On the Analogy between Electrolytes and Ion-Generating Nanomaterials in Liquid Crystals
Abstract
:1. Introduction
2. Model (Analogy between Ion-Generating Nanomaterials and Electrolytes in Liquid Crystals)
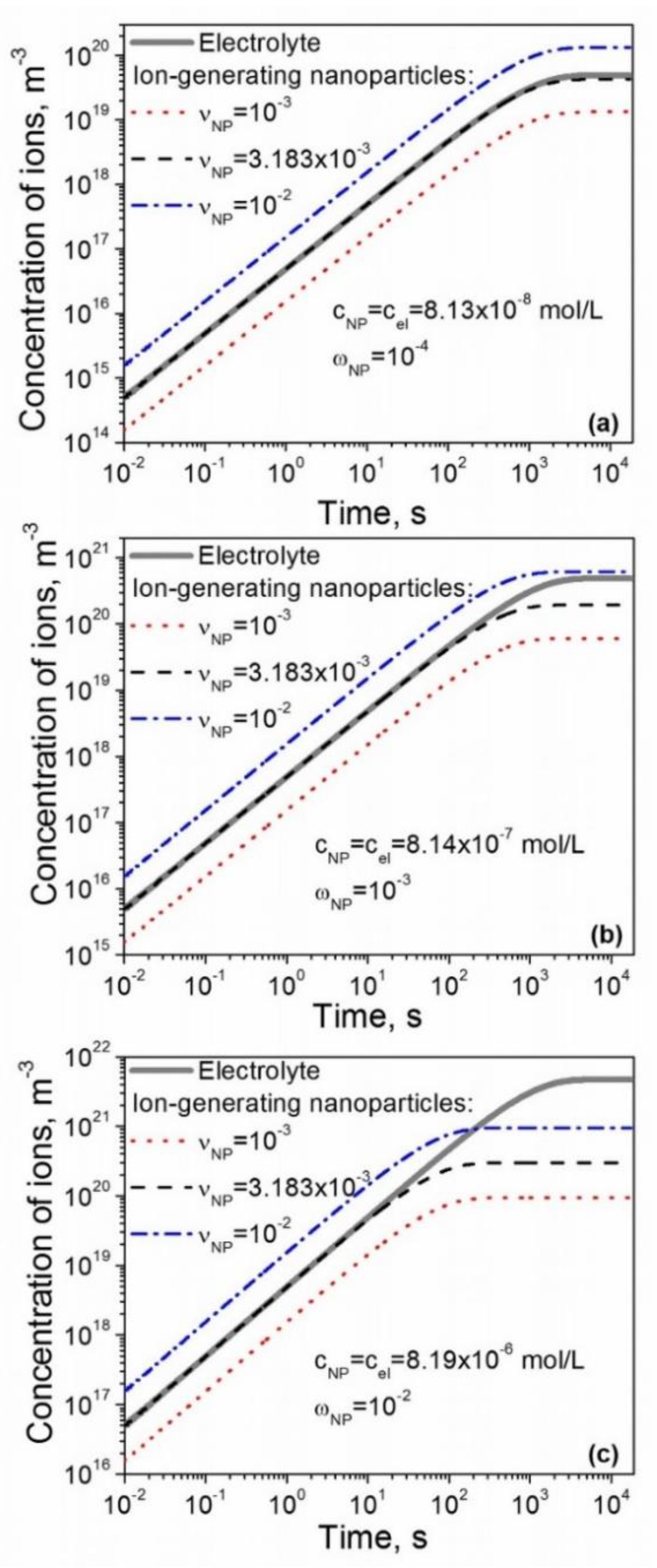
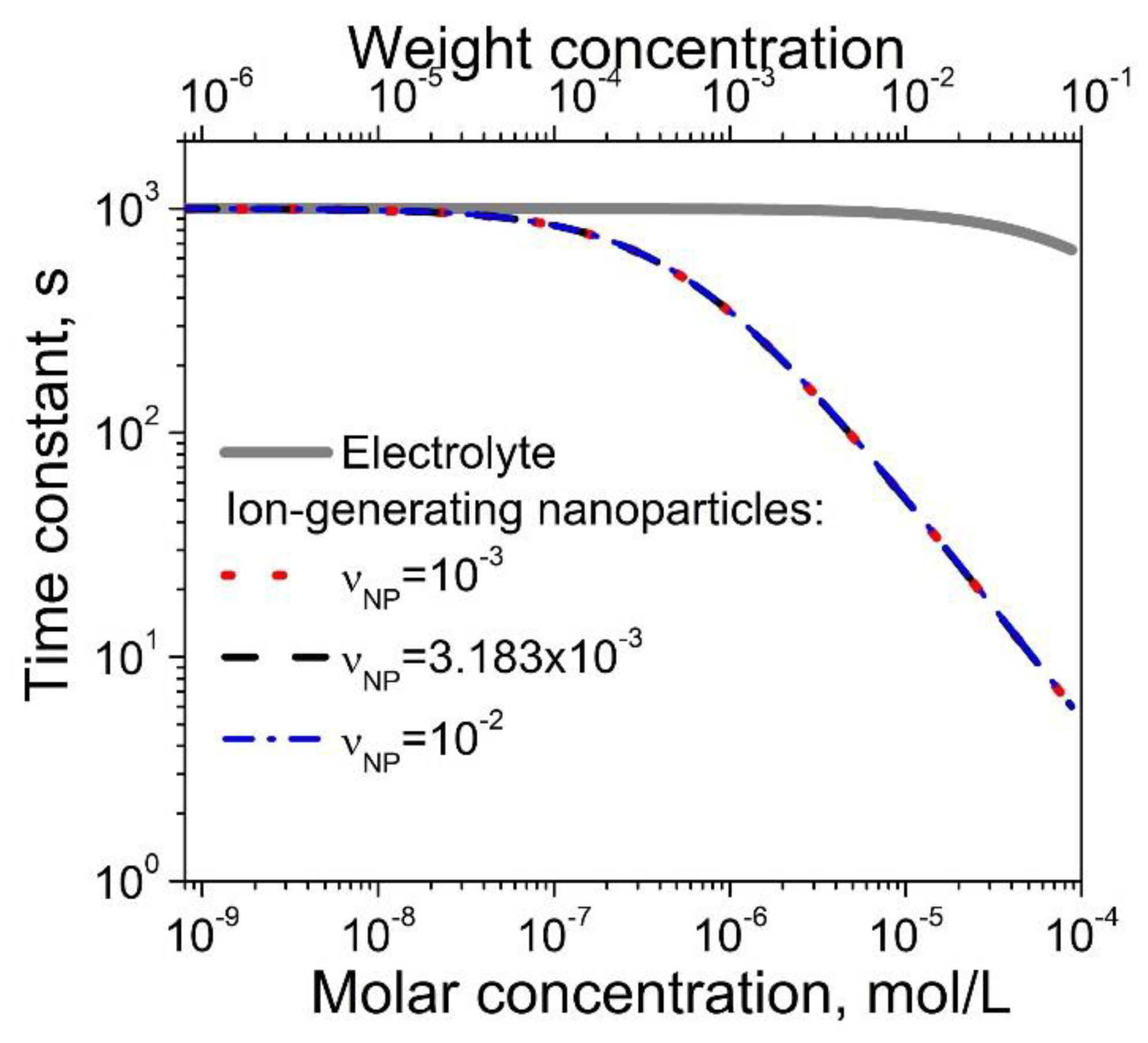
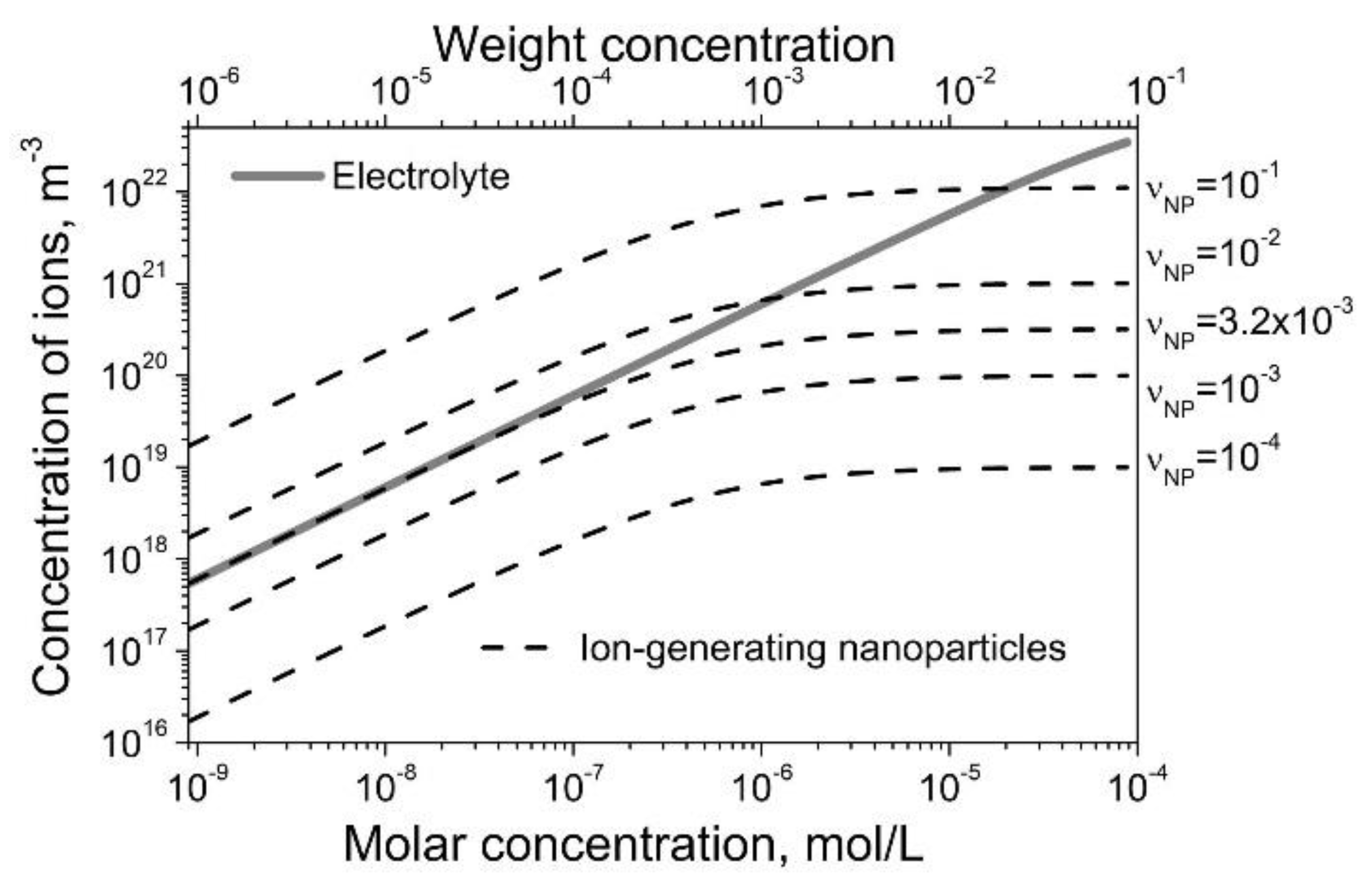
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neyts, K.; Beunis, F. Handbook of Liquid Crystals: Physical Properties and Phase Behavior of Liquid Crystals; Chapter 11, Ion transport in liquid crystals; Wiley-VCH: Weinheim, Germany, 2014; Volume 2, pp. 357–382. [Google Scholar]
- Colpaert, C.; Maximus, B.; Meyere, D. Adequate measuring techniques for ions in liquid crystal layers. Liq. Cryst. 1996, 21, 133–142. [Google Scholar] [CrossRef]
- Williams, R. Domains in Liquid Crystals. J. Chem. Phys. 1963, 39, 384–388. [Google Scholar] [CrossRef]
- Blinov, L.M. Electrohydrodynamic effects in liquid crystals. Sci. Prog. (1933-) 1986, 70, 263–286. [Google Scholar]
- Kramer, L.; Pesch, W. Electrohydrodynamic instabilities in nematic liquid crystals. In Pattern Formation in Liquid Crystals; Partially ordered systems; Buka, A., Kramer, L., Eds.; Springer: New York, NY, USA, 1996; pp. 221–255. [Google Scholar]
- Chang, R.; Richardson, J.M. The anisotropic electrical conductivity of MBBA containing tetrabutyl-ammonium tetraphenyl-boride. Mol. Cryst. Liq. Cryst. 1973, 28, 189–200. [Google Scholar] [CrossRef]
- Barnik, M.I.; Blinov, L.M.; Grebenkin, M.F.; Pikin, S.A.; Chigrinov, V.G. Electrohydrodynamic instability in nematic liquid crystals. Sov. Phys. JETP 1976, 42, 550–553. [Google Scholar]
- Heilmeier, G.H.; Zanoni, L.A.; Barton, L.A. Dynamic scattering in nematic liquid crystals. Appl. Phys. Lett. 1968, 13, 46–47. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Zanoni, L.A.; Barton, L.A. Dynamic scattering: A new electrooptic effect in certain classes of nematic liquid crystals. Proc. IEEE 1968, 56, 1162–1171. [Google Scholar] [CrossRef]
- Chieu, T.C.; Yang, K.H. Transport properties of ions in ferroelectric liquid crystal cells. Jpn. J. Appl. Phys. 1989, 28, 2240–2246. [Google Scholar] [CrossRef]
- Kovalchuk, A.V.; Lavrentovich, O.D.; Linev, V.A. Electrical conductivity of γ-irradiated cholesteric liquid crystals. Sov. Tech. Phys. Lett. 1988, 14, 381–382. [Google Scholar]
