Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Manufacturing of PCL/Fe3O4 Nanocomposite Substrates
2.2. Small Punch Test
2.3. Scanning Electron Microscopy
2.4. Cell Culture
2.4.1. Magnetic Stimulation
2.4.2. Cell Metabolic Activity
2.4.3. Alkaline Phosphatase Activity
2.4.4. Confocal Laser Scanning Microscopy
2.4.5. Immunoblot Analysis
2.5. Statistical Analysis
3. Results
3.1. Small Punch Test
3.2. Scanning Electron Microscopy
3.3. Cell Metabolic Activity
3.4. Alkaline Phosphatase Activity
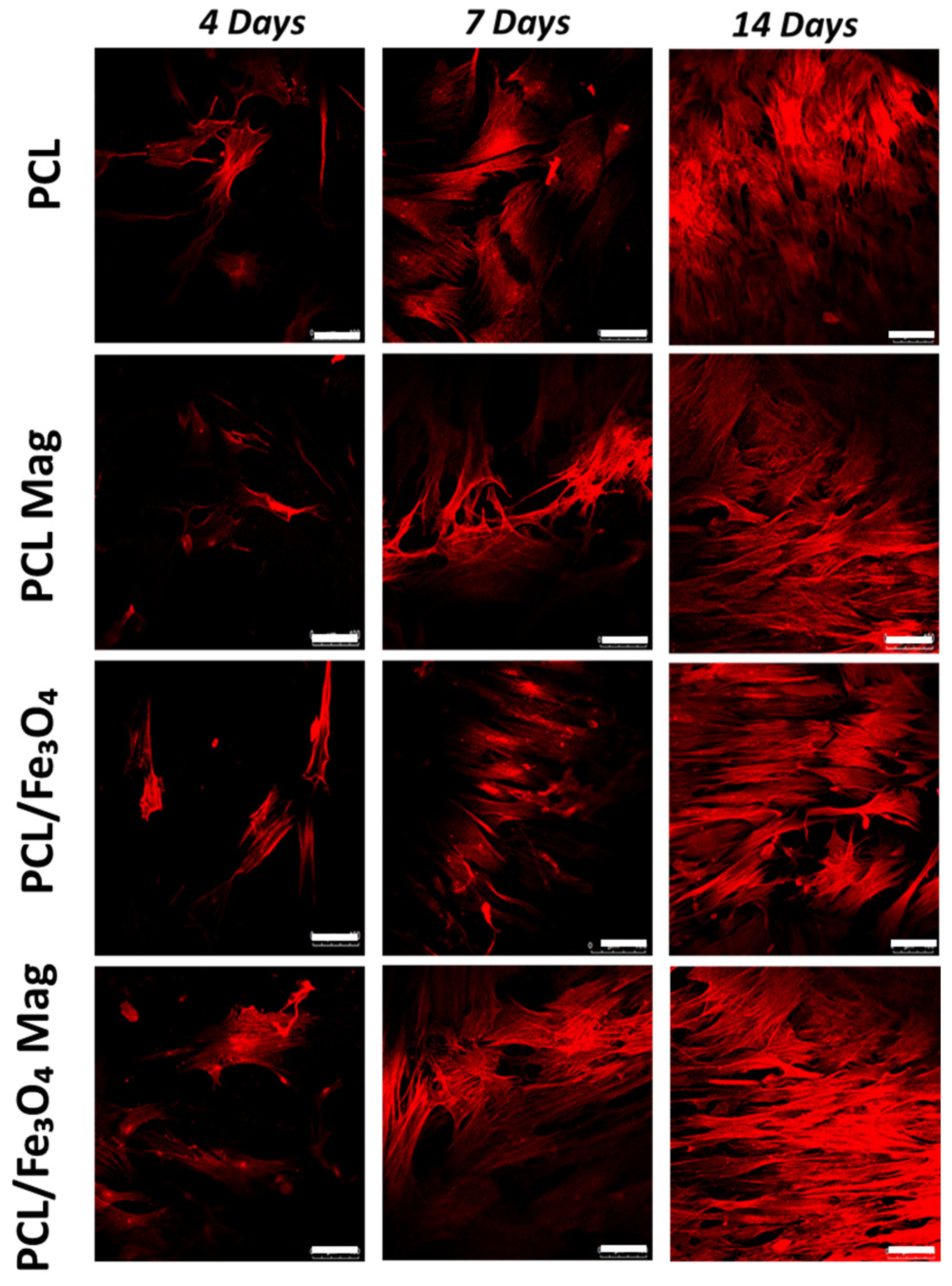
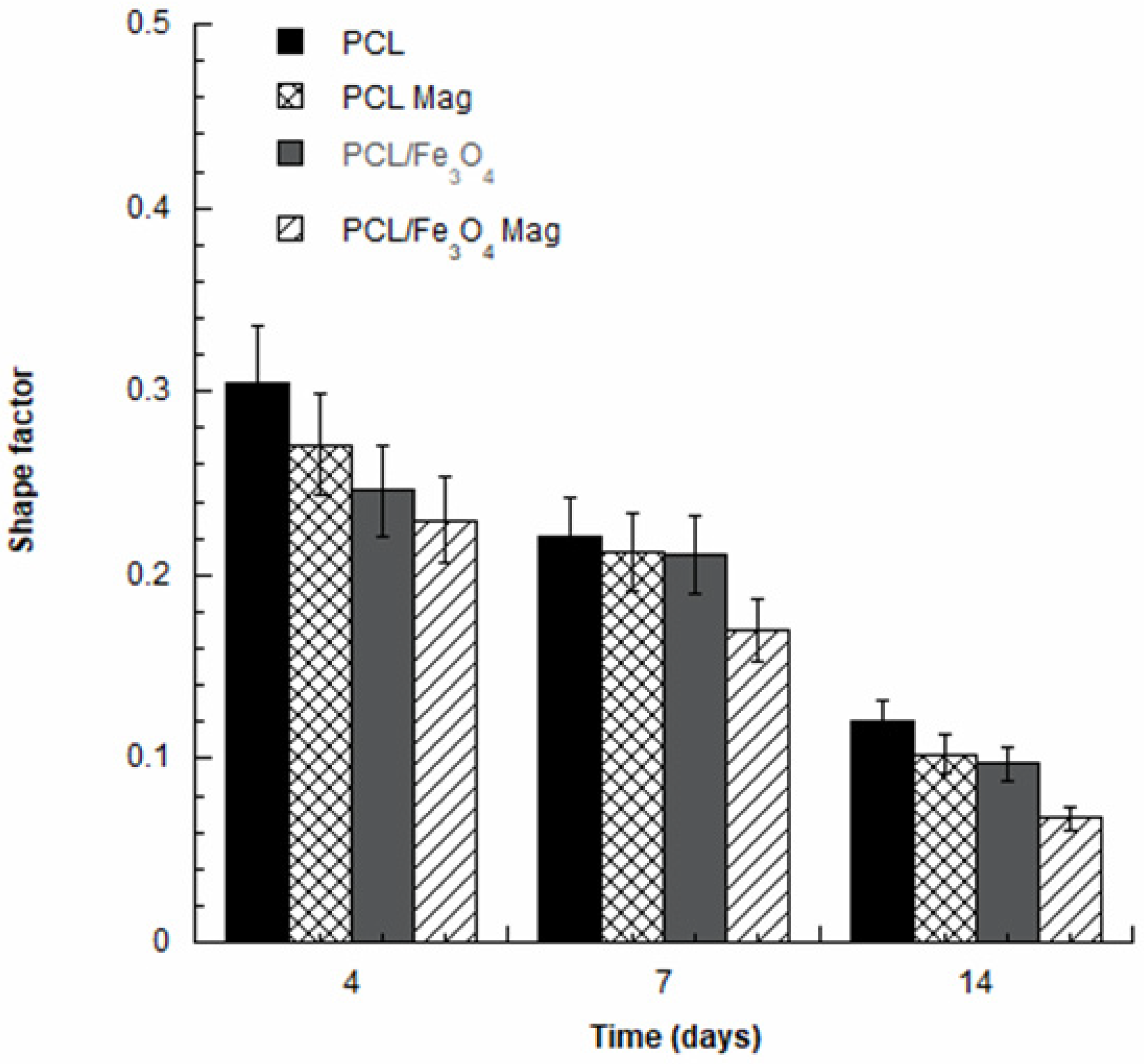
3.5. Confocal Laser Scanning Microscopy
