Green and Economic Fabrication of Zinc Oxide (ZnO) Nanorods as a Broadband UV Blocker and Antimicrobial Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. C. Vulgaris Culture Conditions
2.3. Synthesis of the ZnO Nanorods
2.4. Analytical Methods
2.5. Antimicrobial Activity
3. Results and Discussion
3.1. Characterisation of the ZnO Nanorods
3.1.1. FTIR Spectra Analysis
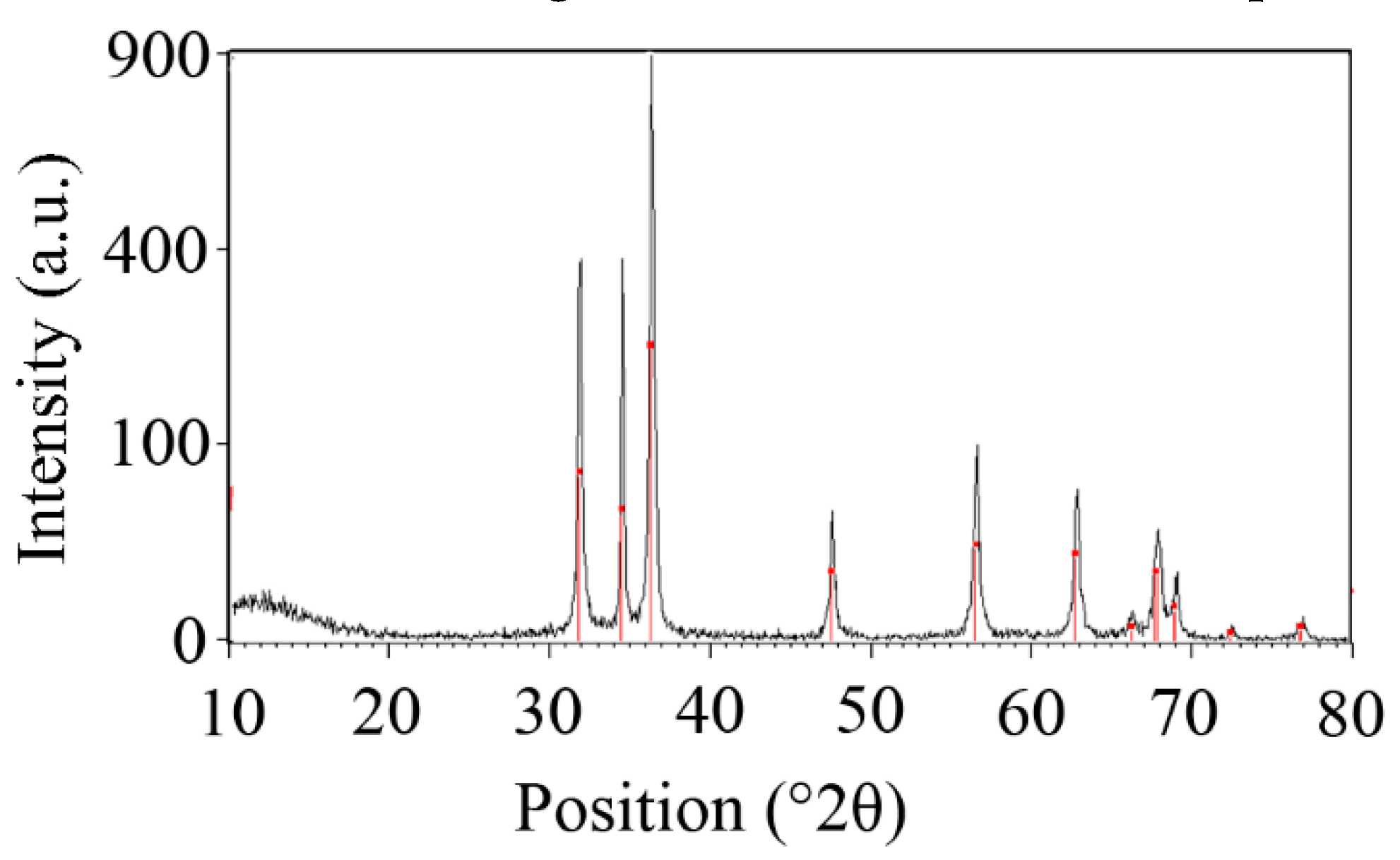
3.1.2. X-ray powder diffraction (XRD) Analysis
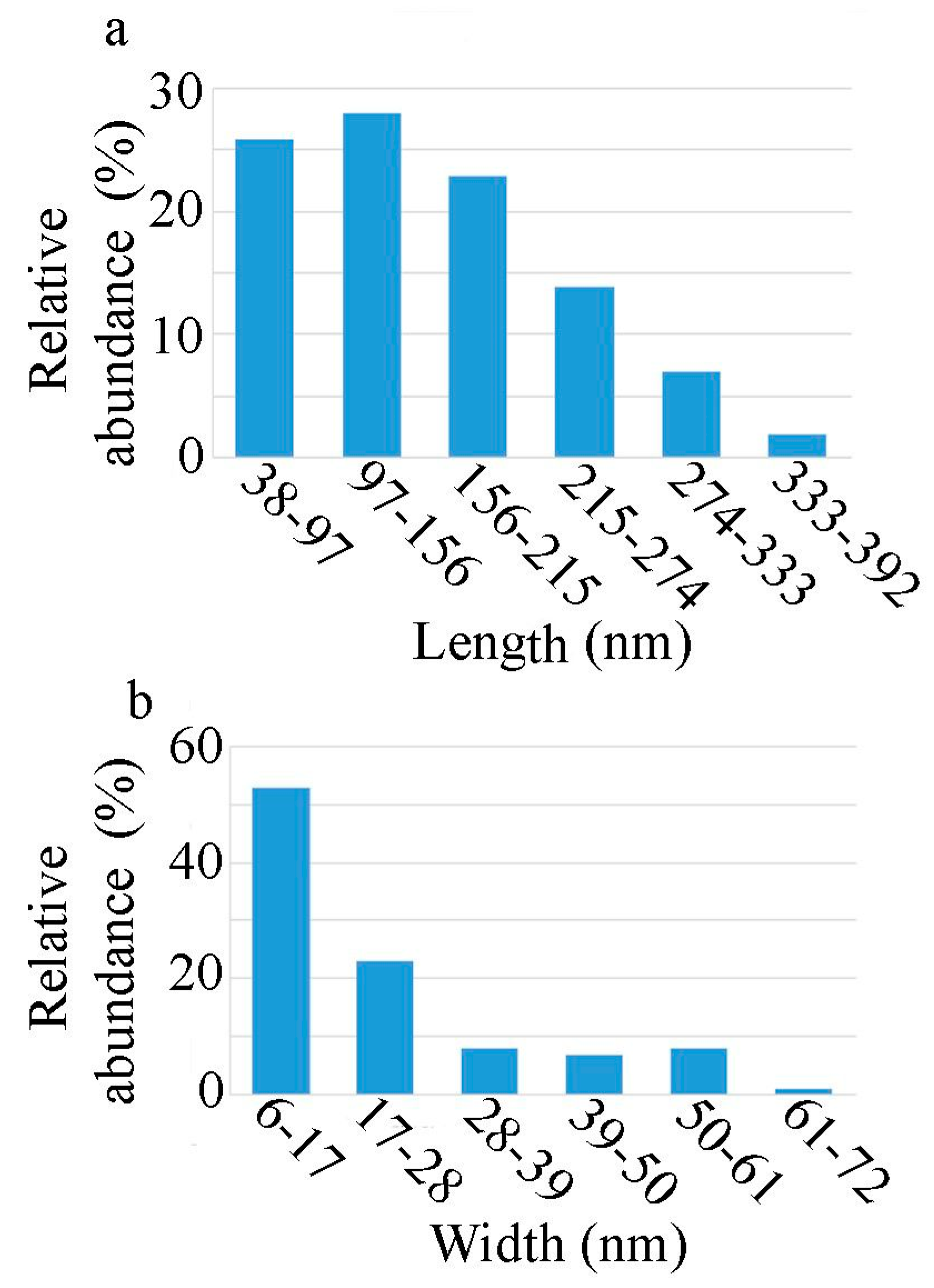
3.1.3. TEM Analysis
3.1.4. UV-Vis Spectra
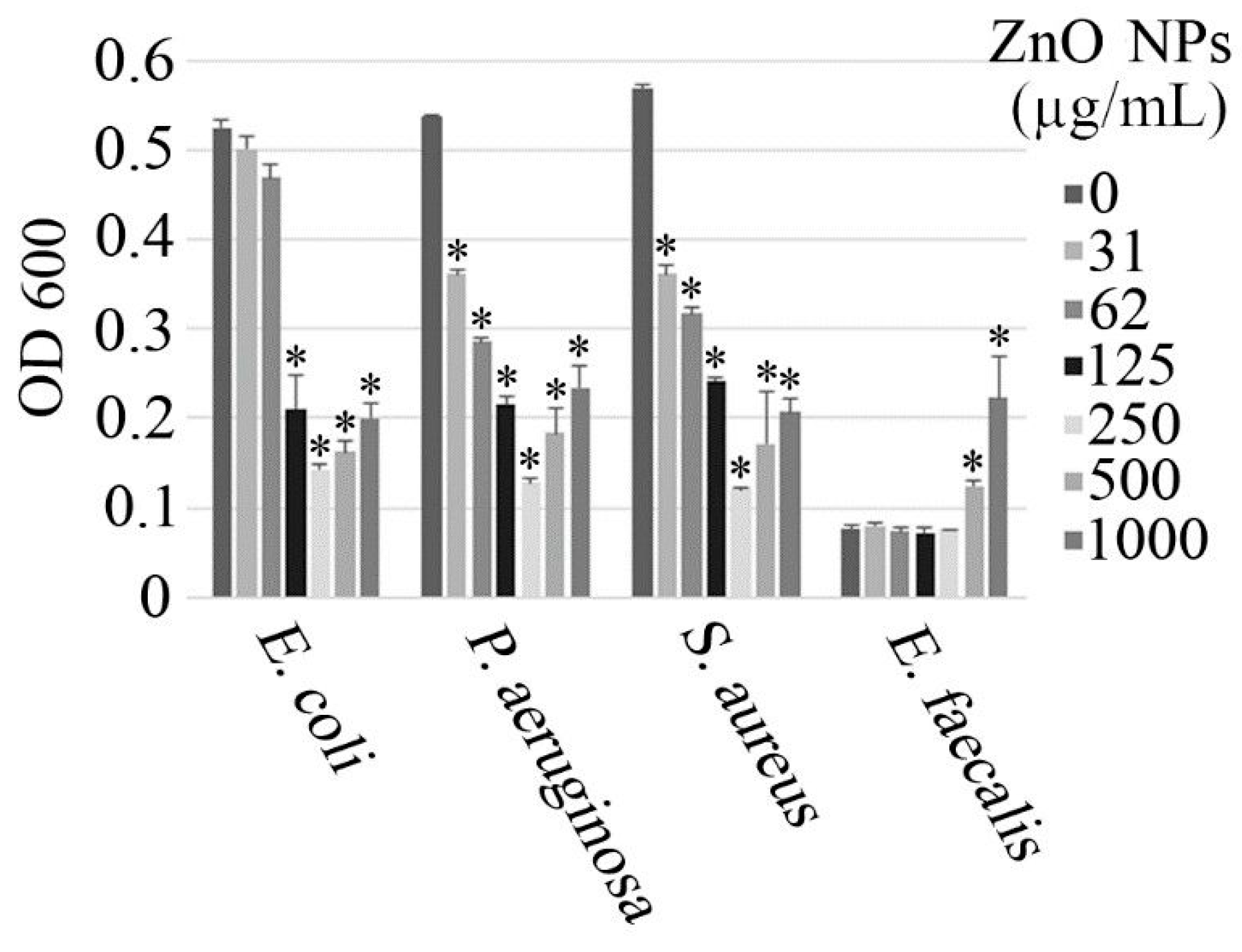
3.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles – An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Parra, M.R.; Haque, F.Z. Poly (Ethylene Glycol) (PEG)-assisted shape-controlled synthesis of one-dimensional ZnO nanorods. Optik 2015, 126, 1562–1566. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.; Ann, L.; Bakhori, S.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Moeeni, F.; Taghizadeh, S.-M.; Seifan, M.; Bautista, C.; Novin, D.; Ghasemi, Y.; Berenjian, A. Xanthan Gum Capped ZnO Microstars as a promising dietary zinc supplementation. Foods 2019, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Haque, F.Z.; Singh, N.; Pandey, P.; Parra, M.R. Study of Zinc Oxide nano/micro rods grown on ITO and glass substrates. Optik 2013, 124, 4167–4171. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lu, H.; Gong, L.; Li, L.; Zheng, J.; Li, H.; Zhu, Z. High sensitive and selective formaldehyde sensors based on nanoparticle-assembled ZnO micro-octahedrons synthesized by homogeneous precipitation method. Sens. Actuators B 2011, 160, 364–370. [Google Scholar] [CrossRef]
- Adhyapak, P.V.; Meshram, S.P.; Amalnerkar, D.P.; Mulla, I.S. Structurally enhanced photocatalytic activity of flower-like ZnO synthesized by PEG-assited hydrothermal route. Ceram. Int. 2014, 40, 1951–1959. [Google Scholar] [CrossRef]