- Murakami, S.; Naito, H. Charge injection and generation in nematic liquid crystal cells. Jpn. J. Appl. Phys. 1997, 36, 773–776. [Google Scholar] [CrossRef]
- De Vleeschouwer, H.; Verschueren, A.; Bougrioua, F.; Van Asselt, R.; Alexander, E.; Vermael, S.; Neyts, K.; Pauwels, H. Long-term ion transport in nematic liquid crystal displays. Jpn. J. Appl. Phys. 2001, 40, 3272–3276. [Google Scholar] [CrossRef]
- Murakami, S.; Naito, H. Electrode and interface polarizations in nematic liquid crystal cells. Jpn. J. Appl. Phys. 1997, 36, 2222–2225. [Google Scholar] [CrossRef]
- Koide, N. The Liquid Crystal Display Story. 50 Years of Liquid Crystal r&d that Lead the Way to the Future; Springer: Tokyo, Japan, 2014. [Google Scholar] [CrossRef]
- Chigrinov, V.G. Liquid Crystal Devices: Physics and Applications; Artech House: Boston, MA, USA, 1999; pp. 1–360. [Google Scholar]
- Naemura, S.; Sawada, A. Ionic conduction in nematic and smectic liquid crystals. Mol. Cryst. Liq. Cryst. 2003, 400, 79–96. [Google Scholar] [CrossRef]
- Hung, H.Y.; Lu, C.W.; Lee, C.Y.; Hsu, C.S.; Hsieh, Y.Z. Analysis of metal ion impurities in liquid crystals using high resolution inductively coupled plasma mass spectrometry. Anal. Methods 2012, 4, 3631–3637. [Google Scholar] [CrossRef]
- Mizusaki, M.; Enomoto, S.; Hara, Y. Generation mechanism of residual direct current voltage for liquid crystal cells with polymer layers produced from monomers. Liq. Cryst. 2017, 44, 609–617. [Google Scholar] [CrossRef]
- Huang, Y.; Bhowmik, A.; Bos, P.J. The effect of salt on ion adsorption on a SiOx alignment film and reduced conductivity of a liquid crystal host. J. Appl. Phys. 2012, 111, 024501. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Xu, J.; Furuta, H.; Murakami, Y.; Kawamoto, S.; Ohkouchi, M.; Hasebe, H.; Takatsu, H. Fabrication and electro-optic characteristics of polymer-stabilized V-mode ferroelectric liquid crystal display and intrinsic H-V-mode ferroelectric liquid crystal displays: Their application to field sequential full colour active matrix liquid crystal displays. Opt. Eng. 2004, 43, 290–298. [Google Scholar]
- Huang, Y.; Bhowmik, A.; Bos, P.J. Characterization of ionic impurities adsorbed onto a 5° SiOx alignment film. Jpn. J. Appl. Phys. 2012, 51, 031701. [Google Scholar]
