Construction of Time-Resolved Luminescence Nanoprobe and Its Application in As(III) Detection
Abstract
:1. Introduction
2. Results
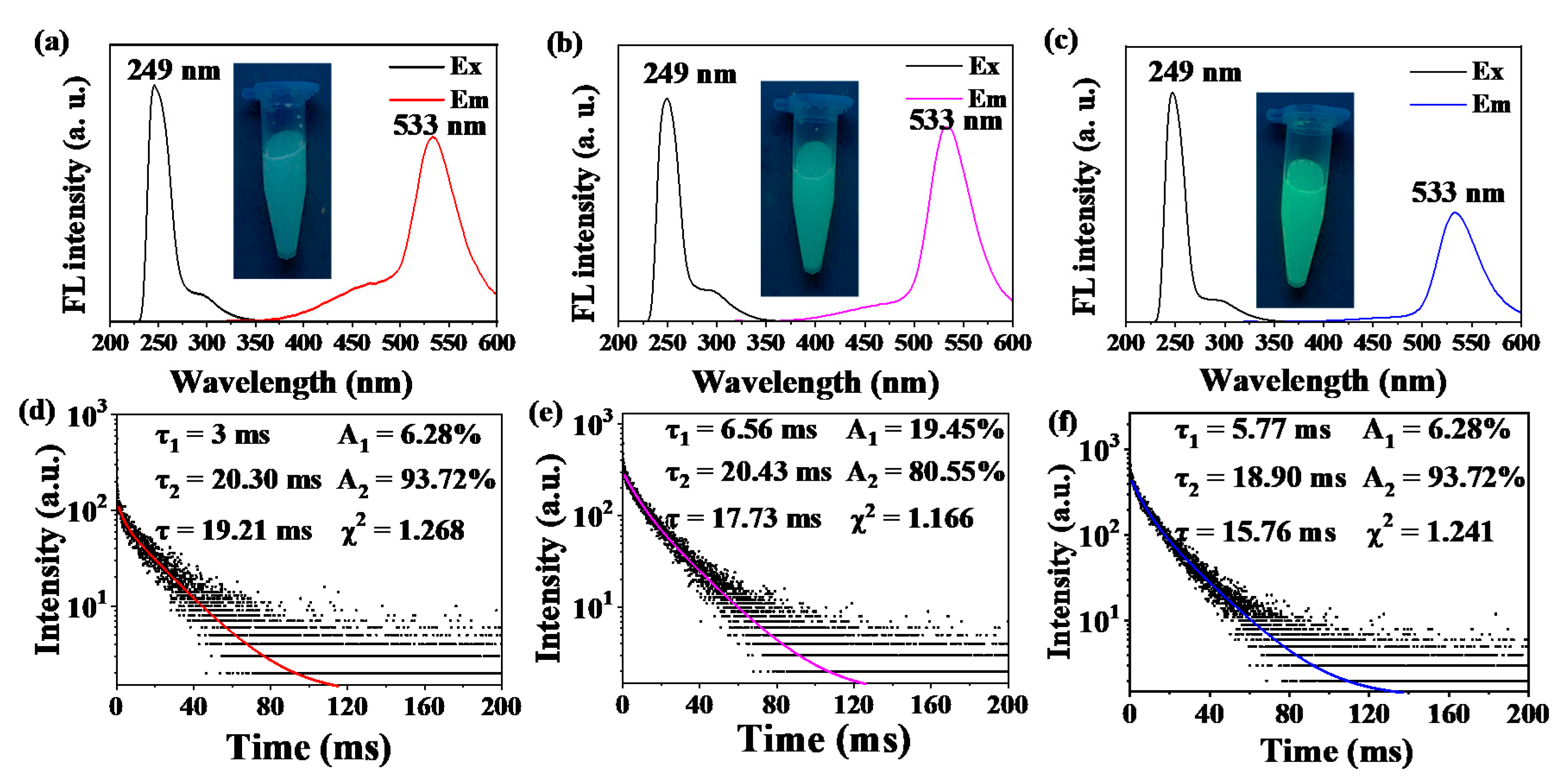
2.1. Characterization of the ZGO: Mn
2.2. Fabrication of the As(III) Probe
2.3. Optimization of Experimental Conditions
2.4. Sensitivity and Selectivity of As(III) Detection
2.5. Testing of Actual Samples
3. Experimental
3.1. Materials and Instruments
3.2. Preparation of the Zn2GeO4: Mn (ZGO: Mn)
3.3. Surface Modification of the ZGO: Mn
3.4. Detection of As(III) Standard Solution
3.5. Preparation of Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, B.; Chen, X.; Liu, R.; Liu, B.; Jiang, C. Target induced aggregation of modified Au@Ag nanoparticles for surface enhanced Raman scattering and its ultrasensitive detection of arsenic(III) in aqueous solution. RSC Adv. 2015, 5, 77755–77759. [Google Scholar] [CrossRef]
- Yong-Guan, Z.; Williams, P.N.; Meharg, A.A. Exposure to inorganic arsenic from rice: A global health issue. Environ. Pollut. 2008, 154, 169–171. [Google Scholar] [CrossRef]
- Yogarajah, N.; Tsai, S.S.H. Detection of trace arsenic in drinking water: Challenges and opportunities for microfluidics. Environ. Sci. Wat. Res. Technol. 2015, 1, 426–447. [Google Scholar] [CrossRef]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef]
- Sloth, J.J.; Julshamn, K. Survey of total and inorganic arsenic content in blue mussels (Mytilus edulis L.) from Norwegian fiords: Revelation of unusual high levels of inorganic arsenic. J. Agric. Food. Chem. 2008, 56, 1269–1273. [Google Scholar] [CrossRef]
- Whaley-Martin, K.J.; Koch, I.; Moriarty, M.; Reimer, K.J. Arsenic speciation in blue mussels (mytilus edulis) along a highly contaminated arsenic gradient. Environ. Sci. Technol. 2012, 46, 3110. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef] [Green Version]
- Osborne, S.E.; Matsumura, I.; Ellington, A.D. Aptamers as therapeutic and diagnostic reagents: Problems and prospects. Curr. Opin. Chem. Biol. 1997, 1, 5–9. [Google Scholar] [CrossRef]
- Shaoliang, D.; Shijia, W.; Nuo, D.; Zhouping, W. A luminescence resonance energy transfer based aptasensor for the mycotoxin Ochratoxin A using upconversion nanoparticles and gold nanorods. Microchim. Acta 2016, 183, 1909–1916. [Google Scholar] [CrossRef]
