Synthesis of Three-Dimensional Carbon Nanostructure/Copper Nanowire for Additive Interface Layer of Ionic Polymer Metal Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Growth of the Three-Dimensional Carbon Nanostructure on Copper Nanowire (3DC Cu-NW)
2.3. Fabrication of the IPMC Actuator based on 3DC Cu-NW
2.4. Characteristics
3. Results
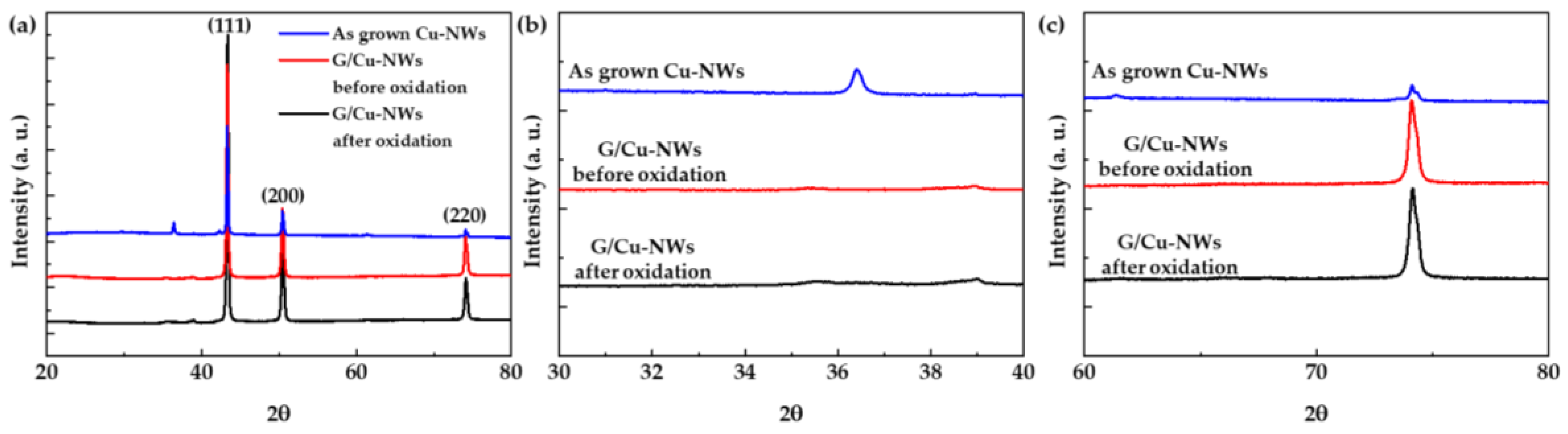
3.1. Characteristics of 3DC Cu-NW
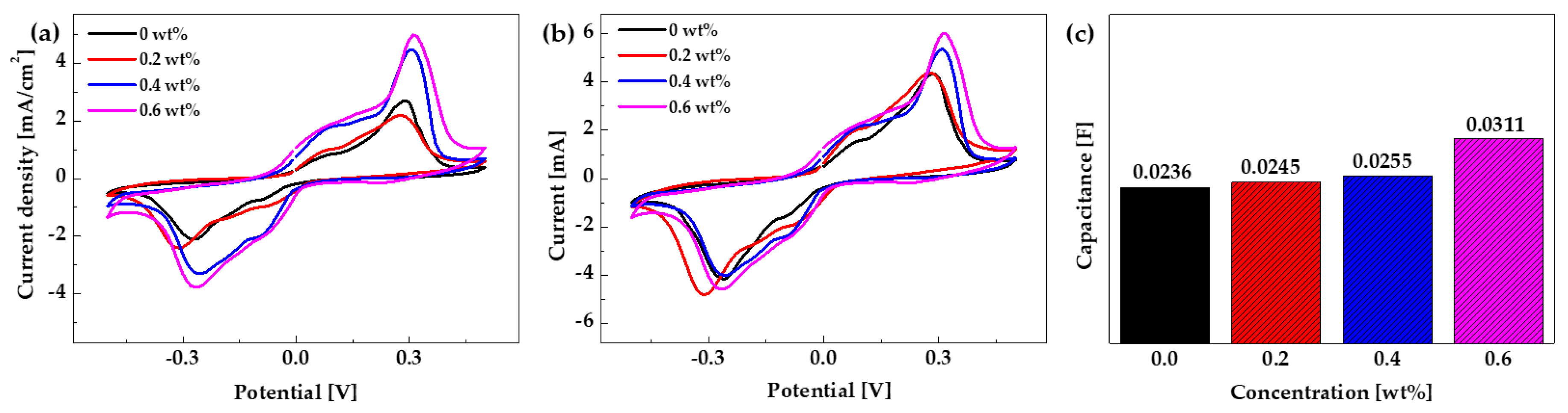
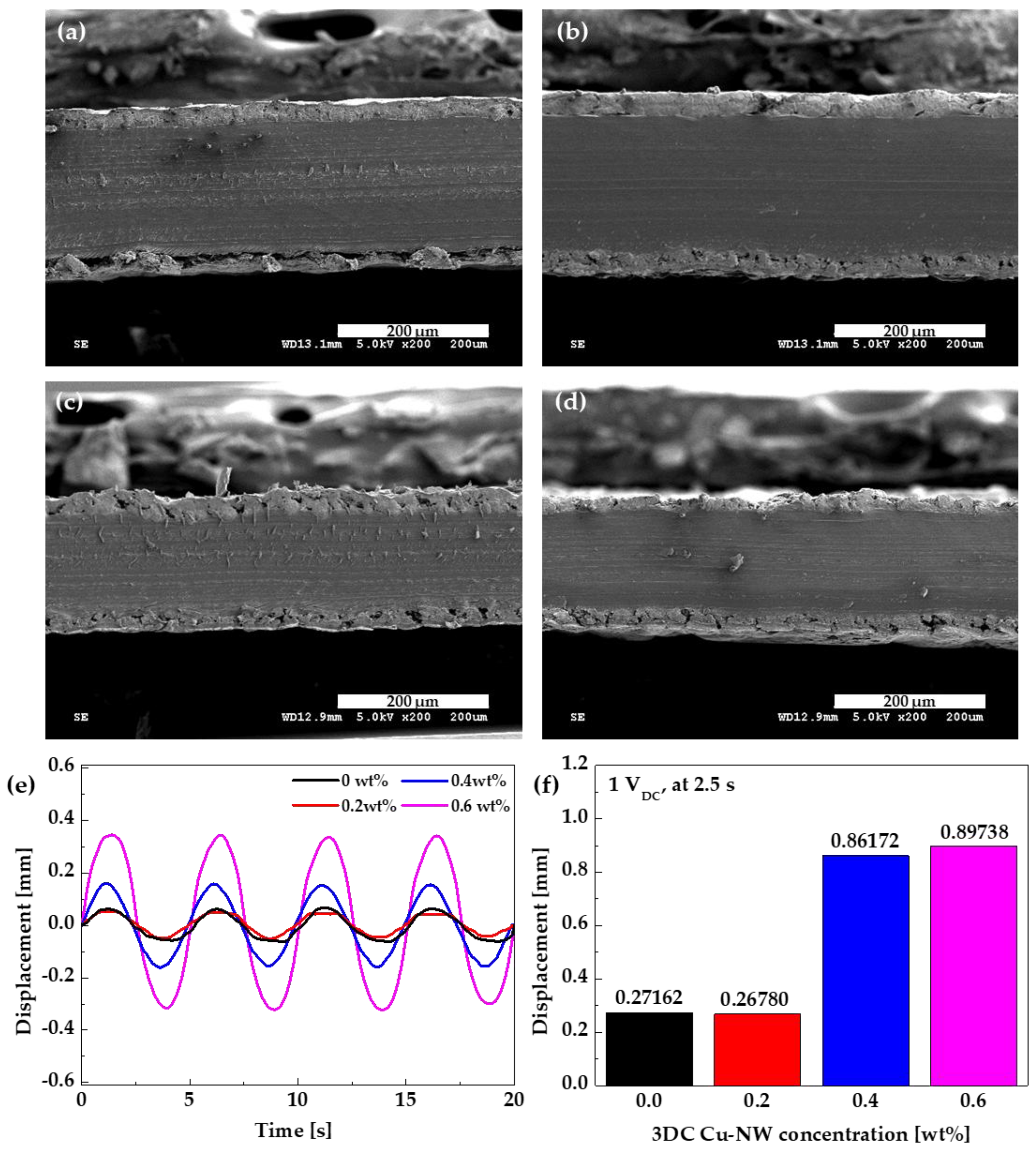
3.2. Characteristics of the IPMC Actuator Based on 3DC Cu-NW
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Li, J.; Xu, Z.; Wang, S.; Wang, Z.; Xu, B.; Sun, Y.; Liu, S.; Zhao, H. Design, analysis, experiments and kinetic model of a high step efficiency piezoelectric actuator. Mechatronics 2019, 59, 61–68. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Wang, H.; Zhao, J. Effect of driving field and temperature on the response behavior of ferroelectric actuator and sensor materials. J. Intell. Mater. Syst. Struct. 1995, 6, 84–93. [Google Scholar] [CrossRef]
- Mehrabi, H.; Aminzahed, I. Design and testing of a microgripper with SMA actuator for manipulation of micro components. Microsyst. Technol. 2019, 26, 531–536. [Google Scholar] [CrossRef]
- Loeian, M.S.; Ziolkowska, D.A.; Khosravi, F.; Jasinski, J.B.; Panchapakesan, B. Exfoliated WS2-Nafion Composite based Electromechanical Actuators. Sci. Rep. 2017, 7, 14599. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Khosravi, F.; Rahneshin, V.; Shanmugam, M.; Loeian, M.; Jasinski, J.; Chon, R.W.; Terentjev, E.; Panchapakesan, B. MoS2 actuators: Reversible mechanical responses of MoS2-polymer nanocomposites to photons. Nanotechnology 2015, 26, 261001. [Google Scholar] [CrossRef] [PubMed]
- Loomis, J.; Fan, X.; Khosravi, F.; Xu, P.; Fletcher, M.; Cohn, R.W.; Panchapakesan, B. Graphene/elastomer composite-based photo-thermal nanopositioners. Sci. Rep. 2013, 3, 1900. [Google Scholar] [CrossRef] [PubMed]
