Novel Architecture Titanium Carbide (Ti3C2Tx) MXene Cocatalysts toward Photocatalytic Hydrogen Production: A Mini-Review
Abstract
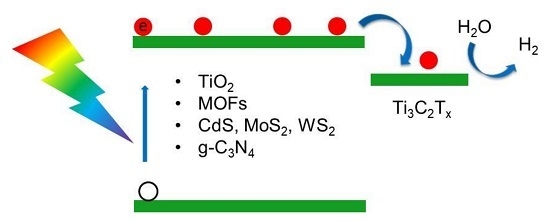
1. Introduction
2. Coupled Morphological and Structural Ti3C2Tx Cocatalysts
3. Modified Ti3C2Tx Cocatalysts with Surface Termination Groups
4. The Design of Ti3C2Tx Composite Photocatalysts
4.1. Couple with Transition Metal Oxide (TMOs)
4.2. Couple with Transient Metal Sulfides (TMSs)
4.3. Couple with the Metal–Organic Framework
4.4. Coupled with Graphitic Carbon Nitride (g–C3N4)
4.5. Ternary Composites
5. Comparison of the Photocatalytic Hydrogen Production
6. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bockris, J. Energy: The Solar-Hydrogen Alternative; Halsted Press: New York, NY, USA, 1975; 381p. [Google Scholar]
- Huang, C.-W.; Nguyen, B.-S.; Wu, J.C.S.; Nguyen, V.-H. A current perspective for photocatalysis towards the hydrogen production from biomass-derived organic substances and water. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Ly, Q.V.; Vo, D.-V.N.; Nguyen, Q.V.; Le, H.S.; Jang, H.W.; Kim, S.Y.; et al. Facile synthesis of WS2 hollow spheres and their hydrogen evolution reaction performance. Appl. Surf. Sci. 2020, 505, 144574. [Google Scholar] [CrossRef]
- Tekalgne, M.A.; Nguyen, K.V.; Nguyen, D.L.T.; Nguyen, V.-H.; Nguyen, T.P.; Vo, D.-V.N.; Trinh, Q.T.; Hasani, A.; Do, H.H.; Lee, T.H.; et al. Hierarchical molybdenum disulfide on carbon nanotube–reduced graphene oxide composite paper as efficient catalysts for hydrogen evolution reaction. J. Alloys Compd. 2020, 823, 153897. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, S.Y.; Lee, T.H.; Jang, H.W.; Le, Q.V.; Kim, I.T. Facile synthesis of W2C@WS2 alloy nanoflowers and their hydrogen generation performance. Appl. Surf. Sci. 2020, 504, 144389. [Google Scholar] [CrossRef]
- Tekalgne, M.A.; Hasani, A.; Heo, D.Y.; Van Le, Q.; Nguyen, T.P.; Lee, T.H.; Ahn, S.H.; Jang, H.W.; Kim, S.Y. SnO2@WS2/p-Si Heterostructure Photocathode for Photoelectrochemical Hydrogen Production. J. Phys. Chem. C 2020, 124, 647–652. [Google Scholar] [CrossRef]
- Hasani, A.; Le, Q.V.; Tekalgne, M.; Choi, M.-J.; Lee, T.H.; Jang, H.W.; Kim, S.Y. Direct synthesis of two-dimensional MoS2 on p-type Si and application to solar hydrogen production. NPG Asia Mater. 2019, 11, 47. [Google Scholar] [CrossRef]
- Hasani, A.; Van Le, Q.; Tekalgne, M.; Choi, M.-J.; Choi, S.; Lee, T.H.; Kim, H.; Ahn, S.H.; Jang, H.W.; Kim, S.Y. Fabrication of a WS2/p-Si Heterostructure Photocathode Using Direct Hybrid Thermolysis. ACS Appl. Mater. Interfaces 2019, 11, 29910–29916. [Google Scholar] [CrossRef] [PubMed]
- Tekalgne, M.; Hasani, A.; Le, Q.V.; Nguyen, T.P.; Choi, K.S.; Lee, T.H.; Jang, H.W.; Luo, Z.; Kim, S.Y. CdSe Quantum Dots Doped WS2 Nanoflowers for Enhanced Solar Hydrogen Production. Phys. Status Solidi A Appl. Res. 2019, 216, 1800853. [Google Scholar] [CrossRef]
- Tekalgne, M.; Hasani, A.; Van Le, Q.; Kim, S.Y. Transition metal dichalcogenide-based composites for hydrogen production. Funct. Compos. Struct. 2019, 1, 012001. [Google Scholar] [CrossRef]
- Guo, W.; Le, Q.V.; Do, H.H.; Hasani, A.; Tekalgne, M.; Bae, S.-R.; Lee, T.H.; Jang, H.W.; Ahn, S.H.; Kim, S.Y. Ni3Se4@MoSe2 Composites for Hydrogen Evolution Reaction. Appl. Sci. 2019, 9, 5035. [Google Scholar] [CrossRef]