- Hao, L.; Gu, H.; Duan, N.; Wu, S.; Ma, X.; Xia, Y.; Tao, Z.; Wang, Z. An enhanced chemiluminescence resonance energy transfer aptasensor based on rolling circle amplification and WS2 nanosheet for Staphylococcus aureus detection. Anal. Chim. Acta 2017, 959, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Agrawal, B.; Chandra, P.; Goyal, R.N. In vitro chloramphenicol detection in a Haemophilus influenza model using an aptamer-polymer based electrochemical biosensor. Biosens. Bioelectron. 2014, 55, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Shijia, W.; Nuo, D.; Zhao, S.; Congcong, F.; Zhouping, W. Dual fluorescence resonance energy transfer assay between tunable upconversion nanoparticles and controlled gold nanoparticles for the simultaneous detection of Pb2+ and Hg2+. Talanta 2014, 128, 327–336. [Google Scholar]
- Jiang, Z.; Fan, Y.; Liang, A.; Wen, G.; Liu, Q.; Li, T. Resonance scattering spectral detection of trace Pb2+ using aptamer-modified aupd nanoalloy as probe. Plasmonics 2010, 5, 375–381. [Google Scholar] [CrossRef]
- Duan, N.; Gong, W.; Wu, S.; Wang, Z. An ssDNA library immobilized SELEX technique for selection of an aptamer against ractopamine. Anal. Chim. Acta 2017, 961, 100–105. [Google Scholar] [CrossRef]
- Xia, Y.; Ou, H.; Li, W.; Han, G.; Li, Z. Efficient blue to red afterglow tuning in a binary nanocomposite plastic film. Nanomaterials 2018, 8, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Hu, X.; Wang, J.; Liu, M.; Wei, W.; Yuan, Q. Direct low-temperature synthesis of ultralong persistent luminescence nanobelts based on a biphasic solution-chemical reaction. Chin. Chem. Lett. 2018, 29, 1641–1644. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wu, X.; Huang, L.; Li, D.; Fan, W.; Han, G. Direct aqueous-phase synthesis of sub-10 nm “luminous pearls” with enhanced in vivo renewable near-infrared persistent luminescence. J. Am. Chem. Soc. 2015, 137, 5304–5307. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, Q.; Zheng, W.; Liu, H.; Yin, C.; Wang, F.; Chen, X.; Yuan, Q.; Tan, W. One-dimensional luminous nanorods featuring tunable persistent luminescence for autofluorescence-free biosensing. ACS Nano 2017, 11, 8185–8191. [Google Scholar] [CrossRef]
- Cong, Y.; He, Y.; Dong, B.; Xiao, Y.; Wang, L. Long afterglow properties of Zn2GeO4:Mn2+, Cr3+ phosphor. Opt. Mater. 2015, 42, 506–510. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Wang, Z.; Cao, H.; Zhang, K.; Li, X.; Yang, Z. Nanomaterial-based aptamer sensors for arsenic detection. Biosens. Bioelectron. 2019, 148, 111785. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. DNA adsorption by magnetic iron oxide nanoparticles and its application for arsenate detection. Chem. Commun. 