- Breedon, M.; Rahmani, M.B.; Keshmiri, S.-H.; Wlodarski, W.; Kalantar-Zadeh, K. Aqueous synthesis of interconnected ZnO nanowires using spray pyrolysis deposited seed layers. Mater. Lett. 2010, 64, 291–294. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Tavakolian, M. Preparation, characterization, and catalysis application of nano-rods zinc oxide in the synthesis of 3-indolyl-3-hydroxy oxindoles in water. Appl. Catal. A 2012, 441–442, 65–71. [Google Scholar] [CrossRef]
- Chen, Y.W.; Liu, Y.C.; Lu, S.X.; Xu, C.S.; Shao, C.L.; Wang, C.; Zhang, J.Y.; Lu, Y.M.; Shen, D.Z.; Fan, X.W. Optical properties of ZnO and ZnO: In nanorods assembled by sol-gel method. J. Chem. Phys. 2005, 123. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ji, Y.L.; Xu, H.; Simon, P.; Wu, Z. Regularly shaped, single-crystalline ZnO nanorods with wurtzite structure. J. Am. Chem. Soc. 2002, 124, 14864–14865. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Mao, S.; Feick, H. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.-L.; Zhu, Y.-J.; Wang, S.-W. Sonochemical and microwave-assisted synthesis of linked single-crystalline ZnO rods. Mater. Chem. Phys. 2004, 88, 421–426. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829. [Google Scholar] [CrossRef]
- Cao, B.; Cai, W.; Li, Y.; Sun, F.; Zhang, L. Ultraviolet-light-emitting ZnO nanosheets prepared by a chemical bath deposition method. Nanotechnology 2005, 16, 1734. [Google Scholar] [CrossRef]
- Fang, X.-S.; Ye, C.-H.; Zhang, L.-D.; Li, Y.; Xiao, Z.-D. Formation and optical properties of thin and wide tin-doped ZnO nanobelts. Chem. Lett. 2005, 34, 436–437. [Google Scholar] [CrossRef]
- Pan, Z.W.; Wang, P. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Srinivasan, S.; He, G.N.; Kang, J.Y.; Wu, S.T.; Ponce, F.A. Synthesis and luminescence properties of ZnO nanostructures produced by the sol–gel method. J. Cryst. Growth 2008, 310, 599–603. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Xiao, G.; Liu, H.; Wang, M. Synthesis and morphology control of ZnO nanostructures in microemulsions. J. Colloid Interface Sci. 2009, 333, 465–473. [Google Scholar] [CrossRef]