- Hasani, A.; Tekalgne, M.; Le, Q.V.; Jang, H.W.; Kim, S.Y. Two-dimensional materials as catalysts for solar fuels: hydrogen evolution reaction and CO2 reduction. J. Mater. Chem. A 2019, 7, 430–454. [Google Scholar] [CrossRef]
- Guo, W.; Le, Q.V.; Hasani, A.; Lee, T.H.; Jang, H.W.; Luo, Z.; Kim, S.Y. MoSe2-GO/rGO Composite Catalyst for Hydrogen Evolution Reaction. Polymers 2018, 10, 1309. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Le, Q.V.; Choi, S.; Lee, T.H.; Hong, S.-P.; Choi, K.S.; Jang, H.W.; Lee, M.H.; Park, T.J.; Kim, S.Y. Surface extension of MeS2 (Me = Mo or W) nanosheets by embedding MeSx for hydrogen evolution reaction. Electrochim. Acta 2018, 292, 136–141. [Google Scholar] [CrossRef]
- Hasani, A.; Nguyen, T.P.; Tekalgne, M.; Van Le, Q.; Choi, K.S.; Lee, T.H.; Jung Park, T.; Jang, H.W.; Kim, S.Y. The role of metal dopants in WS2 nanoflowers in enhancing the hydrogen evolution reaction. Appl. Catal. A 2018, 567, 73–79. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.-H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.-V.N.; Kim, S.Y.; Le, Q.V. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Nitrogen-doped hydrogenated TiO2 modified with CdS nanorods with enhanced optical absorption, charge separation and photocatalytic hydrogen evolution. Chem. Eng. J. 2020, 384, 123275. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Yan, C.; Guo, F. Facile synthesis of 2D/2D Co3(PO4)2/g-C3N4 heterojunction for highly photocatalytic overall water splitting under visible light. Chem. Eng. J. 2020, 382, 122960. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)-based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Fang, X.; Gao, R.; Yang, Y.; Yan, D. A Cocrystal Precursor Strategy for Carbon-Rich Graphitic Carbon Nitride toward High-Efficiency Photocatalytic Overall Water Splitting. iScience 2019, 16, 22–30. [Google Scholar] [CrossRef]
- Ehrmaier, J.; Karsili, T.N.V.; Sobolewski, A.L.; Domcke, W. Mechanism of Photocatalytic Water Splitting with Graphitic Carbon Nitride: Photochemistry of the Heptazine-Water Complex. J. Phys. Chem. A 2017, 121, 4754–4764. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Photocatalytic splitting of water on s-triazine based graphitic carbon nitride: an ab initio investigation. J. Mater. Chem. A 2015, 3, 23011–23016. [Google Scholar] [CrossRef]
- Pan, Z.; Pan, N.; Chen, L.; He, J.; Zhang, M. Flower-like MOF-derived Co–N-doped carbon composite with remarkable activity and durability for electrochemical hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 30075–30083. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pei, J.; He, C.-T.; Wan, J.; Ren, H.; Wang, Y.; Dong, J.; Wu, K.; Cheong, W.-C.; Mao, J.; et al. Single Tungsten Atoms Supported on MOF-Derived N-Doped Carbon for Robust Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1800396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.-K.; Hu, L.; Zheng, J.; Prabhakaran, D.; Wu, S.; Puchtler, T.J.; Li, M.; Wong, K.-Y.; Taylor, R.A.; et al. Photocatalytic water splitting by N-TiO2 on MgO (111) with exceptional quantum efficiencies at elevated temperatures. Nat. Commun. 2019, 10, 4421. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J. Structural engineering of transition metal-based nanostructured electrocatalysts for efficient water splitting. Front. Chem. Sci. Eng. 2018, 12, 838–854. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Dai, J.-H.; Zhang, Y.-M.; Song, Y. Two-Dimensional, Ordered, Double Transition Metal Carbides (MXenes): A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. J. Phys. Chem. C 2018, 122, 28113–28122. [Google Scholar] [CrossRef]