2014, 50, 8568–8570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oroval, M.; Coll, C.; Bernardos, A.; Marcos, M.D.; Martinez-Manez, R.; Shchukin, D.G.; Sancenon, F. Selective fluorogenic sensing of As(III) using aptamer-capped nanomaterials. ACS Appl. Mater. Interfaces 2017, 9, 11332–11336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.K.; Akhtar, N.; Ghosh, S.K. Determination of arsenic in water using fluorescent ZnO quantum dots. Anal. Methods 2016, 8, 445–452. [Google Scholar] [CrossRef]
- Ravikumar, A.; Panneerselvam, P.; Radhakrishnan, K.; Christus, A.A.B.; Sivanesan, S. MoS2 nanosheets as an effective fluorescent quencher for successive detection of arsenic ions in aqueous system. Appl. Surf. Sci. 2018, 449, 31–38. [Google Scholar] [CrossRef]
- Divsar, F.; Habibzadeh, K.; Shariati, S.; Shahriarinour, M. Aptamer conjugated silver nanoparticles for the colorimetric detection of arsenic ions using response surface methodology. Anal. Methods 2015, 7, 4568–4576. [Google Scholar] [CrossRef]
- Nguyen, N.L.T.; Park, C.Y.; Park, J.P.; Kailasa, S.K.; Park, T.J. Synergistic molecular assembly of an aptamer and surfactant on gold nanoparticles for the colorimetric detection of trace levels of As3+ ions in real samples. New J. Chem. 2018, 42, 11530–11538. [Google Scholar] [CrossRef]
- Tang, M.L.; Wen, G.Q.; Liang, A.H.; Jiang, Z.L. A simple and sensitive resonance Rayleigh scattering method for determination of As(III) using aptamer-modified nanogold as a probe. Luminescence 2014, 29, 603–608. [Google Scholar] [CrossRef]
- Lin, S.; Wang, W.; Hu, C.; Yang, G.; Ko, C.-N.; Ren, K.; Leung, C.-H.; Ma, D.-L. The application of a G-quadruplex based assay with an iridium(III) complex to arsenic ion detection and its utilization in a microfluidic chip. J. Mat. Chem. B 2017, 5, 479–484. [Google Scholar] [CrossRef]
- Vega-Figueroa, K.; Santillan, J.; Ortiz-Gomez, V.; Ortiz-Quiles, E.O.; Quinones-Colon, B.A.; Castilla-Casadiego, D.A.; Almodovar, J.; Bayro, M.J.; Rodriguez-Martinez, J.A.; Nicolau, E. Aptamer-based impedimetric assay of arsenite in water: Interfacial properties and performance. ACS Omega 2018, 3, 1437–1444. [Google Scholar] [CrossRef]
- Tan, M.Q.; Song, B.; Wang, G.L.; Yuan, J.L. A new terbium(III) chelate as an efficient singlet oxygen fluorescence probe. Free Radical Biol. Med. 2006, 40, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Q.; Ye, Z.Q.; Wang, G.L.; Yuan, J.L. Preparation and time-resolved fluorometric application of luminescent europium nanoparticles. Chem. Mater. 2004, 16, 2494–2498. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.-T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274–9276. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Lai, Z.; Zhong, L.; Zhang, Z.; Zheng, R.; Su, J.; Huang, Y.; Huang, P.; Song, H.; Yang, N.; et al. A Graphene oxide-based fluorescent aptasensor for the turn-on detection of ccrf-cem. Nanoscale Res. Lett. 2018, 13, 66. [Google Scholar]
