Zinc Oxide Films with High Transparency and Crystallinity Prepared by a Low Temperature Spatial Atomic Layer Deposition Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results
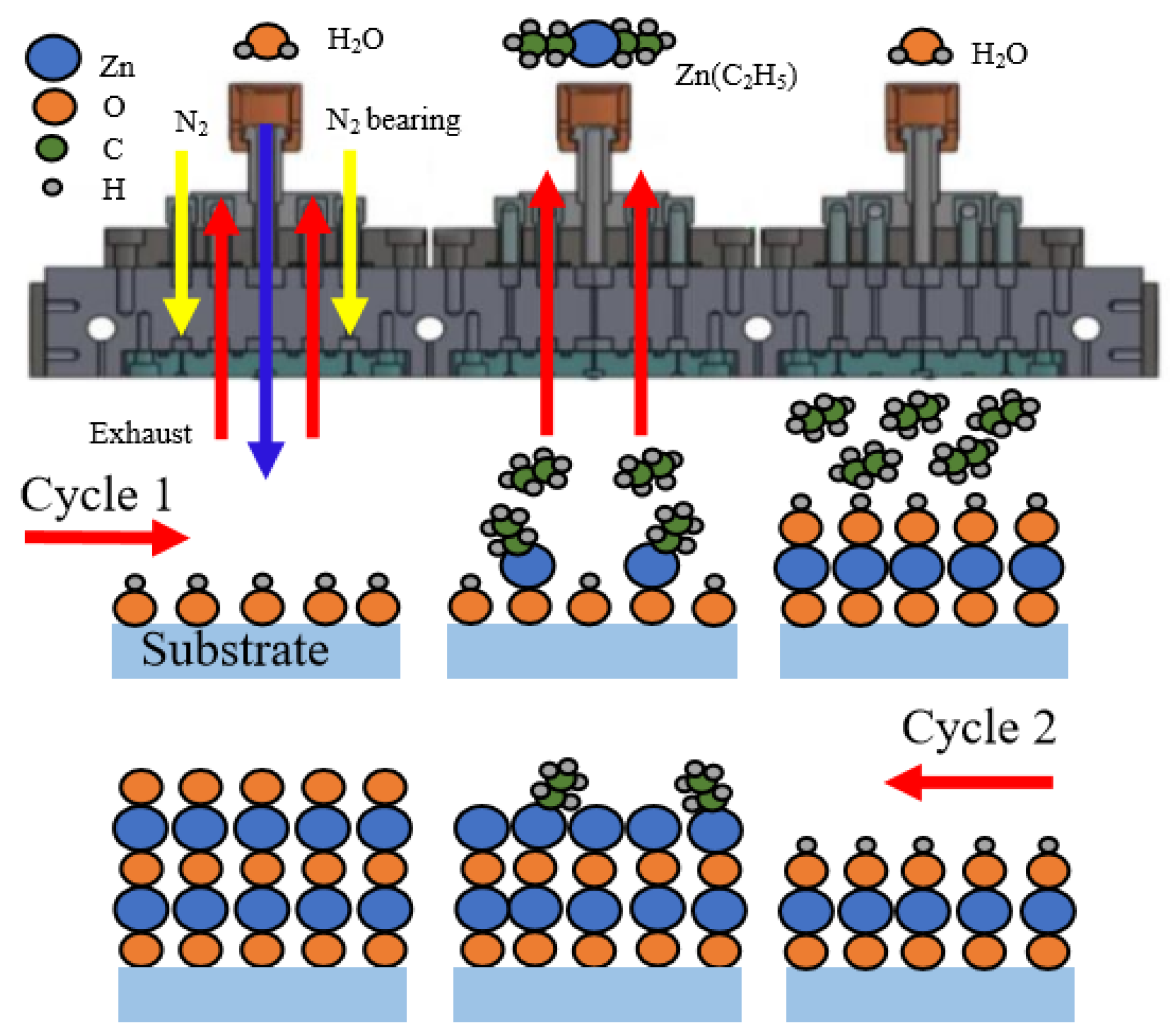
3.1. Deposition Mechanism of ZnO Films by sALD
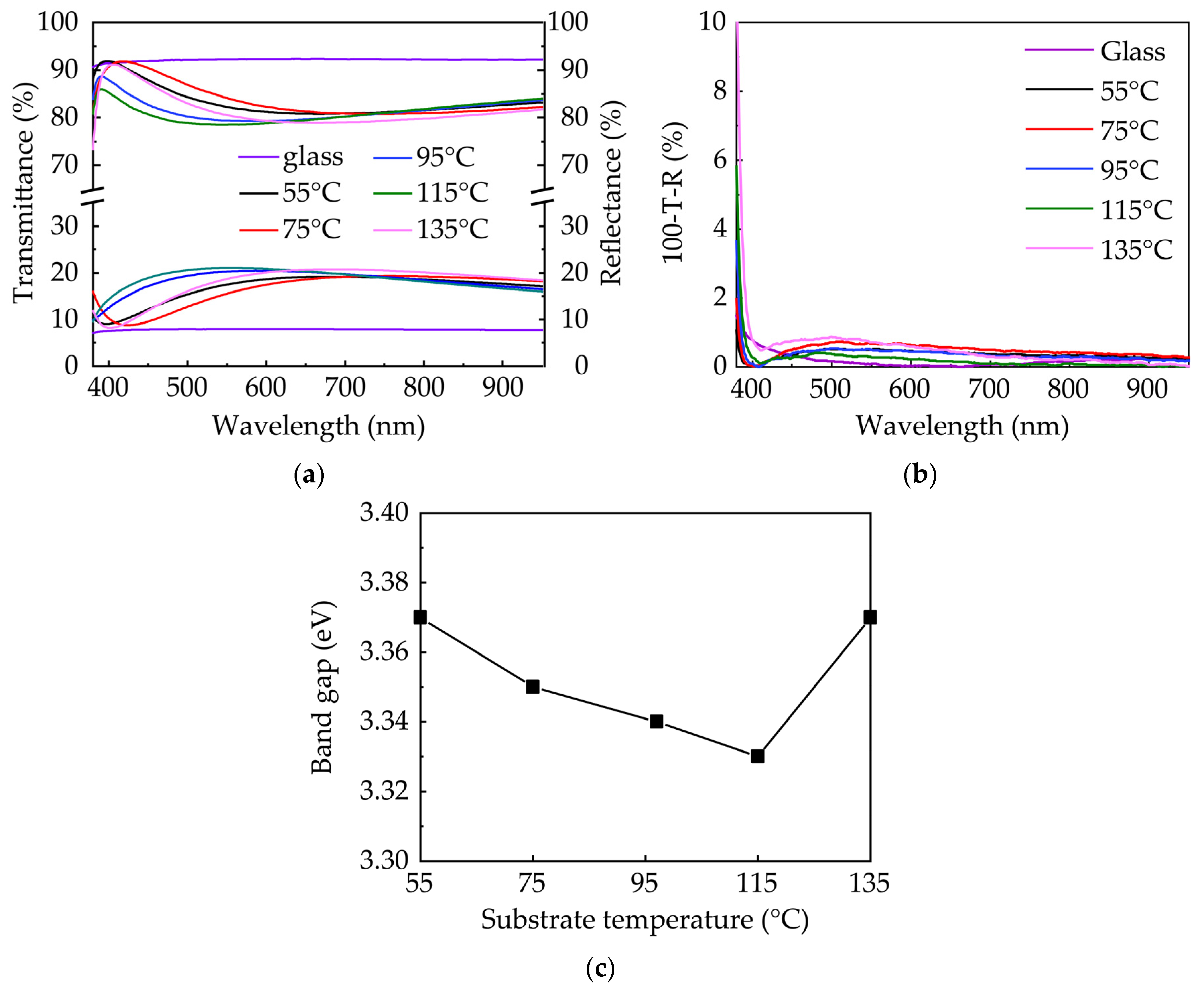
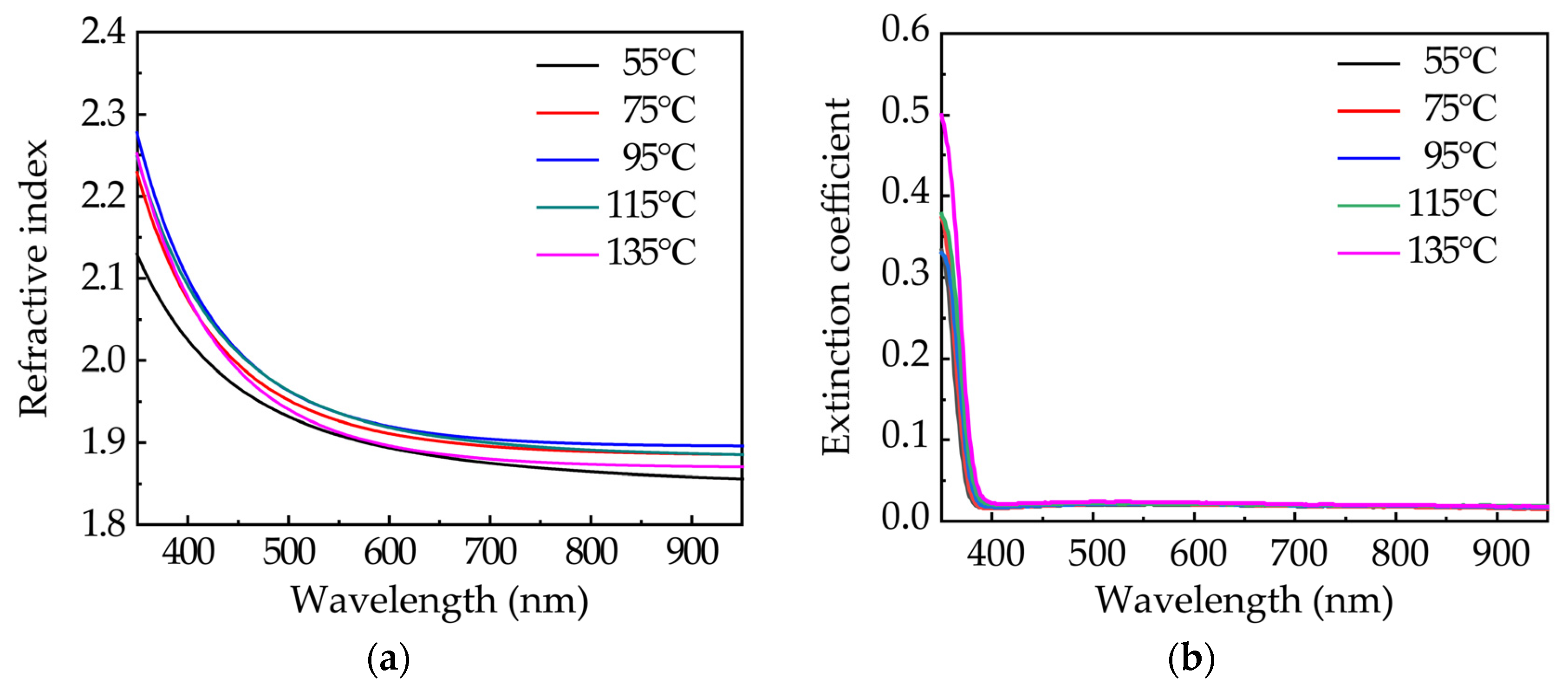
3.2. Optical Properties of ZnO Films
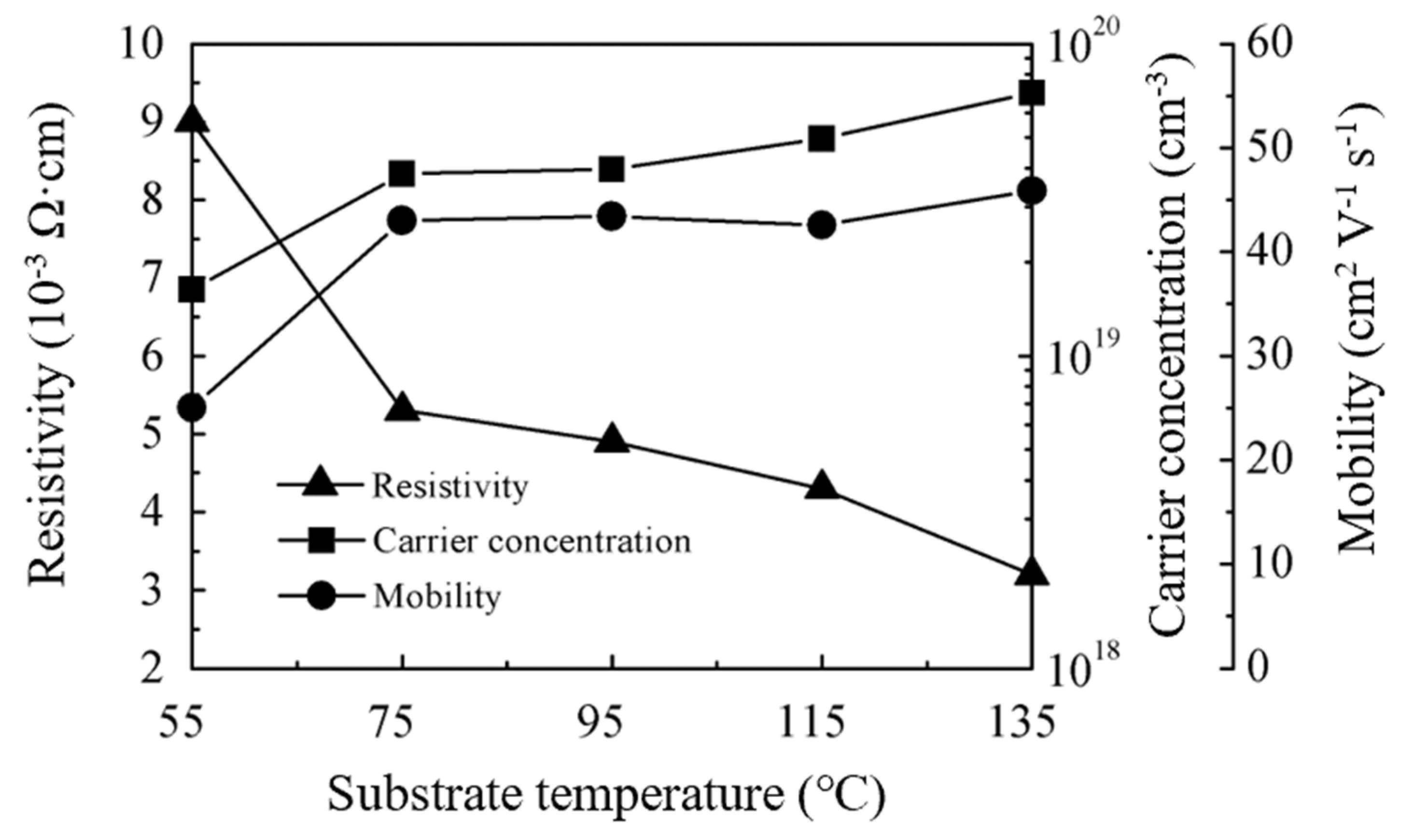
3.3. Electrical Properties of ZnO Films
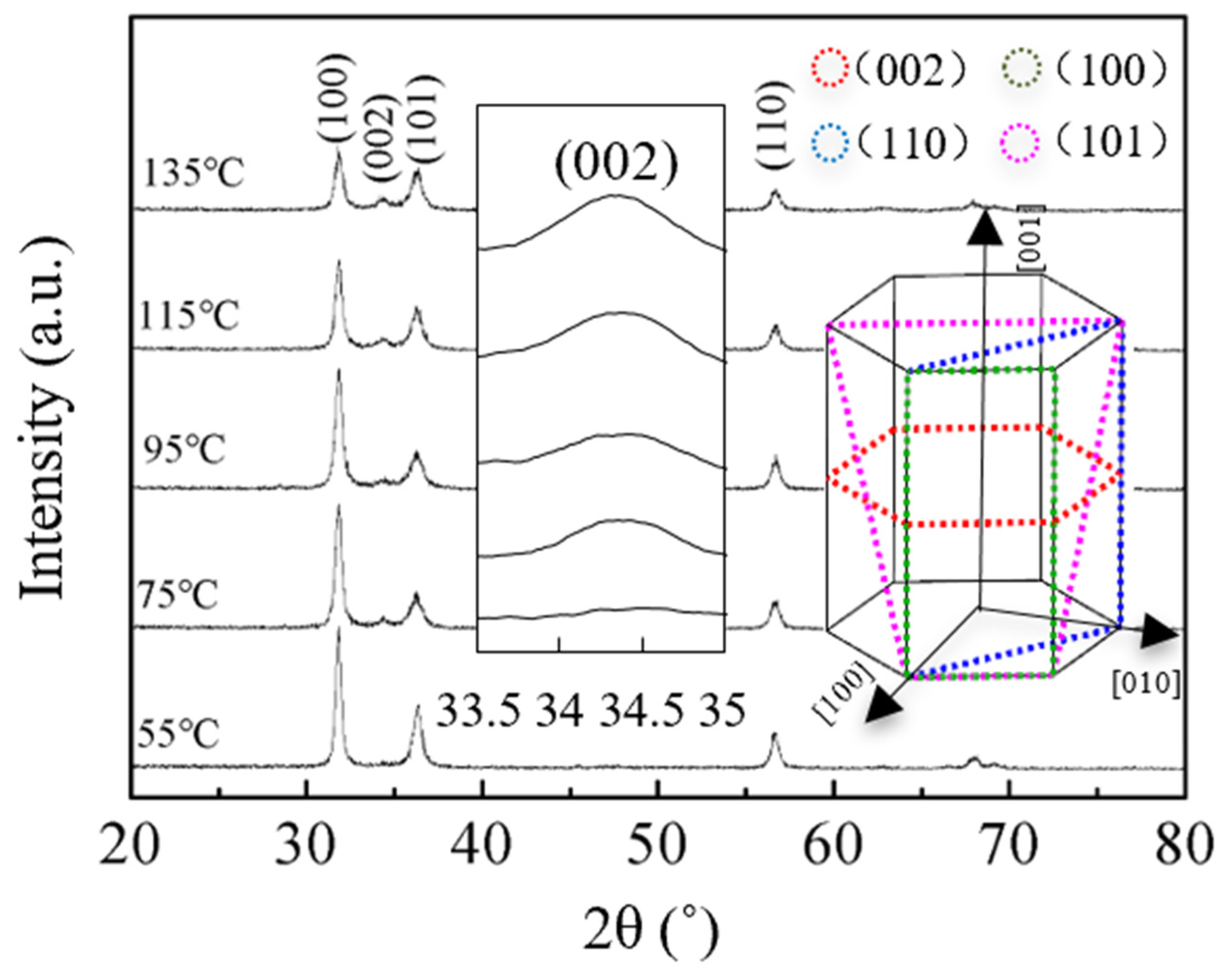
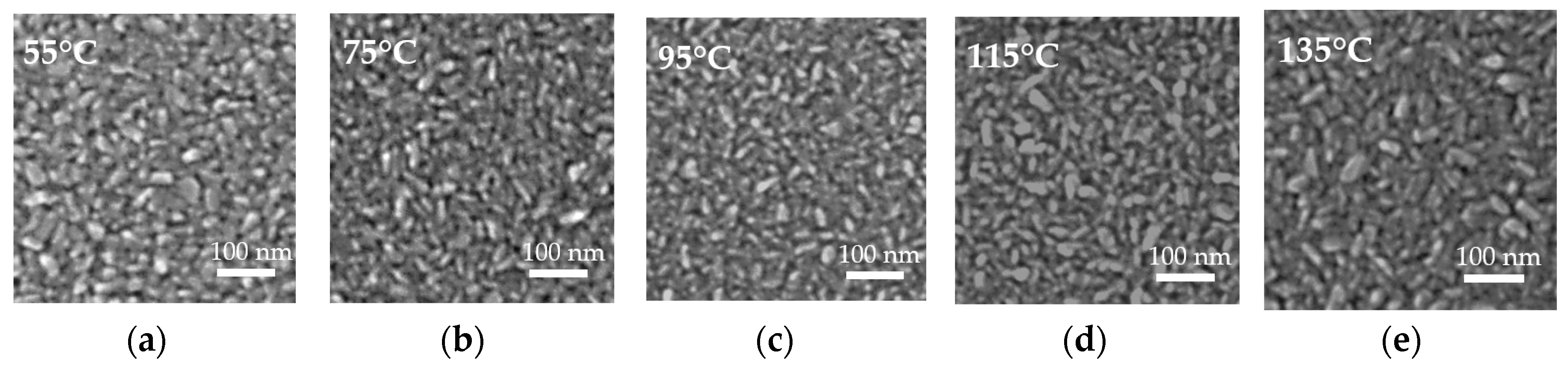
3.4. Structural and Morphological Study of ZnO Films
3.5. ZnO Film on PET Substrate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Ji, L.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite Solar Cells with ZnO Electron-Transporting Materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Liu, F.; Zhang, N.; Liu, K.; Wang, S.; Fang, G. Bidirectional electroluminescence from p -SnO2/i-MgZnO/n-ZnO heterojunction light-emitting diodes. J. Lumin. 2017, 186, 223–228. [Google Scholar] [CrossRef]
- Dong, J.; Han, D.; Li, H.; Yu, W.; Zhang, S.; Zhang, X.; Wang, Y. Effect of Al doping on performance of ZnO thin film transistors. Appl. Surf. Sci. 2018, 433, 836–839. