. Photobiol. A Chem. 2015, 305, 19–28. [Google Scholar] [CrossRef]
- Khattak, S.-R.; Shaikh, D.; Ahmad, I.; Usmanghani, K.; Sheraz, M.A.; Ahmed, S. Photodegradation and Stabilization of Betamethasone-17 Valerate in Aqueous/Organic Solvents and Topical Formulations. AAPS PharmSciTech 2013, 14, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruokolainen, M.; Ollikainen, E.; Sikanen, T.; Kotiaho, T.; Kostiainen, R. Oxidation of Tyrosine-Phosphopeptides by Titanium Dioxide Photocatalysis. J. Am. Chem. Soc. 2016, 138, 7452–7455. [Google Scholar] [CrossRef] [Green Version]



| Ref. | Shape of NPs (Characteristics) | Photosensitizer | Method of Synthesis | Medical/Biological Use |
|---|---|---|---|---|
| [57] | P25 TiO2 (75% anatase and 25% rutile, size 25 nm) | 5,10,15,20-tetrakis(2,6-difluorosulfonylophenyl)porphyrin and its zinc(II) complex | commercial distribution | PACT against S. aureus, E. coli |
| [58] | N-TiO2-NH2 (size: 20–30 nm) | Aluminum(III) phthalocyanine chloride tetrasulfonate | N-doping by calcination of commercially available anatase TiO2 NPs in ammonia atmosphere | PDT against cancer (HeLa and KB cell lines) |
| [59] | N-TiO2-NH2 (size: 20–30 nm) | Aluminum(III) phthalocyanine chloride tetrasulfonate | N-doping by calcination of commercially available anatase TiO2 NPs in ammonia atmosphere | PDT against cancer (HeLa cell line) |
| [60] | anatase (size: 23 nm spheres) | subphthalocyanine derivatives | from TiCl4 and benzyl alcohol; macrocycle deposition overnight in THF | PDT against breast and cervical tumors |
| [61] | anatase (23 nm spheres) | Zinc(II) phthalocyanine derivatives | from TiCl4 and benzyl alcohol; macrocycle deposition overnight in THF | PACT against: S. aureus |
| [62] | anatase (23 nm spheres) | Subphthalocyanine derivative | from TiCl4 and benzyl alcohol; macrocycle deposition overnight in THF | PACT against S. aureus, E. coli |
| [63] | anatase (size—25 nm) | Zinc(II) tetrakis(3-dodecylpyridyloxy)phthalocyanine (mixture of isomers) | deposition in pyridine/ethanol mixture | PACT against MRSA, Salmonella enteritidis |
| [64] | no data presented | Zinc(II) phthalocyanine | sol-gel method | PACT against Leishmania chagasi, Leishmania panamensis; PDT against human liver cancer cell line |
| [65] | anatase/rutile film (600 nm in film thickness, 100 nm grain size) | Copper tetracarboxyphthalocyanines (mixture of isomers) | anodization | PACT against MRSA |
| [66] | TiO2 nanowhiskers (size < 100 nm) | tetrasulphonatophenyl porphyrin | undefined deposition in water | PDT and bioimaging of rheumatoid arthritis |
| [67] | TiO2 nanowhiskers | tetrasulphonatophenyl porphyrin | undefined; deposition in water | PDT of diabetes mellitus |
| [68] | P25 TiO2 (75% anatase and 25% rutile, size—21 nm) | Chlorin e6 | silylation with or without PEGylation | PDT against glioblastoma cell |
| [69] | no data (size—100 nm) | methylene blue used in mixture but without grafting the NPs | commercial distribution | PACT against: S. aureus, E. coli, and Candida albicans |
| Ref. | Shape of Nanoparticles (Characteristics) | Method of Synthesis | Medical/Biological Use |
|---|---|---|---|
| [83] | ZnPc@TiO2_CHCl3 (20 nm) ZnPc@TiO2_THF (125 nm) ZnPc@TiO2_CHCl3/THF (13 nm); mostly anatase with small addition of rutile | NPs—commercially; nanotubes—from titanium(IV) isopropoxide in a sol-gel method followed by hydrothermal treatment; deposition of ZnPc in CHCl3, THF or 1:1 v/v CHCl3/THF | PDT, bioimaging and doxorubicin delivery (tested on HeLa cells) |