- Esposito, D.V.; Hunt, S.T.; Kimmel, Y.C.; Chen, J.G. A New Class of Electrocatalysts for Hydrogen Production from Water Electrolysis: Metal Monolayers Supported on Low-Cost Transition Metal Carbides. J. Am. Chem. Soc. 2012, 134, 3025–3033. [Google Scholar] [CrossRef]
- Miao, M.; Pan, J.; He, T.; Yan, Y.; Xia, B.Y.; Wang, X. Molybdenum Carbide-Based Electrocatalysts for Hydrogen Evolution Reaction. Chem. Eur. 2017, 23, 10947–10961. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, M.W. The MN+1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, S.; Heo, J.; Park, C.M.; Her, N.; Jang, M.; Huang, Y.; Han, J.; Yoon, Y. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res. 2019, 12, 471–487. [Google Scholar] [CrossRef]
- Verger, L.; Natu, V.; Carey, M.; Barsoum, M.W. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2019, 1, 656–669. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The Mn+1AXn phases: Materials science and thin-film processing. Thin Solid Films 2010, 518, 1851–1878. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Y. Introduction to 2D Transition Metal Carbides and Nitrides (MXenes). In 2D Metal Carbides and Nitrides (MXenes): Structure, Properties and Applications; Anasori, B., Gogotsi, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–12. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X.; Zhang, H.; Xiang, Q. Two-Dimensional Transition Metal MXene-Based Photocatalysts for Solar Fuel Generation. J. Phys. Chem. Lett. 2019, 10, 3488–3494. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wang, X.; Liu, E.; Ye, J.; Wang, D. Boosting the Photocatalytic Activity of P25 for Carbon Dioxide Reduction by using a Surface-Alkalinized Titanium Carbide MXene as Cocatalyst. ChemSusChem 2018, 11, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wang, W.; Wang, J.; Duan, H.; Shi, J.; Yu, X. The synergetic effects of Ti3C2 MXene and Pt as co-catalysts for highly efficient photocatalytic hydrogen evolution over g-C3N4. Phys. Chem. Chem. Phys. 2018, 20, 11405–11411. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, L.; Liang, Z.; Xue, Y.; Cui, H.; Tian, J. Synergetic effect of defects rich MoS2 and Ti3C2 MXene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2. Chem. Eng. J. 2020, 383, 123178. [Google Scholar] [CrossRef]
- Zhang, C.; Anasori, B.; Seral-Ascaso, A.; Park, S.-H.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, Flexible, and Conductive 2D Titanium Carbide (MXene) Films with High Volumetric Capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tuan Nguyen, D.M.; Tran, D.L.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q.V. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. Monolayer Ti3C2Tx as an Effective Co-catalyst for Enhanced Photocatalytic Hydrogen Production over TiO2. ACS Appl. Energy Mater. 2019, 2, 4640–4651. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; An, Y.; Wu, H.; Yao, N.; Fan, X.; Guo, X. MXene Nanofibers as Highly Active Catalysts for Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 8976–8982. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Guo, Y.; Liang, Z.; Cui, H.; Tian, J. Boosting the Photocatalytic Ability of g-C3N4 for Hydrogen Production by Ti3C2 MXene Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 41440–41447. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, D.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Wang, X.-F. g-C3N4/Ti3C2Tx (MXenes) composite with oxidized surface groups for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 9124–9131. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Zhao, X.; Tang, Q.; Zhou, Z. Computational studies on structural and electronic properties of functionalized MXene monolayers and nanotubes. J. Mater. Chem. A 2015, 3, 4960–4966. [Google Scholar] [CrossRef]
- Xie, Y.; Kent, P.R.C. Hybrid density functional study of structural and electronic properties of functionalized Tin+1Xn (X = C, N) monolayers. Phys. Rev. B 2013, 87, 235441. [Google Scholar] [CrossRef]
- Berdiyorov, G.R. Effect of surface functionalization on the electronic transport properties of Ti3C2 MXene. EPL (Europhys. Lett.) 2015, 111, 67002. [Google Scholar] [CrossRef]
- Berdiyorov, G.R. Optical properties of functionalized Ti3C2T2 (T = F, O, OH) MXene: First-principles calculations. AIP Advances 2016, 6, 055105. [Google Scholar] [CrossRef]
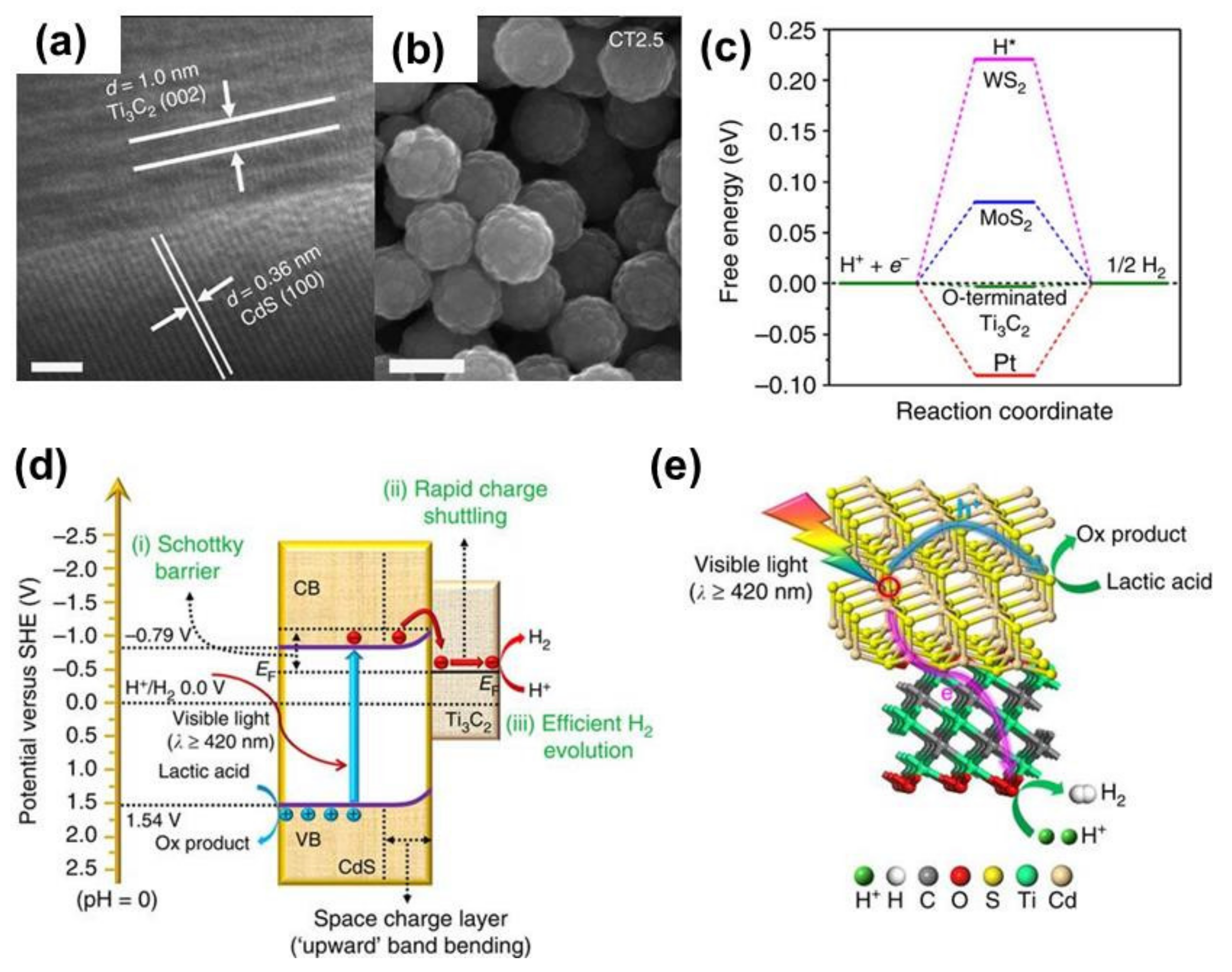
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, D.; Xiang, Q. Plasma-modified Ti3C2Tx/CdS hybrids with oxygen-containing groups for high-efficiency photocatalytic hydrogen production. Nanoscale 2019, 11, 18797–18805. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, D.; Liao, Y.; Wang, G.; Shi, X.; Zhang, H.; Xiang, Q. Synthesis and photocatalytic H2-production activity of plasma-treated Ti3C2Tx MXene modified graphitic carbon nitride. J. Am. Ceram. Soc. 2020, 103, 849–858. [Google Scholar] [CrossRef]
- Miyoshi, A.; Nishioka, S.; Maeda, K. Water Splitting on Rutile TiO2-Based Photocatalysts. Chem. Eur. 2018, 24, 18204–18219. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Li, R.; Weng, Y.; Zhou, X.; Wang, X.; Mi, Y.; Chong, R.; Han, H.; Li, C. Achieving overall water splitting using titanium dioxide-based photocatalysts of different phases. Energy Environ. Sci. 2015, 8, 2377–2382. [Google Scholar] [CrossRef]
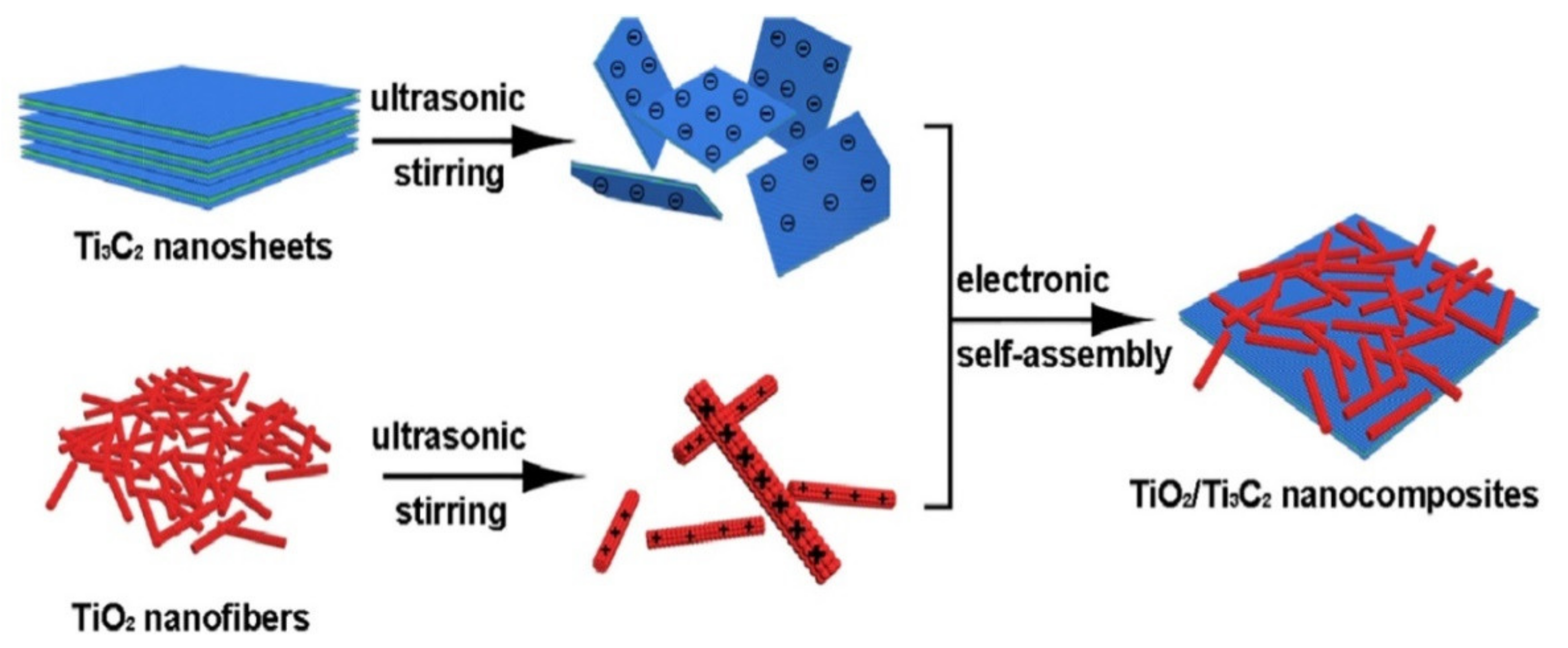
- Zhuang, Y.; Liu, Y.; Meng, X. Fabrication of TiO2 nanofibers/MXene Ti3C2 nanocomposites for photocatalytic H2 evolution by electrostatic self-assembly. Appl. Surf. Sci. 2019, 496, 143647. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2020, 261, 127127. [Google Scholar] [CrossRef]
- Tso, S.; Li, W.-S.; Wu, B.-H.; Chen, L.-J. Enhanced H2 production in water splitting with CdS-ZnO core-shell nanowires. Nano Energy 2018, 43, 270–277. [Google Scholar] [CrossRef]
- Garg, P.; Bhauriyal, P.; Mahata, A.; Rawat, K.S.; Pathak, B. Role of Dimensionality for Photocatalytic Water Splitting: CdS Nanotube versus Bulk Structure. Chemphyschem 2019, 20, 383–391. [Google Scholar] [CrossRef]
- Chen, X.; Shangguan, W. Hydrogen production from water splitting on CdS-based photocatalysts using solar light. Front. Energy 2013, 7, 111–118. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Li, L.; Zheng, J.; Jia, S.; Chen, J.; Liu, B.; Zhu, Z. Fabricating efficient CdSe–CdS photocatalyst systems by spatially resetting water splitting sites. J. Mater. Chem. A 2017, 5, 20131–20135. [Google Scholar] [CrossRef]