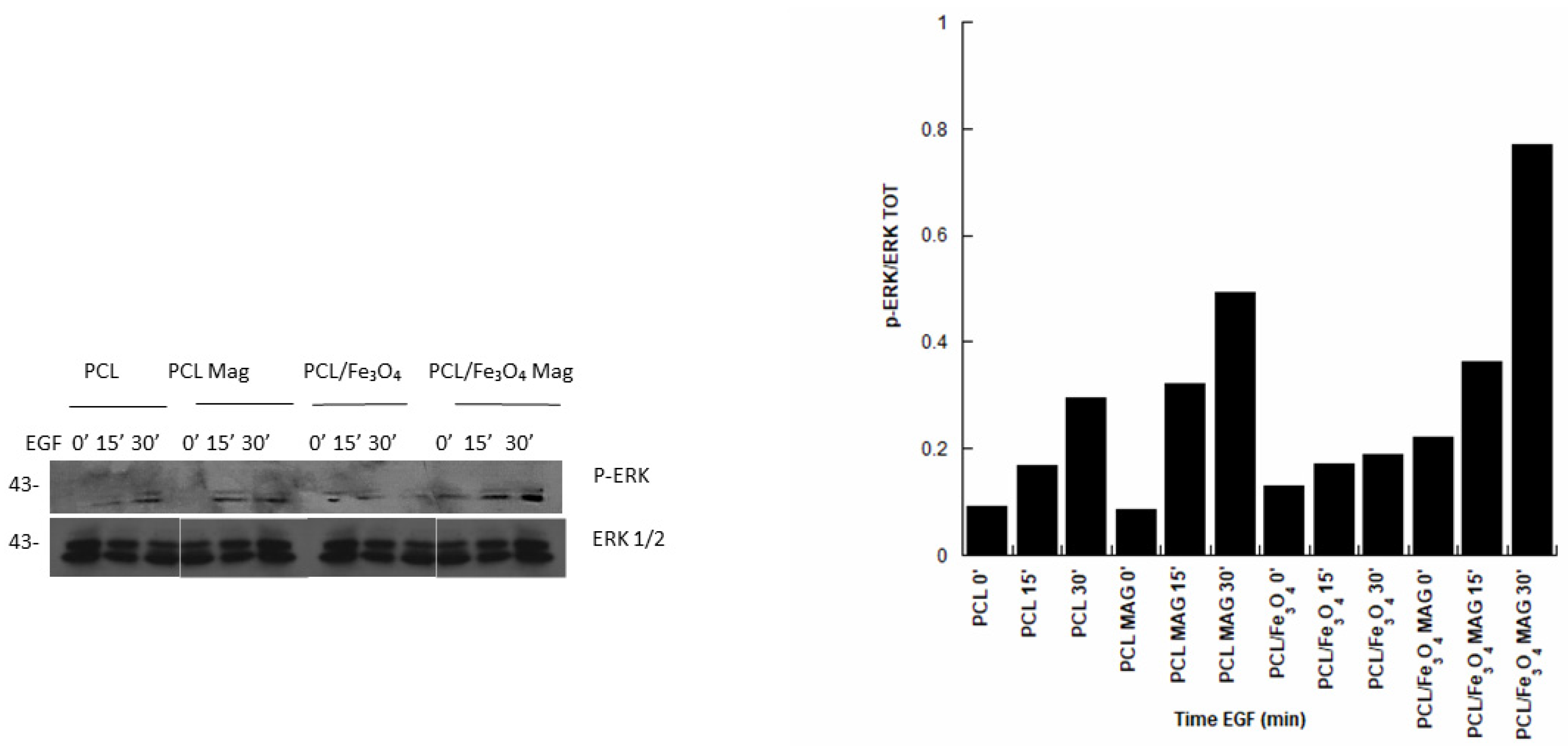
3.6. Immunoblot Analysis
4. Discussion
5. Conclusions
- The possibility to develop PCL/Fe3O4 (80/20 w/w) substrates with improved mechanical properties (higher strength than PCL without negatively affecting the work to failure) was demonstrated by the small punch test.