- Geis, M.W.; Bos, P.J.; Liberman, V.; Rothschild, M. Broadband optical switch based on liquid crystal dynamic scattering. Opt. Express 2016, 24, 13812–13823. [Google Scholar] [CrossRef]
- Dabrowski, R.; Dziaduszek, J.; Bozetka, J.; Piecek, W.; Mazur, R.; Chrunik, M.; Perkowski, P.; Mrukiewicz, M.; Żurowska, M.; Weglowska, D. Fluorinated smectics–New liquid crystalline medium for smart windows and memory displays. J. Mol. Liq. 2017, 267, 415–427. [Google Scholar] [CrossRef]
- Konshina, E.A.; Shcherbinin, D.P. Study of dynamic light scattering in nematic liquid crystal and its optical, electrical and switching characteristics. Liq. Cryst. 2018, 45, 292–302. [Google Scholar] [CrossRef]
- Madhuri, P.L.; Martin-Palma, R.J.; Martín-Adrados, B.; Abdulhalim, I. Voltage controlled scattering from porous silicon Mie-particles in liquid crystals. J. Mol. Liq. 2019, 281, 108–116. [Google Scholar]
- Abdulhalim, I.; Madhuri, P.; Diab, M.; Mokari, T. Novel easy to fabricate liquid crystal composite with potential for electrically or thermally controlled transparency windows. Opt. Express 2019, 27, 17387–17401. [Google Scholar] [CrossRef]
- Zhan, Y.; Lu, H.; Jin, M.; Zhou, G. Electrohydrodynamic instabilities for smart window applications. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Chen, H.W.; Lee, J.H.; Lin, B.Y.; Chen, S.; Wu, S.T. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Light Sci. Appl. 2018, 7, 17168. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, E.; Chen, R.; Wu, S.-T. Liquid-Crystal-on-Silicon for Augmented Reality Displays. Appl. Sci. 2018, 8, 2366. [Google Scholar] [CrossRef] [Green Version]
- Chigrinov, V.G. Liquid Crystal Photonic; Nova Science Pub Inc.: New York, NY, USA, 5 November 2014; pp. 1–204. [Google Scholar]
- He, Z.; Tan, G.; Chanda, D.; Wu, S.-T. Novel liquid crystal photonic devices enabled by two-photon polymerization [Invited]. Opt. Express 2019, 27, 11472–11491. [Google Scholar] [CrossRef]
- Abdulhalim, I. Non-display bio-optic applications of liquid crystals. Liq. Cryst. Today 2011, 20, 44–60. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wang, Y.; Reshetnyak, V. Liquid crystal lenses with tunable focal length. Liq. Cryst. Rev. 2017, 5, 111–143. [Google Scholar] [CrossRef]
- De Sio, L.; Roberts, D.E.; Liao, Z.; Hwang, J.; Tabiryan, N.; Steeves, D.M.; Kimball, B.R. Beamshaping diffractive wave plates. Appl. Opt. 2018, 57, A118–A121. [Google Scholar] [CrossRef]