- Loomis, J.; King, B.; Burkhead, T.; Xu, P.; Bessler, N.; Terentjev, E.; Panchapakesan, B. Graphene-nanoplatelet-based photomechanical actuators. Nanotechnology 2012, 23, 045501. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Guo, S. Development and Evaluation of a Venus Flytrap-Inspired Microrobot Microsyst. Technology 2016, 22, 1949–1958. [Google Scholar]
- Shahinpoor, M.; Kim, K.J. Ionic polymer–metal composites: IV. Industrial and medical applications. Smart Mater. Struct. 2004, 14, 197. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, Y.; Mou, J. Recent Developments About IPMCs (Ionic Polymer-Metal) Composites: A Review of Performances for Different Conditions. Fluid Dyn. Mater. Process. 2018, 14, 243–258. [Google Scholar] [CrossRef]
- Bhandari, B.; Lee, G.Y.; Ahn, S.H. A review on IPMC material as actuators and sensors: Fabrications, characteristics and applications. Int. J. Precis. Eng. Manuf. 2012, 13, 141–163. [Google Scholar] [CrossRef]
- Chung, C.K.; Fung, P.K.; Hong, Y.Z.; Ju, M.S.; Lin, C.C.K.; Wu, T.C. A novel fabrication of ionic polymer-metal composites (IPMC) actuator with silver nano-powders. Sens. Actuators B Chem. 2006, 117, 367–375. [Google Scholar] [CrossRef]
- Bian, K.; Liu, H.; Tai, G.; Zhu, K.; Xiong, K. Enhanced actuation response of nafion-based ionic polymer metal composites by doping BaTiO3 nanoparticles. J. Phys. Chem. C 2016, 120, 12377–12384. [Google Scholar] [CrossRef]
- Jung, J.H.; Jeon, J.H.; Sridhar, V.; Oh, I.K. Electro-active graphene–Nafion actuators. Carbon 2011, 49, 1279–1289. [Google Scholar] [CrossRef]
- Guo, D.J.; Liu, R.; Cheng, Y.; Zhang, H.; Zhou, L.M.; Fang, S.M.; Elliott, W.H.; Tan, W. Reverse adhesion of a gecko-inspired synthetic adhesive switched by an ion-exchange polymer–metal composite actuator. ACS Appl. Mater. Interfaces 2015, 7, 5480–5487. [Google Scholar] [CrossRef] [Green Version]