- The combination of a time-dependent magnetic field with PCL/Fe3O4 nanocomposites (PCL/Fe3O4 Mag) impacted the behavior of hMSCs, especially resulting in a prolonged cell differentiation.
- The effect of a time-dependent magnetic field in increasing ERK phosphorylation levels and, hence, in the activation of the MAPK pathway, was reported, also corroborating previous findings on the combination of a magnetic field and magnetic nanocomposite structures.
- The role of the material–magnetic field combination was revealed, as the highest ERK phosphorylation levels were found in the case of PCL/Fe3O4 Mag.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gloria, A.; De Santis, R.; Ambrosio, L. Polymer-based composite scaffolds for tissue engineering. J. Appl. Biomater. Biomech. 2010, 8, 57–67. [Google Scholar]
- Gloria, A.; Frydman, B.; Lamas, M.L.; Serra, A.C.; Martorelli, M.; Coelho, J.F.J.; Fonseca, A.C.; Domingos, M. The influence of poly (ester amide) on the structural and functional features of 3D additive manufactured poly(ε-caprolactone) scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Devin, J.E.; Attawia, M.A.; Laurencin, C.T. Three-dimensional degradable porous polymer–ceramic matrices for use in bone repair. J. Biomater. Sci. Polym. 1996, 7, 661–669. [Google Scholar] [CrossRef]
- Mathieu, L.M.; Mueller, T.L.; Bourban, P.E.; Pioletti, D.P.; Müller, R.; Månson, J.A.E. Architecture and properties of anisotropic polymer composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Gloria, A.; Ronca, D.; Russo, T.; D’Amora, U.; Chierchia, M.; De Santis, R.; Nicolais, L.; Ambrosio, L. Technical features and criteria in designing fiber-reinforced composite materials: From the aerospace and aeronautical field to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Gloria, A.; D’Antò, V.; D’Amora, U.; Ametrano, G.; Bollino, F.; De Santis, F.; Ausanio, G.; Catauro, M.; Rengo, S.; et al. Poly(ε-caprolactone) reinforced with sol-gel synthesized organic-inorganic hybrid fillers as composite substrates for tissue engineering. J. Appl. Biomater. Biomech. 2010, 8, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Rogel, M.R.; Qiu, H.; Ameer, G.A. The role of nanocomposites in bone regeneration. J. Mater. Chem. 2008, 18, 4233–4241. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology properties relationships. Compos. Part A 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Baradaran, T.; Shafiei, S.S.; Mohammadi, S.; Moztarzadeh, F. Poly(ε-caprolactone)/layered double hydroxide microspheres-aggregated nanocomposite scaffold for osteogenic differentiation of mesenchymal stem cell. Mater. Today Commun. 2020, 23, 100913. [Google Scholar] [CrossRef]
- Shapourzadeh, A.; Atyabi, S.-M.; Irani, S.; Bakhshi, H. Osteoinductivity of polycaprolactone nanofibers grafted functionalized with carboxymethyl chitosan: Synergic effect of β-carotene and electromagnetic field. Int. J. Biol. Macromol. 2020, 150, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Röcken, M. Static Magnetic Field (SMF) as a Regulator of Stem Cell Fate – New Perspectives in Regenerative Medicine Arising from an Underestimated Tool. Stem Cell Rev. Rep. 2018, 14, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef]