| [84] | UCNPs@SiO2@TiO2 (TiO2 shell thickness—5–6 nm) | TiO2 was grown on UCNPs@SiO2-NH2 NPs from titanium diisopropoxide bis(acetylacetonate); further hydrothermal treatment yielded crystalline structure | PDT in cancer treatment mixed with doxorubicin (tested on HeLa cells) |
| [85] | diamond-shaped mesoporous TiO2 (220 nm in width, 250 nm in length, 40 nm thick, pore size—4.1 nm) | from Ti(IV) isopropoxide at 28 °C, followed by silylation and PEGylation | pH-responsive drug delivery vehicles for cancer therapy |
| [86] | TiO2 nanowhiskers (width 80 nm, length range—200–5000 nm) | K2CO3 with TiO2 heated at 810 °C, soaked in distilled water for about 7 days, dried, and calcinated | PDT with daunorubicin delivery against hepatocarcinoma cells |
| [87] | 0.3 µm TiO2 nanotube array (single nanotube diameter—90 nm) | growth of TiO2 nanotubes in a glycerol/water/NH4F mixture, then annealing to form anatase | Visible-light-triggered release of ampicillin |
| [88] | NaYF4:Yb/Tm-TiO2 (sphere-shaped) (20–40 nm) | TiO2 NPs prepared by solvothermal method from tetrabutyl titanate; trifluoroacetates of lantanides were mixed with TiO2 NPs and thermally treated; further functionalization included PEGylation, silylation and conjugation of folic acid | PDT with doxorubicin delivery tested on drug-resistant breast cancers |
| [89] | UCNPs@mSiO2/TiO2 (30 nm of silica/titania shell thickness) | silica coating was synthesized on UCNPs with tetraethylorthosilicate, silylated and reacted with tetrabutyl titanate followed by calcination to yield anatase phase | PDT mixed with doxorubicin delivery against HeLa cells) |
| [90] | TiO2 (anatase, 10 nm) Au-TiO2 (1–30 nm) | TiO2 from butyl titanate by solvothermal method; Au-TiO2 by solvothermal method using mixture of butyl titanate and HAuCl4; both were followed by calcination. | PDT and doxorubicin delivery tested on breast cancer cells |
| Ref. | Shape of Nanoparticles (Characteristics) | Method of Synthesis | Medical/Biological Use |
|---|---|---|---|
| [92] | TiO2 (anatase, 25 nm) TiO2/Ag NPs | commercial distribution of TiO2 anatase powder was mixed with silver nitrate, reduced and heated at 300 °C | toxicity reduction of teeth whitening gels |
| [93] | TiO2 (anatase, ≤15 µm) Eggshell-TiO2 composite (irregular, spherical shape particles, ≤13 nm) | undefined/commercial distribution of TiO2, eggshell powder with TiO2 was ground in ball mill | occluding opened dentine tubules |
| [94] | TiO2 (anatase, 10 nm) | undefined/commercial distribution | improving of endoprotheses biocompatibility |
| [95] | P25 (anatase/rutile 8:2, 21 nm) | commercial distribution | photocatalytic degradation of phenol |
| [96] | TiO2 (anatase, 20–50 nm) TiO2 (rutile, 50–100 nm) TiO2 mixed phase (anatase/rutile 83:17, 20–50 nm) | commercial distribution | photocatalytic degradation of atenolol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. https://doi.org/10.3390/nano10020387
Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, Glowacka-Sobotta A, Stanisz B, Goslinski T, Sobotta L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials. 2020; 10(2):387. https://doi.org/10.3390/nano10020387
Chicago/Turabian StyleZiental, Daniel, Beata Czarczynska-Goslinska, Dariusz T. Mlynarczyk, Arleta Glowacka-Sobotta, Beata Stanisz, Tomasz Goslinski, and Lukasz Sobotta. 2020. "Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine" Nanomaterials 10, no. 2: 387. https://doi.org/10.3390/nano10020387
APA StyleZiental, D., Czarczynska-Goslinska, B., Mlynarczyk, D. T., Glowacka-Sobotta, A., Stanisz, B., Goslinski, T., & Sobotta, L. (2020). Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials, 10(2), 387. https://doi.org/10.3390/nano10020387