- Ao, W.; Li, J.; Yang, H.; Zeng, X.; Ma, X. Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technol. 2006, 168, 148–151. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef] [PubMed]
- Duraimurugan, J.; Kumar, G.S.; Venkatesh, M.; Maadeswaran, P.; Girija, E. Morphology and size controlled synthesis of zinc oxide nanostructures and their optical properties. J. Mater. Sci. Mater. Electron. 2018, 29, 9339–9346. [Google Scholar] [CrossRef]
- Yu, W.; Li, X.; Gao, X. Catalytic synthesis and structural characteristics of high-quality tetrapod-like ZnO nanocrystals by a modified vapor transport process. Cryst. Growth Des. 2005, 5, 151–155. [Google Scholar] [CrossRef]
- Viswanathan, R.; Lilly, G.D.; Gale, W.F.; Gupta, R.B. Formation of zinc oxide−Titanium dioxide composite nanoparticles in supercritical water. Ind. Eng. Chem. Res. 2003, 42, 5535–5540. [Google Scholar] [CrossRef]
- Rataboul, F.; Nayral, C.; Casanove, M.-J.; Maisonnat, A.; Chaudret, B. Synthesis and characterization of monodisperse zinc and zinc oxide nanoparticles from the organometallic precursor [Zn (C6H11) 2]. J. Organomet. Chem. 2002, 643, 307–312. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mazaheri, M. Preparation of ZnO nanoparticles from [bis(acetylacetonato)zinc(II)]–oleylamine complex by thermal decomposition. Mater. Lett. 2008, 62, 1890–1892. [Google Scholar] [CrossRef]
- Sato, T.; Tanigaki, T.; Suzuki, H.; Saito, Y.; Kido, O.; Kimura, Y.; Kaito, C.; Takeda, A.; Kaneko, S. Structure and optical spectrum of ZnO nanoparticles produced in RF plasma. J. Cryst. Growth 2003, 255, 313–316. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Bi, S.; Luo, G. Preparation of ZnO nanoparticles using the direct precipitation method in a membrane dispersion micro-structured reactor. Powder Technol. 2010, 202, 130–136. [Google Scholar] [CrossRef]