- Barry, S.E. Challenges in the development of magnetic particles for therapeutic applications. Int. J. Hyperth. 2008, 24, 451–466. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Muthana, M.; Scott, S.D.; Farrow, N.; Morrow, F.; Murdoch, C.; Grubb, S.; Brown, N.; Dobson, J.; Lewis, C.E. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 2008, 15, 902–910. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Lee, J.H.; Lee, E.J.; Kim, J.H.; Kim, T.H.; Kim, H.W. Potential of magnetic nanofiber scaffolds with mechanical and biological properties applicable for bone regeneration. PLoS ONE 2014, 9, e91584. [Google Scholar] [CrossRef] [Green Version]
- Díaz, E.; Valle, M.B.; Ribeiro, S.; Lanceros-Mendez, S.; Barandiarán, J.M. 3D Cytocompatible Composites of PCL/Magnetite. Materials 2019, 12, 3843. [Google Scholar] [CrossRef] [Green Version]
- Banobre-Lopez, M.; Pineiro-Redondo, Y.; Sandri, M.; Tampieri, A.; De Santis, R.; Dediu, V.A.; Rivas, J. Hyperthermia Induced in Magnetic Scaffolds for Bone Tissue Engineering. IEEE Trans. Magn. 2014, 50, 1–7. [Google Scholar] [CrossRef]
- Markaki, A.E.; Clyne, W.T. Magneto-mechanical actuation of bonded ferromagnetic fibre arrays. Acta Mater. 2005, 53, 877–889. [Google Scholar] [CrossRef]
- Mannix, R.J.; Kumar, S.; Cassiola, F.; Montoya-Zavala, M.; Feinstein, E.; Prentiss, M.; Ingber, D.E. Nanomagnetic actuation of receptor-mediated signal transduction. Nat. Nanotechnol. 2008, 3, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; McBain, S.; Dobson, J.; El Ha, A.J. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface 2008, 5, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Sura, H.S.; Magnay, J.; Attridge, K.; Green, D.; Oreffo, R.O.C.; Dobson, J.P.; El Haj, A.J. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Eng. 2010, 16, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ino, K.; Hayashida, M.; Kobayashi, T.; Matsunuma, H.; Kagami, H.; Ueda, M.; Honda, H. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 1553–1561. [Google Scholar] [CrossRef]
- Ito, A.; Hibino, E.; Kobayashi, C.; Terasaki, H.; Kagami, H.; Ueda, M.; Kobayashi, T.; Honda, H. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 489–496. [Google Scholar] [CrossRef]
- Pislaru, S.V.; Harbuzariu, A.; Agarwal, G.; Witt Aas Cvt Latg, T.; Gulati, R.; Sandhu, N.P.; Mueske Aa, C.; Kalra, M.; Simari, R.D.; Sandhu, G.S. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation 2006, 114, I314–I318. [Google Scholar] [CrossRef] [Green Version]
- Dobson, J.; Cartmell, S.H.; Keramane, A.; El Haj, A.J. Principles and design of a novel magnetic force mechanical conditioning bioreactor for tissue engineering, stem cell conditioning, and dynamic in vitro screening. IEEE Trans. NanoBiosci. 2006, 5, 173–177. [Google Scholar] [CrossRef]
- Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 2008, 3, 139–143. [Google Scholar] [CrossRef]
- Mack, J.J.; Cox, B.N.; Sudre, O.; Corrin, A.A.; dos Santos, S.L.; Lucato, C.M.; Andrew, J.S. Achieving nutrient pumping and strain stimulus by magnetic actuation of tubular scaffolds. Smart Mater. Struct. 2009, 18, 104 025–104 040. [Google Scholar] [CrossRef]
- Perea, H.; Aigner, J.; Hopfner, U.; Wintermantel, E. Direct magnetic tubular cell seeding: A novel approach for vascular tissue engineering. Cells Tissues Organs. 2006, 183, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ito, A.; Arinobe, M.; Murase, Y.; Iwata, Y.; Narita, Y.; Kagami, H.; Ueda, M.; Honda, H. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J. Biosci. Bioeng. 2007, 103, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Banobre-Lopez, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; Dionigi, C.; Sytcheva, A.; Herrmannasdorfer, T.; Dediu, V.; Ambrosio, L. A basic approach toward the development of nanocomposite magnetic scaffolds for advanced bone tissue engineering. J. Appl. Polym. Sci. 2011, 122, 3599–3605. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, 198–206. [Google Scholar] [CrossRef]
- Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; D’Alessandro, T.; Sandri, M.; Bañobre-Lopez, M.; Piñeiro-Redondo, Y.; Uhlarz, M.; Tampieri, A.; et al. Magnetic poly(ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J. R. Soc. Interface 2013, 10, 20120833. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kawasumi, M.; Saito, M. Effect of static magnetic field on cell migration. Electr. Eng. Jpn. 2007, 160, 46–52. [Google Scholar] [CrossRef]
- Miyakoshi, J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005, 87, 213–223. [Google Scholar] [CrossRef]
- Rosen, A.D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell. Biochem. Biophys. 2003, 39, 163–173. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Marcacci, M.; Tampieri, A. Evaluation of the effects of a moderate intensity static magnetic field application on human osteoblast-like cells. Am. J. Biomed. Eng. 2012, 2, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Delle Donne, R.; Sepe, M.; Porpora, M.; Garbi, C.; Chiuso, F.; Gallo, A.; Parisi, S.; Russo, L.; Bachmann, V.; et al. Praja2 regulates KSR1 stability and mitogenic signaling. Cell Death Dis. 2016, 7, e2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amora, U.; Russo, T.; Gloria, A.; Rivieccio, V.; D’Antò, V.; Negri, G.; Ambrosio, L.; De Santis, R. 3D additive-manufactured nanocomposite magnetic scaffolds: Effect of the application mode of a time-dependent magnetic field on hMSCs behavior. Bioact. Mater. 2017, 2, 138–145. [Google Scholar]
- Domingos, M.; Intranuovo, F.; Russo, T.; De Santis, R.; Gloria, A.; Ambrosio, L.; Ciurana, J.; Bartolo, P. The first systematic analysis of 3D rapid prototyped poly(ε-caprolactone) scaffolds manufactured through BioCell printing: The effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability. Biofabrication 2013, 5, 045004. [Google Scholar] [CrossRef]
- Tampieri, A.; Sprio, S.; Sandri, M.; Valentini, F. Mimicking natural bio-mineralization processes: A new tool for osteochondral scaffold development. Trends Biotechnol. 2011, 29, 526–535. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-J.; Singh, R.K.; Seo, S.-J.; Kim, T.-H.; Kim, J.-H.; Lee, E.-J.; Kim, H.-W. Magnetic scaffolds of polycaprolactone with functionalized magnetite nanoparticles: Physicochemical, mechanical, and biological properties effective for bone regeneration. RSC Adv. 2014, 4, 17325–17336. [Google Scholar] [CrossRef]
- Zhang, B.; Tu, Z.; Zhao, F.; Wang, J. Superparamagnetic iron oxide nanoparticles prepared by using an improved polyol method. Appl. Surf. Sci. 2013, 266, 375–379. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, A.; Gloria, A.; D’Amora, U.; Russo, T.; Panseri, S.; Sandri, M.; Tampieri, A.; Marcacci, M.; Dediu, V.A.; et al. Towards the design of 3D fiber-deposited poly(ɛ-caprolactone)/iron-doped hydroxyapatite nanocomposite magnetic scaffolds for bone regeneration. J. Biomed. Nanotechnol. 2015, 11, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; Tampieri, A.; Herrmannsdörfer, T.; Ambrosio, L. A route toward the development of 3D magnetic scaffolds with tailored mechanical and morphological properties for hard tissue regeneration: Preliminary study. Virtual Phys. Prototyp. 2011, 6, 189–195. [Google Scholar] [CrossRef]