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Zhang, Z.; Briscoe, J.; Warwick, M.E.A.; Li, H.; Hu, P. Aerosol assisted chemical vapour deposition of conformal ZnO compact layers for efficient electron transport in perovskite solar cells. Mater. Lett. 2018, 217, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Shewale, P.S.; Lee, S.H.; Yu, Y.S. UV sensitive pulsed laser deposited ZnO thin films: Influence of growth temperature. J. Alloys Compd. 2018, 744, 849–858. [Google Scholar] [CrossRef]
- Suja, M.; Bashar, S.B.; Morshed, M.M.; Liu, J. Realization of Cu-Doped p-Type ZnO Thin Films by Molecular Beam Epitaxy. ACS Appl. Mater. Interfaces 2015, 7, 8894–8899. [Google Scholar] [CrossRef]
- Bang, K.; Son, G.-C.; Son, M.; Jun, J.-H.; An, H.; Baik, K.H.; Myoung, J.-M.; Ham, M.-H. Effects of Li doping on the structural and electrical properties of solution-processed ZnO films for high-performance thin-film transistors. J. Alloys Compd. 2018, 739, 41–46. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, K.-M.; Kim, M.; Park, H.-H. Low temperature method to passivate oxygen vacancies in un-doped ZnO films using atomic layer deposition. Thin Solid Film. 2018, 660, 852–858. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Muñoz-Rojas, D.; Nelson, S.F.; Illiberi, A.; Poodt, P.; Roozeboom, F.; MacManus-Driscoll, J.L. Research Update: Atmospheric pressure spatial atomic layer deposition of ZnO thin films: Reactors, doping, and devices. APL Mater. 2015, 3, 040701. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Resende, J.; Jiménez, C.; Deschanvres, J.-L.; Carroy, P.; Muñoz, D.; Bellet, D.; Muñoz-Rojas, D. Deposition of ZnO based thin films by atmospheric pressure spatial atomic layer deposition for application in solar cells. J. Renew. Sustain. Energy 2017, 9, 021203. [Google Scholar] [CrossRef]
- Illiberi, A.; Scherpenborg, R.; Theelen, M.; Poodt, P.; Roozeboom, F. On the environmental stability of ZnO thin films by spatial atomic layer deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 31, 061504. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Routkevitch, D.; Varaksa, N.; George, S.M. Spatial atomic layer deposition on flexible porous substrates: ZnO on anodic aluminum oxide films and Al2O3 on Li ion battery electrodes. J. Vac. Sci. Technol. A 2016, 34, 01A146. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Hsu, C.-C.; Tseng, M.-H.; Shyue, J.-J.; Tsai, F.-Y. Stable and High-Performance Flexible ZnO Thin-Film Transistors by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2015, 7, 22610–22617. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, R.; Wang, H.; Wu, X.; Wu, J.; Wu, X.; Qin, W. Enhanced Performance in Al-Doped ZnO Based Transparent Flexible Transparent Thin-Film Transistors Due to Oxygen Vacancy in ZnO Film with Zn–Al–O Interfaces Fabricated by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 11711–11720. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Witkowski, B.S.; Wachnicki, L.; Jakiela, R.; Virt, I.S.; Andrzejczuk, M.; Lewandowska, M.; Godlewski, M. Electrical and mechanical stability of aluminum-doped ZnO films grown on flexible substrates by atomic layer deposition. Mater. Sci. Eng. B 2014, 186, 15–20. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Xiong, X.; Song, J.; Gu, C.; Zhan, D.; Hu, Q.; Li, S.; Wu, Y. High-Performance Flexible ZnO Thin-Film Transistors by Atomic Layer Deposition. IEEE Electron Device Lett. 2019, 40, 419–422. [Google Scholar] [CrossRef]
- Lee, W.-J.; Bera, S.; Song, P.K.; Lee, J.W.; Dai, W.; Kim, H.C.; Kim, C.S.; Kwon, S.-H. Optimization of bending durability of Ti-ZnO thin films on flexible glass substrates with highly enhanced optoelectronic characteristics by atomic layer deposition. Jpn. J. Appl. Phys. 2019, 58, 075501. [Google Scholar] [CrossRef]