- Zhang, Z.; You, Z.; Chu, D. Fundamentals of phase-only liquid crystal on silicon (LCOS) devices. Light Sci. Appl. 2014, 3, e213. [Google Scholar] [CrossRef]
- Lazarev, G.; Chen, P.-J.; Strauss, J.; Fontaine, N.; Forbes, A. Beyond the display: Phase-only liquid crystal on Silicon devices and their applications in photonics. Opt. Express 2019, 27, 16206–16249. [Google Scholar] [CrossRef] [PubMed]
- Otón, J.M.; Otón, E.; Quintana, X.; Geday, M.A. Liquid-crystal phase-only devices. J. Mol. Liq. 2018, 267, 469–483. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Time-dependent electrical properties of liquid crystal cells: Unravelling the origin of ion generation. Liq. Cryst. 2018, 45, 1540–1548. [Google Scholar] [CrossRef]
- Korniychuk, P.P.; Gabovich, A.M.; Singer, K.; Voitenko, A.I.; Reznikov, Y.A. Transient and steady electric currents through a liquid crystal cell. Liq. Cryst. 2010, 37, 1171–1181. [Google Scholar] [CrossRef]
- Stamatoiu, O.; Mirzaei, J.; Feng, X.; Hegmann, T. Nanoparticles in liquid crystals and liquid crystalline nanoparticles. Top. Curr. Chem. 2012, 318, 331–394. [Google Scholar]
- Garbovskiy, Y.; Glushchenko, A. Liquid crystalline colloids of nanoparticles: Preparation, properties, and applications. Solid State Phys. 2010, 62, 1–74. [Google Scholar]
- Bisoyi, H.K.; Kumar, S. Liquid-crystal nanoscience: An emerging avenue of soft self-assembly. Chem. Soc. Rev. 2011, 40, 306–319. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. (Eds.) Liquid Crystals with Nano and Microparticles; World Scientific: Singapore, 2016; Volumes 2, ISBN 978-981-4619-25-7. [Google Scholar]
- Shen, Y.; Dierking, I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef] [Green Version]
- Dierking, I. Nanomaterials in Liquid Crystals. Nanomaterials 2018, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Dierking, I. From colloids in liquid crystals to colloidal liquid crystals. Liq. Cryst. 2019, 46, 2057–2074. [Google Scholar] [CrossRef]
- Shukla, R.K.; Liebig, C.M.; Evans, D.R.; Haase, W. Electro-optical behaviour and dielectric dynamics of harvested ferroelectric LiNbO3 nanoparticle-doped ferroelectric liquid crystal nanocolloids. RSC Adv. 2014, 4, 18529–18536. [Google Scholar] [CrossRef]