- Lian, H.; Qian, W.; Estevez, L.; Liu, H.; Liu, Y.; Jiang, T.; Wanga, W.G.; Guoe, W.; Giannelis, E.P. Enhanced actuation in functionalized carbon nanotube–Nafion composites. Sens. Actuators B Chem. 2011, 156, 187–193. [Google Scholar] [CrossRef]
- Lee, P.C.; Hyun, J.E.; Jeoung, S.K.; Nam, J.D.; Hwang, T.; Kim, K.J.; Solasa, K.C. Ionic polymer metal composites for use as an organic electrolyte supercapacitor. Smart Mater. Struct. 2019, 28, 054003. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, S.J.; Kim, K.J.; Lee, D.Y. Characteristics of ionic polymer–metal composite with chemically doped TiO2 particles. Smart Mater. Struct. 2011, 20, 124004. [Google Scholar] [CrossRef]
- Bhanushali, S.; Ghosh, P.; Ganesh, A.; Cheng, W. 1D copper nanostructures: Progress, challenges and opportunities. Small 2015, 11, 1232–1252. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Stewart, I.E.; Ha, Y.C.; Wilson, A.R.; Chen, Z.; Wiley, B.J. A rapid synthesis of high aspect ratio copper nanowires for high-performance transparent conducting films. Chem. Commun. 2014, 50, 2562–2564. [Google Scholar] [CrossRef]
- Chang, Y.; Lye, M.L.; Zeng, H.C. Large-scale synthesis of high-quality ultralong copper nanowires. Langmuir 2005, 21, 3746–3748. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Jeong, Y.; Lee, D.; Lee, Y. Copper nanowire–graphene core–shell nanostructure for highly stable transparent conducting electrodes. ACS Nano 2015, 9, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zou, M.; Li, Z.; Chen, D.; Zhang, H.; Yuan, Y.; Pei, Y.; Cao, A. Robust and Stable Cu Nanowire@ Graphene Core–Shell Aerogels for Ultraeffective Electromagnetic Interference Shielding. Small 2018, 14, 1800634. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, H.; Wu, C.; Lin, N.; Soomro, A.M.; Guo, H.; Liu, C.; Yang, X.; Wu, Y.; Cai, D.; et al. Direct synthesis of graphene 3D-coated Cu nanosilks network for antioxidant transparent conducting electrode. Nanoscale 2015, 7, 10613–10621. [Google Scholar] [CrossRef]