- Fleury, E.; Ha, J.S. Small punch tests to estimate the mechanical properties of steels for steam power plant: I. Mechanical strength. Int. J. Press. Vessel. Pip. 1998, 75, 699–706. [Google Scholar] [CrossRef]
- Tang, W.; Santare, M.H.; Advani, S.G. Melt processing and mechanical property characterization of multi-walled carbon nanotube/high density polyethylene (MWNT/HDPE) composite films. Carbon 2003, 41, 2779–2785. [Google Scholar] [CrossRef]
- Yang, S.F.; Leong, K.F.; Du, Z.H.; Chua, C.K. The design of scaffolds for use in tissue engineering: Part 1. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Kawazoe, N.; Chen, G. Dependence of Spreading and Differentiation of Mesenchymal Stem Cell son Micropatterned Surface Area. J. Nanomater. 2011, 265251. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Wang, Y. Influence of Cell Spreading Area on the Osteogenic Commitment and Phenotype Maintenance of Mesenchymal Stem Cells. Sci. Rep. 2019, 9, 6891. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.-M.; Lee, E.-S.; Kim, M.-J.; Kim, J.-J.; Lee, J.-H.; Lee, H.-H.; Park, K.-R.; Yi, J.-K.; Kim, H.-W.; Kim, E.-C. Magnetic Nanocomposite Scaffold-Induced Stimulation of Migration and Odontogenesis of Human Dental Pulp Cells through Integrin Signaling Pathways. PLoS ONE 2015, 10, e0138614. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Song, Z.; Wang, Q.; Ma, T.; Li, Z.; Lu, Y. Kun Zhang Role of RUNX2 in osteogenic differentiation of mesenchymal stem cells induced by BMP9. Int. J. Clin. Exp. Med. 2019, 12, 12243–12249. [Google Scholar]
- Hu, J.; Liao, H.; Ma, Z.; Chen, H.; Huang, Z.; Zhang, Y.; Yu, M.; Chen, Y.; Xu, J. Focal Adhesion Kinase Signaling Mediated the Enhancement of Osteogenesis of Human Mesenchymal Stem Cells Induced by Extracorporeal Shockwave. Sci. Rep. 2016, 6, 20875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ye, D.; Li, M.; Ma, M.; Gu, N. Adaptive Materials Based on Iron Oxide Nanoparticles for Bone Regeneration. Chemphyschem 2018, 19, 1965–1979. [Google Scholar] [CrossRef] [PubMed]




| Materials | Peak Load (N) | Work to Failure (mJ) |
|---|---|---|
| PCL | 28.1 ± 2.1 | 31.4 ± 4.2 |
| PCL/Fe3O4 (80/20 w/w) | 34.4 ± 2.6 | 30.1 ± 5.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, T.; Peluso, V.; Gloria, A.; Oliviero, O.; Rinaldi, L.; Improta, G.; De Santis, R.; D’Antò, V. Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior. Nanomaterials 2020, 10, 577. https://doi.org/10.3390/nano10030577
Russo T, Peluso V, Gloria A, Oliviero O, Rinaldi L, Improta G, De Santis R, D’Antò V. Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior. Nanomaterials. 2020; 10(3):577. https://doi.org/10.3390/nano10030577
Chicago/Turabian StyleRusso, Teresa, Valentina Peluso, Antonio Gloria, Olimpia Oliviero, Laura Rinaldi, Giovanni Improta, Roberto De Santis, and Vincenzo D’Antò. 2020. "Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior" Nanomaterials 10, no. 3: 577. https://doi.org/10.3390/nano10030577
APA StyleRusso, T., Peluso, V., Gloria, A., Oliviero, O., Rinaldi, L., Improta, G., De Santis, R., & D’Antò, V. (2020). Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior. Nanomaterials, 10(3), 577. https://doi.org/10.3390/nano10030577