- Koh, Y.W.; Lin, M.; Tan, C.K.; Foo, Y.L.; Loh, K.P. Self-assembly and selected area growth of zinc oxide nanorods on any surface promoted by an aluminum precoat. J. Phys. Chem. B 2004, 108, 11419–11425. [Google Scholar] [CrossRef]
- Lee, W.; Leem, J.-Y. Size control of ZnO nanorods using the hydrothermal method in conjunction with substrate rotation. J. Nanosci. Nanotechnol. 2017, 17, 7952–7956. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Moeini, F. Nano copper (I) oxide/zinc oxide catalyzed N-arylation of nitrogen-containing heterocycles with aryl halides and arylboronic acids in air. RSC Adv. 2014, 4, 7321–7329. [Google Scholar] [CrossRef]
- Medina, J.; Bolaños, H.; Mosquera-Sanchez, L.P.; Rodriguez-Paez, J. Controlled synthesis of ZnO nanoparticles and evaluation of their toxicity in Mus musculus mice. Int. Nano Lett. 2018, 8, 165–179. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhang, M.; Guo, M.; Wang, X. Studies on the PEG-assisted hydrothermal synthesis and growth mechanism of ZnO microrod and mesoporous microsphere arrays on the substrate. Cryst. Growth Des. 2010, 10, 1500–1507. [Google Scholar] [CrossRef]
- Lin, S.-T.; Thirumavalavan, M.; Lee, J.-F. A comprehensive study on the mechanism for controlled synthesis of ZnO-based nanomaterials via various polysaccharides as chelates. Results Phys. 2018, 9, 1596–1601. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Bagheri, M.; Taghizadeh, S.-M.; Berenjian, A.; Ghasemi, Y. Biomimetic synthesis of silver nanoparticles using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015018. [Google Scholar] [CrossRef]