- Mehta, R.; Chugh, S.; Chen, Z. Enhanced electrical and thermal conduction in graphene-encapsulated copper nanowires. Nano Lett. 2015, 15, 2024–2030. [Google Scholar] [CrossRef]
- Kim, J.; Bae, S.H.; Kotal, M.; Stalbaum, T.; Kim, K.J.; Oh, I.K. Soft but Powerful Artificial Muscles Based on 3D Graphene–CNT–Ni Heteronanostructures. Small 2017, 13, 1701314. [Google Scholar] [CrossRef]
- Park, M.; Ahn, S.K.; Hwang, S.; Park, S.; Kim, S.; Jeon, M. Synthesis of Nitrogen-Doped Graphene on Copper Nanowires for Efficient Thermal Conductivity and Stability by Using Conventional Thermal Chemical Vapor Deposition. Nanomaterials 2019, 9, 984. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.; Park, M.; Kim, S.; Chung, P.S.; Jeon, M. Graphene Oxide-Silver Nanowires Paper Electrodes with Poly (3, 4-ethylenedioxythiophene)-Poly (styrenesulfonate) to Enhance the Driving Properties of Ionic Electroactive Polymer Actuators. Nanosci. Nanotechnol. Lett. 2018, 10, 1107–1112. [Google Scholar] [CrossRef]
- Park, M.; Kim, J.; Song, H.; Kim, S.; Jeon, M. Fast and Stable Ionic Electroactive Polymer Actuators with PEDOT:PSS/(Graphene–Ag-Nanowires) Nanocomposite Electrodes. Sensors 2018, 18, 3126. [Google Scholar] [CrossRef] [Green Version]
- Geng, D.; Yang, S.; Zhang, Y.; Yang, J.; Liu, J.; Li, R.; Sham, T.-K.; Sun, X.; Ye, S.; Knights, S. Nitrogen doping effects on the structure of graphene. Appl. Surf. Sci. 2011, 257, 9193–9198. [Google Scholar] [CrossRef]
- Li, L.; Dou, Y.; Wang, L.; Luo, M.; Liang, J. One-step synthesis of high-quality N-doped graphene/Fe3O4 hybrid nanocomposite and its improved supercapacitor performances. RSC Adv. 2014, 4, 25658–25665. [Google Scholar] [CrossRef]