- Basu, R.; Garvey, A. Effects of ferroelectric nanoparticles on ion transport in a liquid crystal. Appl. Phys. Lett. 2014, 105, 151905. [Google Scholar] [CrossRef] [Green Version]
- Garbovskiy, Y.; Glushchenko, I. Ion trapping by means of ferroelectric nanoparticles, and the quantification of this process in liquid crystals. Appl. Phys. Lett. 2015, 107, 041106. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Huang, S.M.; Yeh, E.R.; Lee, W. Temperature dependent electrical and dielectric properties of nematic liquid crystals doped with ferroelectric particles. Displays 2016, 44, 61–65. [Google Scholar] [CrossRef]
- Kumar, P.; Debnath, S.; Rao, N.V.; Sinha, A. Nanodoping: A route for enhancing electro-optic performance of bent core nematic system. J. Phys. Condens. Matter 2018, 30, 095101. [Google Scholar] [CrossRef]
- Al-Zangana, S.; Turner, M.; Dierking, I. A comparison between size dependent paraelectric and ferroelectric BaTiO3 nanoparticle doped nematic and ferroelectric liquid crystals. J. Appl. Phys. 2017, 121, 085105. [Google Scholar] [CrossRef] [Green Version]
- Garbovskiy, Y.; Glushchenko, A. Ferroelectric Nanoparticles in Liquid Crystals: Recent Progress and Current Challenges. Nanomaterials 2017, 7, 361. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.P.; Malik, P.; Raina, K.K. Electro-optic, dielectric and optical studies of NiFe2O4-ferroelectric liquid crystal: A soft magnetoelectric material. Liq. Cryst. 2016, 43, 1671–1681. [Google Scholar]
- Mertelj, A.; Lisjak, D. Ferromagnetic nematic liquid crystals. Liq. Cryst. Rev. 2017, 5, 1–33. [Google Scholar] [CrossRef]
- Pandey, F.P.; Rastogi, A.; Manohar, R.; Dhar, R.; Singh, S. Dielectric and electro-optical properties of zinc ferrite nanoparticles dispersed nematic liquid crystal 4’-Heptyl-4-biphenylcarbonnitrile. Liq. Cryst. 2019, 40, 1–16. [Google Scholar] [CrossRef]
- Urbanski, M.; Lagerwall, J. Nanoparticles dispersed in liquid crystals: Impact on conductivity, low-frequency relaxation and electro-optical performance. J. Mater. Chem. C 2016, 4, 3485–3491. [Google Scholar] [CrossRef]
- Middha, M.; Kumar, R.; Raina, K.K. Photoluminescence tuning and electro-optical memory in chiral nematic liquid crystals doped with silver nanoparticles. Liq. Cryst. 2016, 43, 1002–1008. [Google Scholar] [CrossRef]