- Ghanbariasad, A.; Taghizadeh, S.-M.; Show, P.L.; Nomanbhay, S.; Berenjian, A.; Ghasemi, Y.; Ebrahiminezhad, A. Controlled synthesis of iron oxyhydroxide (FeOOH) nanoparticles using secretory compounds from Chlorella vulgaris microalgae. Bioengineered 2019, 10, 390–396. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Tengh Edition. CLSI Document Mo7-A10; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 35, ISBN 1-56238-988-2. (Electronic). [Google Scholar]
- Ambika, S.; Sundrarajan, M. Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J. Photochem. Photobiol. B 2015, 146, 52–57. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A 2015, 136, 864–870. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Rajkuberan, C.; Manikandan, K.; Prabukumar, S.; Danieljohn, J.; Sivaramakrishnan, S. Facile biosynthesis of antimicrobial zinc oxide (ZnO) nanoflakes using leaf extract of Couroupita guianensis Aubl. Mater. Lett. 2017, 188, 383–386. [Google Scholar] [CrossRef]
- Xie, J.; Lee, J.Y.; Wang, D.I.; Ting, Y.P. Silver nanoplates: From biological to biomimetic synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Su, K.; Zhang, R.; Han, H.; Deng, Y.; Luo, S. Green synthesis of ZnO nano particles using chlorella vulgaris extract as additives. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kunming, China, 25–29 May 2019; p. 012005. [Google Scholar]
- Sathiyanarayanan, G.; Vignesh, V.; Saibaba, G.; Vinothkanna, A.; Dineshkumar, K.; Viswanathana, M.B.; Selvin, J. Synthesis of carbohydrate polymer encrusted gold nanoparticles using bacterial exopolysaccharide: A novel and greener approach. RSC Adv. 2014, 4, 22817–22827. [Google Scholar] [CrossRef]
- Sepulveda-Guzman, S.; Reeja-Jayan, B.; de La Rosa, E.; Torres-Castro, A.; Gonzalez-Gonzalez, V.; Jose-Yacaman, M. Synthesis of assembled ZnO structures by precipitation method in aqueous media. Mater. Chem. Phys. 2009, 115, 172–178. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Banoee, M.; Seif, S.; Nazari, Z.E.; Jafari-Fesharaki, P.; Shahverdi, H.R.; Moballegh, A.; Moghaddam, K.M.; Shahverdi, A.R. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J. Biomed. Mater. Res. Part B 2010, 93, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr. J. Microbiol. Res. 2011, 5, 1368–1373. [Google Scholar]