- Kim, J.-M.; Nam, T.; Lim, S.J.; Seol, Y.G.; Lee, N.-E.; Kim, D.; Kim, H. Atomic layer deposition ZnO:N flexible thin film transistors and the effects of bending on device properties. Appl. Phys. Lett. 2011, 98, 142113. [Google Scholar] [CrossRef]
- Illiberi, A.; Roozeboom, F.; Poodt, P. Spatial Atomic Layer Deposition of Zinc Oxide Thin Films. ACS Appl. Mater. Interfaces 2012, 4, 268–272. [Google Scholar] [CrossRef]
- Nandakumar, N.; Dielissen, B.; Garcia-Alonso, D.; Liu, Z.; Gortzen, R.; Kessels, W.M.M.; Aberle, A.G.; Hoex, B. Resistive Intrinsic ZnO Films Deposited by Ultrafast Spatial ALD for PV Applications. IEEE J. Photovolt. 2015, 5, 1462–1469. [Google Scholar] [CrossRef]
- Cai, J.; Ma, Z.; Wejinya, U.; Zou, M.; Liu, Y.; Zhou, H.; Meng, X. A revisit to atomic layer deposition of zinc oxide using diethylzinc and water as precursors. J. Mater. Sci. 2019, 54, 5236–5248. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ko Park, S.H.; Jeong, H.Y.; Park, C.; Choi, S.Y.; Choi, J.Y.; Han, S.H.; Yoon, T.H. Orientations of Polycrystalline ZnO at the Buried Interface of Oxide Thin Film Transistors (TFTs): A Grazing Incidence X-ray Diffraction Study. Bull. Korean Chem. Soc. 2008, 29, 727–728. [Google Scholar]