- Li, M.; Cui, Z.; Li, E. Silver-modified MoS2 nanosheets as a high-efficiency visible-light photocatalyst for water splitting. Ceram. Int. 2019, 45, 14449–14456. [Google Scholar] [CrossRef]
- Lin, L.; Huang, S.; Zhu, Y.; Du, B.; Zhang, Z.; Chen, C.; Wang, X.; Zhang, N. Construction of CdS/MoS2 heterojunction from core–shell MoS2@Cd-MOF for efficient photocatalytic hydrogen evolution. Dalton Trans. 2019, 48, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Hu, Y.H. MoS2 as a co-catalyst for photocatalytic hydrogen production from water. Energy Sci. Eng. 2016, 4, 285–304. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical Layered WS2/Graphene-Modified CdS Nanorods for Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2016, 9, 996–1002. [Google Scholar] [CrossRef]
- Kumar, R.; Das, D.; Singh, A.K. C2N/WS2 van der Waals type-II heterostructure as a promising water splitting photocatalyst. J. Catal. 2018, 359, 143–150. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Ma, R.; Kumar, D.P.; Lim, M.; Kim, T.K. Heterostructured WS2-MoS2 Ultrathin Nanosheets Integrated on CdS Nanorods to Promote Charge Separation and Migration and Improve Solar-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 2017, 10, 1563–1570. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B 2019, 268, 118382. [Google Scholar] [CrossRef]
- Tie, L.; Yang, S.; Yu, C.; Chen, H.; Liu, Y.; Dong, S.; Sun, J.; Sun, J. In situ decoration of ZnS nanoparticles with Ti3C2 MXene nanosheets for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 545, 63–70. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Q.; Li, J.; Liu, H. Boosting the photocatalytic activity of CdLa2S4 for hydrogen production using Ti3C2 MXene as a co-catalyst. Appl. Catal. B 2019, 267, 118379. [Google Scholar] [CrossRef]
- Tian, P.; He, X.; Zhao, L.; Li, W.; Fang, W.; Chen, H.; Zhang, F.; Huang, Z.; Wang, H. Ti3C2 nanosheets modified Zr-MOFs with Schottky junction for boosting photocatalytic HER performance. Sol. Energy 2019, 188, 750–759. [Google Scholar] [CrossRef]
- Dong, J.; Shi, Y.; Huang, C.; Wu, Q.; Zeng, T.; Yao, W. A New and stable Mo-Mo2C modified g-C3N4 photocatalyst for efficient visible light photocatalytic H2 production. Appl. Catal. B 2019, 243, 27–35. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, D.; Sun, D.; Mao, S.; He, C.; Wang, Z.; Ji, X.; Shi, J.-W. Carbon nanosheet facilitated charge separation and transfer between molybdenum carbide and graphitic carbon nitride toward efficient photocatalytic H2 production. Appl. Surf. Sci. 2019, 473, 91–101. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. 2D/2D heterojunction of Ti3C2/g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Nanoscale 2019, 11, 8138–8149. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Shen, J.; Yu, X.; Liu, Q.; Li, D.; Tang, H. Construction of Ti3C2 MXene/O-doped g-C3N4 2D-2D Schottky-junction for enhanced photocatalytic hydrogen evolution. Ceram. Int. 2019, 45, 24656–24663. [Google Scholar] [CrossRef]