- Tan, M.Q.; Wang, G.L.; Hai, X.D.; Ye, Z.Q.; Yuan, J.L. Development of functionalized fluorescent europium nanoparticles for biolabeling and time-resolved fluorometric applications. J. Mater. Chem. 2004, 14, 2896–2901. [Google Scholar] [CrossRef]
- Wang, X.L.; Huang, Y.K.; Wu, S.J.; Duan, N.; Xu, B.C.; Wang, Z.P. Simultaneous detection of staphylococcus aureus and salmonella typhimurium using multicolor time-resolved fluorescence nanoparticles as labels. Int. J. Food Microbiol. 2016, 237, 172–179. [Google Scholar] [CrossRef]






| Signal | Nanomaterial | LOD (ng·mL−1) | Water Sample | Food Sample | Reference |
|---|---|---|---|---|---|
| Fluorescence | Fe3O4 NPs | 3.75 | Yes | No | [23] |
| Fluorescence | MSNs | 0.90 | Yes | No | [24] |
| Fluorescence | ZnO QDs | 27 | Yes | No | [25] |
| Fluorescence | MoS2 | 1.35 | Yes | No | [26] |
| Colorimetry | Ag NPs | 5.98 | Yes | No | [27] |
| Colorimetry | Au NPs | 1.27 | Yes | No | [28] |
| RRS | Ag NPs | 1.9 | Yes | No | [29] |
| Fluorescence | ZGO: Mn | 0.5 | Yes | Yes | This work |
| Sample | Added | Found | RSD | Sample |
|---|---|---|---|---|
| Lake water | 50 ng·mL−1 | 63.85 ng·mL−1 | 15.7 | 127.70 |
| Drinking water | 50 ng·mL−1 | 66.62 ng·mL−1 | 15.0 | 133.24 |
| Scallop meat | 1 mg·kg−1 | 1.19 mg·kg−1 | 21.5 | 119.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Wang, H.; Wang, Z.; Tan, M. Construction of Time-Resolved Luminescence Nanoprobe and Its Application in As(III) Detection. Nanomaterials 2020, 10, 551. https://doi.org/10.3390/nano10030551
Chen T, Wang H, Wang Z, Tan M. Construction of Time-Resolved Luminescence Nanoprobe and Its Application in As(III) Detection. Nanomaterials. 2020; 10(3):551. https://doi.org/10.3390/nano10030551
Chicago/Turabian StyleChen, Teng, Haitao Wang, Zhouping Wang, and Mingqian Tan. 2020. "Construction of Time-Resolved Luminescence Nanoprobe and Its Application in As(III) Detection" Nanomaterials 10, no. 3: 551. https://doi.org/10.3390/nano10030551
APA StyleChen, T., Wang, H., Wang, Z., & Tan, M. (2020). Construction of Time-Resolved Luminescence Nanoprobe and Its Application in As(III) Detection. Nanomaterials, 10(3), 551. https://doi.org/10.3390/nano10030551