- Simbrunner, J.; Hofer, S.; Schrode, B.; Garmshausen, Y.; Hecht, S.; Resel, R.; Salzmann, I. Indexing grazing-incidence X-ray diffraction patterns of thin films: Lattices of higher symmetry. J. Appl. Cryst. 2019, 52, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Tanner, B.K.; Hase, T.P.A.; Lafford, T.A.; Goorsky, M.S. Grazing incidence in-plane X-ray diffraction in the laboratory. Powder Diffr. 2004, 19, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Yousfi, E.B.; Fouache, J.; Lincot, D. Study of atomic layer epitaxy of zinc oxide by in-situ quartz crystal microgravimetry. Appl. Surf. Sci. 2000, 153, 223–234. [Google Scholar] [CrossRef]
- Nelson, S.F.; Levy, D.H.; Tutt, L.W.; Burberry, M. Cycle time effects on growth and transistor characteristics of spatial atomic layer deposition of zinc oxide. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 01A154. [Google Scholar] [CrossRef]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond. Sci. Technol. 2014, 29, 043001. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Yao, J.K.; Huang, H.L.; Ma, J.Y.; Jin, Y.X.; Zhao, Y.A.; Shao, J.D.; He, H.B.; Yi, K.; Fan, Z.X.; Zhang, F.; et al. High refractive index TiO2 film deposited by electron beam evaporation. Surf. Eng. 2009, 25, 257–260. [Google Scholar] [CrossRef]