- Tian, P.; He, X.; Zhao, L.; Li, W.; Fang, W.; Chen, H.; Zhang, F.; Huang, Z.; Wang, H. Enhanced charge transfer for efficient photocatalytic H2 evolution over UiO-66-NH2 with annealed Ti3C2Tx MXenes. Int. J. Hydrog. Energy 2019, 44, 788–800. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Li, X.; Liu, Y.; Cao, Y.; Wang, H.; Yu, H.; Peng, F.; Zhang, L.; Zhang, B.; et al. High efficiency photocatalytic hydrogen production over ternary Cu/TiO2@Ti3C2Tx enabled by low-work-function 2D titanium carbide. Nano Energy 2018, 53, 97–107. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Liang, Z.; Xue, Y.; Cui, H.; Tian, J. 1T-MoS2 nanopatch/Ti3C2 MXene/TiO2 nanosheet hybrids for efficient photocatalytic hydrogen evolution. Mater. Chem. Front. 2019, 3, 2673–2680. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Yin, S.; Liang, Z.; Xue, Y.; Wang, X.; Cui, H.; Tian, J. Photocatalytic H2 Evolution on TiO2 Assembled with Ti3C2 MXene and Metallic 1T-WS2 as Co-catalysts. Nano-Micro Lett. 2019, 12, 6. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Liu, J.; Sun, F.; Yang, P.; Qiu, J. A hierarchically porous and hydrophilic 3D nickel–iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities. Nano Energy 2019, 63, 103880. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, J.; Rajendran, S.; Zhang, X.; Liu, R. Heterostructured d-Ti3C2/TiO2/g-C3N4 Nanocomposites with Enhanced Visible-Light Photocatalytic Hydrogen Production Activity. ChemSusChem 2018, 11, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Varadhan, P.; Fu, H.-C.; Kim, H.; Zhang, D.; Chen, S.; Song, L.; Ma, D.; Wang, Y.; Alshareef, H.N.; et al. Heteroatom-Mediated Interactions between Ruthenium Single Atoms and an MXene Support for Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1903841. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, Z.; Ji, G.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, X.; Cui, H. 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B 2019, 246, 12–20. [Google Scholar] [CrossRef]
- Su, T.; Peng, R.; Hood, Z.D.; Naguib, M.; Ivanov, I.N.; Keum, J.K.; Qin, Z.; Guo, Z.; Wu, Z. One-Step Synthesis of Nb2O5/C/Nb2C (MXene) Composites and Their Use as Photocatalysts for Hydrogen Evolution. ChemSusChem 2018, 11, 688–699. [Google Scholar] [CrossRef]
- Pan, L.; Mei, H.; Liu, H.; Pan, H.; Zhao, X.; Jin, Z.; Zhu, G. High-efficiency carrier separation heterostructure improve the photocatalytic hydrogen production of sulfide. J. Alloys Compd. 2020, 817, 153242. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Dall’Agnese, C.; Wang, X.-F. Eosin Y-sensitized partially oxidized Ti3C2 MXene for photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 310–315. [Google Scholar] [CrossRef]







| No. | Photocatalysts | Light Source | Reaction Temp. | Scavenger | Reactant Medium | H2 Production Rate (μmol·gcat−1·h−1) | Ref/(Year) |
|---|---|---|---|---|---|---|---|
| 1 | TiO2 nanofibers/ Ti3C2Tx nanosheets (3 wt %) | 300 W Xe lamp | Room temperature (RT) | Methanol | CH3OH/H2O (l, 1:9) | 6979 | [67]/2019 |
| 2 | TiO2 nanofibers | 1831 | |||||
| 3 | Ti3C2Tx nanosheets | ND | |||||
| 4 | F–Ti3C2Tx/TiO2 hybrids | 350 W Xe arc lamp | RT | Glycerin | C3H8O3/H2O (l, 1:9) | 127.1 | [37]/2019 |
| 5 | OH–Ti3C2Tx/TiO2 hybrids | 61.4 | |||||
| 6 | CdS (CT0) | 300 W Xe arc lamp: λ ≥ 420 nm; 80 mW·cm−2 | RT | Lactic acid | C3H6O3/H2O (l, 17.6:62.4) | 105 | [61]/2017 |
| 7 | Ti3C2Tx nanoparticles | ND | |||||
| 8 | 0.05 wt % Ti3C2Tx nanoparticles/CdS (CT0.05) | 993 | |||||
| 9 | 0.1 wt % Ti3C2Tx nanoparticles/CdS (CT0.1) | 1278 | |||||
| 10 | 2.5 wt % Ti3C2Tx nanoparticles/CdS (CT2.5) | 14,342 | |||||
| 11 | 5 wt %Ti3C2Tx nanoparticles/CdS (CT5) | 3377 | |||||
| 12 | Pt/CdS | 10,978 | |||||