- Balaji, S.S.; Elavarasan, A.; Sathish, M. High performance supercapacitor using N-doped graphene prepared via supercritical fluid processing with an oxime nitrogen source. Appl. Surf. Sci. 2013, 266, 360–367. [Google Scholar] [CrossRef]
- Yadav, S.K.; Cho, J.W. Functionalized graphene nanoplatelets for enhanced mechanical and thermal properties of polyurethane nanocomposites. Cogent Eng. 2015, 2, 1094017. [Google Scholar] [CrossRef]
- Mani, V.; Chen, S.M.; Lou, B.S. Three dimensional graphene oxide-carbon nanotubes and graphene-carbon nanotubes hybrids. Int. J. Electrochem. Sci. 2013, 8, e60. [Google Scholar]
- Zhoua, N.; An, Q.; Xiaoa, Z.; Zhai, S.; Shi, Z. Solvothermal synthesis of three-dimensional, Fe2O3 NPs-embedded CNT/N-doped graphene composites with excellent microwave absorption performance. RSC Adv. 2017, 7, 45156–45169. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, M.; Kim, S.; Jeon, M. Synthesis of Three-Dimensional Carbon Nanostructure/Copper Nanowire for Additive Interface Layer of Ionic Polymer Metal Composite. Nanomaterials 2020, 10, 423. https://doi.org/10.3390/nano10030423
Park S, Park M, Kim S, Jeon M. Synthesis of Three-Dimensional Carbon Nanostructure/Copper Nanowire for Additive Interface Layer of Ionic Polymer Metal Composite. Nanomaterials. 2020; 10(3):423. https://doi.org/10.3390/nano10030423
Chicago/Turabian StylePark, Seongjun, Minjeong Park, Seonpil Kim, and Minhyon Jeon. 2020. "Synthesis of Three-Dimensional Carbon Nanostructure/Copper Nanowire for Additive Interface Layer of Ionic Polymer Metal Composite" Nanomaterials 10, no. 3: 423. https://doi.org/10.3390/nano10030423
APA StylePark, S., Park, M., Kim, S., & Jeon, M. (2020). Synthesis of Three-Dimensional Carbon Nanostructure/Copper Nanowire for Additive Interface Layer of Ionic Polymer Metal Composite. Nanomaterials, 10(3), 423. https://doi.org/10.3390/nano10030423