- Lujala, V.; Skarp, J.; Tammenmaa, M.; Suntola, T. Atomic layer epitaxy growth of doped zinc oxide thin films from organometals. Appl. Surf. Sci. 1994, 82–83, 34–40. [Google Scholar] [CrossRef]
- Pung, S.Y.; Choy, K.L.; Hou, X. Growth of (002)-oriented ZnO thin films on largely lattice-mismatched substrates using atomic layer deposition. IJNT 2013, 10, 247. [Google Scholar] [CrossRef]
- Shaban, M.; Zayed, M.; Hamdy, H. Nanostructured ZnO thin films for self-cleaning applications. RSC Adv. 2017, 7, 617–631. [Google Scholar] [CrossRef]
- Gao, Z.; Banerjee, P. Review Article: Atomic layer deposition of doped ZnO films. J. Vac. Sci. Technol. A 2019, 37, 050802. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.A.; Cui, J.B. Highly Tunable Electrical Properties in Undoped ZnO Grown by Plasma Enhanced Thermal-Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2012, 4, 3122–3128. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Substrate temperature (°C) | 55–135 |
| Substrate holder move speed (cm/s) | 15 |
| Distance between spray and sub. (mm) | 0.3 |
| H2O carry gas flow rate (sccm) | 400 |
| H2O dilute gas flow rate (sccm) | 800 |
| DEZ carry gas flow rate (sccm) | 100 |
| DEZ dilute gas flow rate (sccm) | 3000 |