- Narayanan, P.; Wilson, W.S.; Abraham, A.T.; Sevanan, M. Synthesis, characterization, and antimicrobial activity of zinc oxide nanoparticles against human pathogens. Bionanosci. 2012, 2, 329–335. [Google Scholar] [CrossRef]
- Siddiqi, K.; Ur Rahman, A.; Tajuddin, A.; Husen, A. properties of Zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Iram, S.; Khan, J.; Aman, N.; Nadhman, A.; Zulfiqar, Z.; Yameen, M. Enhancing the anti-enterococci activity of different antibiotics by combining with metal oxide nanoparticles. Jundishapur J. Microbiol. 2016, 9, 1I. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; He, Y.; Irwin, P.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of Zinc oxide nanoparticles against campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghizadeh, S.-M.; Lal, N.; Ebrahiminezhad, A.; Moeini, F.; Seifan, M.; Ghasemi, Y.; Berenjian, A. Green and Economic Fabrication of Zinc Oxide (ZnO) Nanorods as a Broadband UV Blocker and Antimicrobial Agent. Nanomaterials 2020, 10, 530. https://doi.org/10.3390/nano10030530
Taghizadeh S-M, Lal N, Ebrahiminezhad A, Moeini F, Seifan M, Ghasemi Y, Berenjian A. Green and Economic Fabrication of Zinc Oxide (ZnO) Nanorods as a Broadband UV Blocker and Antimicrobial Agent. Nanomaterials. 2020; 10(3):530. https://doi.org/10.3390/nano10030530
Chicago/Turabian StyleTaghizadeh, Seyedeh-Masoumeh, Neha Lal, Alireza Ebrahiminezhad, Fatemeh Moeini, Mostafa Seifan, Younes Ghasemi, and Aydin Berenjian. 2020. "Green and Economic Fabrication of Zinc Oxide (ZnO) Nanorods as a Broadband UV Blocker and Antimicrobial Agent" Nanomaterials 10, no. 3: 530. https://doi.org/10.3390/nano10030530