- Podgornov, F.V.; Wipf, R.; Stuhn, B.; Ryzhkova, A.V.; Haase, W. Low-frequency relaxation modes in ferroelectric liquid crystal/gold nanoparticle dispersion: Impact of nanoparticle shape. Liq. Cryst. 2016, 43, 1536–1547. [Google Scholar] [CrossRef]
- Urbanski, M.; Lagerwall, J.P.F. Why organically functionalized nanoparticles increase the electrical conductivity of nematic liquid crystal dispersions. J. Mater. Chem. C 2017, 5, 8802–8809. [Google Scholar] [CrossRef]
- Podgornov, F.V.; Gavrilyak, M.; Karaawi, A.; Boronin, V.; Haase, W. Mechanism of electrooptic switching time enhancement in ferroelectric liquid crystal/gold nanoparticles dispersion. Liq. Cryst. 2018, 45, 1594–1602. [Google Scholar] [CrossRef]
- Shivaraja, S.J.; Gupta, R.K.; Kumar, S.; Manjuladevi, V. Effect of functionalised silver nanoparticle on the elastic constants and ionic transport of a nematic liquid crystal. Liq. Cryst. 2019, 46, 1868–1876. [Google Scholar]
- Chandran, A.; Prakash, J.; Gangwar, J.; Joshi, T.; Srivastava, A.K.; Haranath, D.; Biradar, A.M. Low-voltage electro-optical memory device based on NiO nanorods dispersed in a ferroelectric liquid crystal. RSC Adv. 2016, 6, 53873–53881. [Google Scholar] [CrossRef]
- Ha, Y.-S.; Kim, H.; Park, H.-G.; Seo, D.S. Enhancement of electrooptic properties in liquid crystal devices via titanium nanoparticle doping. Opt. Express 2012, 20, 6448–6455. [Google Scholar] [CrossRef]
- Shcherbinin, D.P.; Konshina, E.A. Ionic impurities in nematic liquid crystal doped with quantum dots CdSe/ZnS. Liq. Cryst. 2017, 44, 648–655. [Google Scholar] [CrossRef]
- Konshina, E.; Shcherbinin, D.; Kurochkina, M. Comparison of the properties of nematic liquid crystals doped with TiO2 and CdSe/ZnS nanoparticles. J. Mol. Liq. 2018, 267, 308–314. [Google Scholar] [CrossRef]
- Shcherbinin, D.P.; Konshina, E.A. Impact of titanium dioxide nanoparticles on purification and contamination of nematic liquid crystals. Beilstein J. Nanotechnol. 2017, 8, 2766–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakasha, J.; Khana, S.; Chauhana, S.; Biradar, A.M. Metal oxide-nanoparticles and liquid crystal composites: A review of recent progress. J. Mol. Liq. 2019, 112052. [Google Scholar] [CrossRef]
- Lee, C.W.; Shih, W.P. Quantification of ion trapping effect of carbon nanomaterials in liquid crystals. Mater. Lett. 2010, 64, 466–468. [Google Scholar] [CrossRef]