| Precursor concentration (cc/cm3) | 40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.-J.; Sun, Z.-T.; Hsu, C.-H.; Huang, P.-H.; Zhang, X.-Y.; Wu, W.-Y.; Gao, P.; Qiu, Y.; Lien, S.-Y.; Zhu, W.-Z. Zinc Oxide Films with High Transparency and Crystallinity Prepared by a Low Temperature Spatial Atomic Layer Deposition Process. Nanomaterials 2020, 10, 459. https://doi.org/10.3390/nano10030459
Zhao M-J, Sun Z-T, Hsu C-H, Huang P-H, Zhang X-Y, Wu W-Y, Gao P, Qiu Y, Lien S-Y, Zhu W-Z. Zinc Oxide Films with High Transparency and Crystallinity Prepared by a Low Temperature Spatial Atomic Layer Deposition Process. Nanomaterials. 2020; 10(3):459. https://doi.org/10.3390/nano10030459
Chicago/Turabian StyleZhao, Ming-Jie, Zhi-Tao Sun, Chia-Hsun Hsu, Pao-Hsun Huang, Xiao-Ying Zhang, Wan-Yu Wu, Peng Gao, Yu Qiu, Shui-Yang Lien, and Wen-Zhang Zhu. 2020. "Zinc Oxide Films with High Transparency and Crystallinity Prepared by a Low Temperature Spatial Atomic Layer Deposition Process" Nanomaterials 10, no. 3: 459. https://doi.org/10.3390/nano10030459
APA StyleZhao, M.-J., Sun, Z.-T., Hsu, C.-H., Huang, P.-H., Zhang, X.-Y., Wu, W.-Y., Gao, P., Qiu, Y., Lien, S.-Y., & Zhu, W.-Z. (2020). Zinc Oxide Films with High Transparency and Crystallinity Prepared by a Low Temperature Spatial Atomic Layer Deposition Process. Nanomaterials, 10(3), 459. https://doi.org/10.3390/nano10030459