| 13 | NiS/CdS | 12,953 | |||||
| 14 | Ni/CdS | 8649 | |||||
| 15 | MoS2/CdS | 6183 | |||||
| 16 | Ti3C2Tx nanosheets modified Zr–MOFs (UiO-66-NH2) | 350 W Xe lamp | RT | S2−/SO32− | 0.1 M Na2S and 0.1 M Na2SO3 | 204 | [82]/2019 |
| 17 | 2 wt % Pt/UiO-66-NH2 | 123 | |||||
| 18 | UiO-66-NH2 | 25.6 | |||||
| 19 | Zn2In2S5/Ti3C2Tx hybrids | 300 W Xe arc lamp: λ ≥ 420 nm; | RT | S2−/SO32− | 0.35 M Na2S and 0.25 M Na2SO3 | 2596.8 | [92]/2019 |
| 20 | Ti3C2Tx/TiO2/UiO-66-NH2 hybrid | 300 W Xe lamp (PerkinElmer): 350 < λ < 780 nm | 5 °C | S2−/SO32− | 0.1 M Na2S and 0.1 M Na2SO3 | 1980 | [88]/2019 |
| 21 | Ti3C2Tx/UiO-66-NH2 | 1320 | |||||
| 22 | UiO-66-NH2 | 942.9 | |||||
| 23 | MoxS@TiO2@Ti3C2Tx composite | 300 W Xe arc lamp: an AM1.5 filter; 180 mW·cm−2 within a range of 200–1200 nm. | 25 °C | Triethanolamine (TEOA) | TEOA in aqueous acetone | 10505.8 | [46]/2020 |
| 24 | Cu/TiO2@Ti3C2Tx | 300W Xe lamp (CEL-HXF 300E) | RT | Methanol | CH3OH/H2O (l, 1:14) | 764 | [89]/ 2018 |
| 25 | TiO2@Ti3C2Tx | 65 | |||||
| 26 | 1T–MoS2 nanopatch/Ti3C2Tx/TiO2 nanosheet | 300 W Xe arc lamp: an AM1.5 filter; 180 mW·cm−2 within a range of 200–1200 nm. | 25 °C | TEOA | TEOA/Acetone/H2O (l, 1:3:16) | 9738 | [90]/2019 |
| 27 | Ti3C2Tx/TiO2 nanosheet | 898 | |||||
| 28 | TiO2 nanosheet | 74 | |||||
| 29 | 1T–WS2@TiO2@ Ti3C2Tx | 300 W Xe arc lamp: an AM-1.5 filter | 25 °C | TEOA | TEOA/Acetone/H2O (l, 1:3:16) | 3409.8 | [91]/2019 |
| 30 | TiO2 | 67.8 | |||||
| 31 | ternary Cu2O/(001) TiO2@Ti3C2Tx | 300 W Xe lamp (CEL-HXF 300E) | RT | Methanol | CH3OH/H2O (l, 1:14) | 1496 | [92]/2019 |
| 32 | (001) TiO2@ Ti3C2Tx | 165 | |||||
| 33 | Ti3C2Tx@TiO2@MoS2 composites | 300 W Xe arc lamp (CELHXF300): an AM1.5 filter | 25 °C | TEOA | TEOA in aqueous acetone | 6425.3 | [95]/2019 |
| 34 | Ti3C2Tx@TiO2 | 898.1 | |||||
| 35 | TiO2/Ti3C2Tx/CoS | 300 W Xe arc lamp | RT | Methanol | CH3OH/H2O (l, 1:4) | 950 | [95]/2019 |
| 36 | TiO2 | 140 | |||||
| 37 | CoS | 10 | |||||
| 38 | TiO2/Ti3C2Tx | 330 | |||||
| 39 | TiO2/CoS | 540 | |||||
| 40 | g–C3N4/Ti3C2Tx/Pt | 300 W Xe arc lamp | RT | TEOA | TEOA/H2O (l, 1:9) | 5100 | [45]/2018 |
| 41 | g–C3N4/Ti3C2Tx | 1700 | |||||
| 42 | g–C3N4/Pt | 1275 | |||||
| 43 | g–C3N4@Ti3C2Tx quantum dots | 300 W Xe arc lamp (CELHXF300): an AM-1.5 filter | RT | TEOA | TEOA/H2O (l, 3:17) | 5111.8 | [55]/2019 |
| 44 | g–C3N4 | 196.8 | |||||
| 45 | Pt/g–C3N4 | 1896.4 | |||||
| 46 | Ti3C2Tx/O-doped g–C3N4 | 300 W Xe lamp | RT | TEOA | TEOA (l) | 25,124 | [87]/2019 |
| 47 | O-doped g–C3N4 | 13,745 | |||||
| 48 | Ti3C2Tx/g–C3N4 | 15,573 | |||||
| 49 | Ti3C2Tx/TiO2/g–C3N4 nanocomposites | 300 W Xe lamp: λ > 420 nm | 25 °C | TEOA | TEOA/H2O (l, 2:17) | 1620 | [93]/2018 |
| 50 | g–C3N4 | 670 | |||||
| 51 | CdLa2S4/Ti3C2Tx nanocomposite | 300 W Xe lamp: a high-pass filter (λ > 420 nm) | RT | S2−/SO32− | 0.35 M Na2S and 0.25 M Na2SO3 | 11,182.4 | [81]/2019 |
| 52 | Pt/CdLa2S4 | 1734.7 | |||||
| 53 | CdLa2S4 | 832 | |||||
| 54 | Ti3C2Tx | ND | |||||
| 55 | CdS nanorod/ Ti3C2Tx nanosheet | 300 W Xe lamp (PerkinElmer): a cut-off filter (λ > 420 nm) | 6 °C | Lactic acid | C3H6O3/H2O (l, 1:9) | 2407 | [79]/2019 |
| 56 | CdS nanorod | 360 | |||||
| 57 | ZnS nanoparticles/Ti3C2Tx nanosheets | 300 W Xe lamp | RT | Lactic acid | C3H6O3/H2O (l, 1:4) | 502.6 | [80]/2019 |
| 58 | ZnS nanoparticles | 124.6 | |||||