- Tomylko, S.; Yaroshchuk, O.; Kovalchuk, O.; Maschke, U.; Yamaguchi, R. Dielectric properties of nematic liquid crystal modified with diamond nanoparticles. Ukr. J. Phys. 2012, 57, 239–243. [Google Scholar]
- Samoilov, A.N.; Minenko, S.S.; Fedoryako, A.P.; Lisetski, L.N.; Lebovka, N.I.; Soskin, M.S. Multiwalled vs. single-walled carbon nanotube dispersions in nematic liquid crystals: Comparative studies of optical transmission and dielectric properties. Funct. Mater. 2014, 21, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Jian, B.R.; Tang, C.Y.; Lee, W. Temperature-dependent electrical properties of dilute suspensions of carbon nanotubes in nematic liquid crystals. Carbon 2011, 49, 910–914. [Google Scholar] [CrossRef]
- Wu, P.C.; Lisetski, L.N.; Lee, W. Suppressed ionic effect and low-frequency texture transitions in a cholesteric liquid crystal doped with graphene nanoplatelets. Opt. Express 2015, 23, 11195–11204. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.P.; Singh, S. Carbon nanotube dispersion in nematic liquid crystals: An overview. Prog. Mater. Sci. 2016, 80, 38–76. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Glushchenko, I. Nano-objects and ions in liquid crystals: Ion trapping effect and related phenomena. Crystals 2015, 5, 501–533. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Nanomaterials in Liquid Crystals as Ion-Generating and Ion-Capturing Objects. Crystals 2018, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Garbovskiy, Y. Switching between purification and contamination regimes governed by the ionic purity of nanoparticles dispersed in liquid crystals. Appl. Phys. Lett. 2016, 108, 121104. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Electrical properties of liquid crystal nano-colloids analysed from perspectives of the ionic purity of nano-dopants. Liq. Cryst. 2016, 43, 648–653. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Nanoparticle enabled thermal control of ions in liquid crystals. Liq. Cryst. 2017, 44, 948–955. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Nanoparticle—Enabled Ion Trapping and Ion Generation in Liquid Crystals. Adv. Condens. Matter Phys. 2018, 2018, 8914891. [Google Scholar] [CrossRef] [Green Version]
- Briere, G.; Gaspard, F.; Herino, R. Ionic residual conduction in the isotropic phase of a nematic liquid crystal. Chem. Phys. Lett. 1971, 9, 285–288. [Google Scholar] [CrossRef]
- Blinov, L.M. Structure and Properties of Liquid Crystals; Springer: New York, NY, USA, 2010. [Google Scholar]
- Garbovskiy, Y. Adsorption/desorption of ions in liquid crystal nano-colloids: The applicability of the Langmuir isotherm, impact of high electric fields, and effects of the nanoparticle’s size. Liq. Cryst. 2016, 43, 853–860. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Ions and size effects in nanoparticle/liquid crystal colloids sandwiched between two substrates. The case of two types of fully ionized species. Chem. Phys. Lett. 2017, 679, 77–85. [Google Scholar] [CrossRef]
- Garbovskiy, Y. Kinetics of Ion-Capturing/Ion-Releasing Processes in Liquid Crystal Devices Utilizing Contaminated Nanoparticles and Alignment Films. Nanomaterials 2018, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Barbero, G.; Evangelista, L.R. Adsorption Phenomena and Anchoring Energy in Nematic Liquid Crystals; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Steffen, V.; Cardozo-Filho, L.; Silva, E.A.; Evangelista, L.R.; Guirardello, R.; Mafra, M.R. Equilibrium modeling of ion adsorption based on Poisson–Boltzmann equation. Colloids Surf. A 2015, 468, 159–166. [Google Scholar] [CrossRef]
- Batalioto, F.; Figueiredo Neto, A.M.; Barbero, G. Ion trapping on silica nanoparticles: Effect on the ζ-potential. J. App. Phys. 2017, 122, 164303. [Google Scholar] [CrossRef]
- Steffen, V.; Silva, E.A.; Evangelista, L.R.; Cardozo-Filho, L. Debya-Huckel approximation for simplification of ions adsorption equilibrium model based on Poisson-Boltzmann equation. Surf. Interfaces 2018, 10, 144–148. [Google Scholar] [CrossRef]
| Ion-Generating Nanomaterials | Electrolytes |
|---|---|
| n | n |
(where ) | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbovskiy, Y. On the Analogy between Electrolytes and Ion-Generating Nanomaterials in Liquid Crystals. Nanomaterials 2020, 10, 403. https://doi.org/10.3390/nano10030403
Garbovskiy Y. On the Analogy between Electrolytes and Ion-Generating Nanomaterials in Liquid Crystals. Nanomaterials. 2020; 10(3):403. https://doi.org/10.3390/nano10030403
Chicago/Turabian StyleGarbovskiy, Yuriy. 2020. "On the Analogy between Electrolytes and Ion-Generating Nanomaterials in Liquid Crystals" Nanomaterials 10, no. 3: 403. https://doi.org/10.3390/nano10030403