| 59 | ZnO nanorods /Ti3C2Tx hybrids | 300 W Xe lamp: λ > 420 nm | RT | Ethanol | C2H5OH/H2O (l, 3:16) | 456 | [68]/2020 |
| 60 | ZnO nanorods | ND | |||||
| 61 | CdS/MoS2/Ti3C2Tx composites | 300 W Xe lamp (CELHXF300): a cut-off filter (λ > 420 nm) | RT | S2−/SO32− | 0.25 M Na2S and 0.35 M Na2SO3 | 9679 | [94]/2019 |
| 62 | plasma-Ti3C2Tx/CdS hybrids | 300 W arc Xe lamp (PLSSXE300): a UV cut-off filter (λ > 420 nm); | RT | Lactic acid | C3H6O3/H2O (l, 1:9) | 825 | [62]/2019 |
| 63 | Ti3C2Tx/CdS hybrids | 473 | |||||
| 64 | g–C3N4/plasma-Ti3C2Tx | 350 W Xe lamp: a UV cut-off filter (λ > 400 nm); 70 mW·cm−2 | RT | TEOA | TEOA/H2O (l, 1:9) | 17.8 | [63]/2020 |
| 65 | g–C3N4/Ti3C2Tx | 7.5 | |||||
| 66 | g–C3N4 | 0.7 | |||||
| 67 | TiO2/Ti3C2Tx@AC-48 h composite | 350 W Xe lamp (AHD 350): a cut-off filter (λ > 400 nm) | RT | Ascorbic acid (AA) | 29 mg·mL−1 AA with the sensitization of 1 mM EY in aqueous solution | 33.4 | [98]/2019 |
| 68 | 1% Pt/TiO2 | 0.7 | |||||
| 69 | TiO2/Ti3C2Tx@AC-48 h composite | 29 mg·mL−1 AA in aqueous solution | 0.3 |
| No. | Photocatalysts | Light Source | Reaction Temp. | Scavenger | Reactant Medium | H2 Production Rate (μmol·gcat−1·h−1) | Ref./(Year) |
|---|---|---|---|---|---|---|---|
| 1 | Ti3C2Tx/O-doped g–C3N4 | 300 W Xe lamp | RT | TEOA | TEOA (l) | 25,124 | [87]/2019 |
| 2 | CdLa2S4/Ti3C2Tx nanocomposite | 300 W Xe lamp: a high-pass filter (λ > 420 nm) | RT | S2−/SO32− | 0.35 M Na2S and 0.25 M Na2SO3 | 11,182.4 | [81]/2019 |
| 3 | 2.5 wt % Ti3C2Tx nanoparticles/CdS (CT2.5) | 300 W Xe arc lamp: λ ≥ 420 nm; 80 mW·cm−2 | RT | Lactic acid | C3H6O3/H2O (l, 17.6:62.4) | 14,342 | [61]/2017 |
| 4 | Nb2O5/C/Nb2CTx Composites | 200 W Hg lamp: λ = 285–325 nm; 120 mW·cm−2 | 25 °C | Methanol | CH3OH/H2O (l, 1:3) | 7.81 | [96]/2018 |
| 5 | Zn0.5Cd0.5S/Ti2C/TiO2 | 300 W Xe lamp: λ ≥ 400 nm; | RT | S2−/SO32− | 0.3 M Na2S and 0.3 M Na2SO3 | 32,560 | [97]/2020 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.-H.; Nguyen, B.-S.; Hu, C.; Nguyen, C.C.; Nguyen, D.L.T.; Nguyen Dinh, M.T.; Vo, D.-V.N.; Trinh, Q.T.; Shokouhimehr, M.; Hasani, A.; et al. Novel Architecture Titanium Carbide (Ti3C2Tx) MXene Cocatalysts toward Photocatalytic Hydrogen Production: A Mini-Review. Nanomaterials 2020, 10, 602. https://doi.org/10.3390/nano10040602
Nguyen V-H, Nguyen B-S, Hu C, Nguyen CC, Nguyen DLT, Nguyen Dinh MT, Vo D-VN, Trinh QT, Shokouhimehr M, Hasani A, et al. Novel Architecture Titanium Carbide (Ti3C2Tx) MXene Cocatalysts toward Photocatalytic Hydrogen Production: A Mini-Review. Nanomaterials. 2020; 10(4):602. https://doi.org/10.3390/nano10040602
Chicago/Turabian StyleNguyen, Van-Huy, Ba-Son Nguyen, Chechia Hu, Chinh Chien Nguyen, Dang Le Tri Nguyen, Minh Tuan Nguyen Dinh, Dai-Viet N. Vo, Quang Thang Trinh, Mohammadreza Shokouhimehr, Amirhossein Hasani, and et al. 2020. "Novel Architecture Titanium Carbide (Ti3C2Tx) MXene Cocatalysts toward Photocatalytic Hydrogen Production: A Mini-Review" Nanomaterials 10, no. 4: 602. https://doi.org/10.3390/nano10040602
APA StyleNguyen, V.-H., Nguyen, B.-S., Hu, C., Nguyen, C. C., Nguyen, D. L. T., Nguyen Dinh, M. T., Vo, D.-V. N., Trinh, Q. T., Shokouhimehr, M., Hasani, A., Kim, S. Y., & Le, Q. V. (2020). Novel Architecture Titanium Carbide (Ti3C2Tx) MXene Cocatalysts toward Photocatalytic Hydrogen Production: A Mini-Review. Nanomaterials, 10(4), 602. https://doi.org/10.3390/nano10040602