Hydrothermal SiO2 Nanopowders: Obtaining Them and Their Characteristics
Abstract
:1. Introduction
2. Materials and Methods
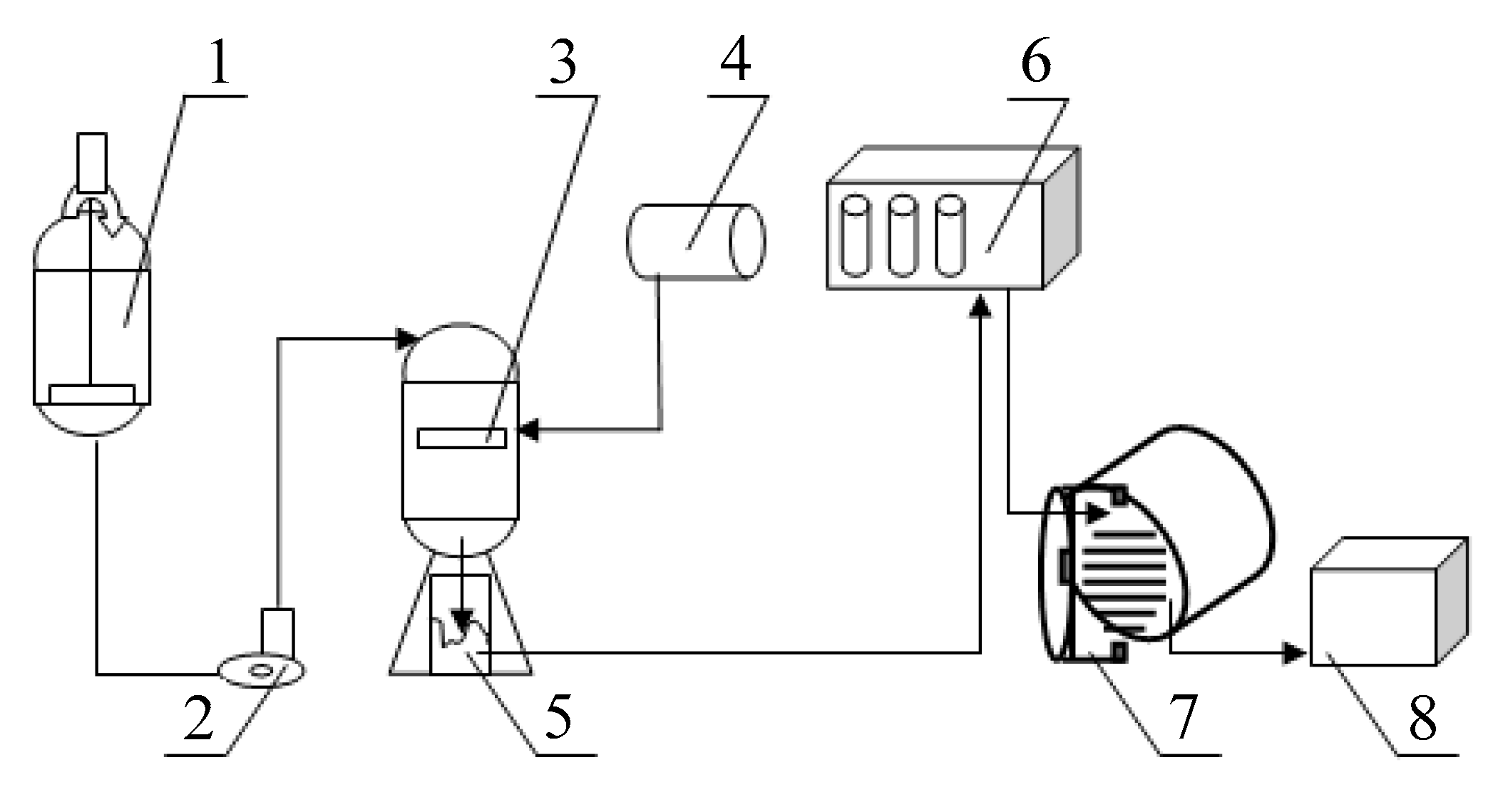
2.1. Methods for Producing Nanopowders
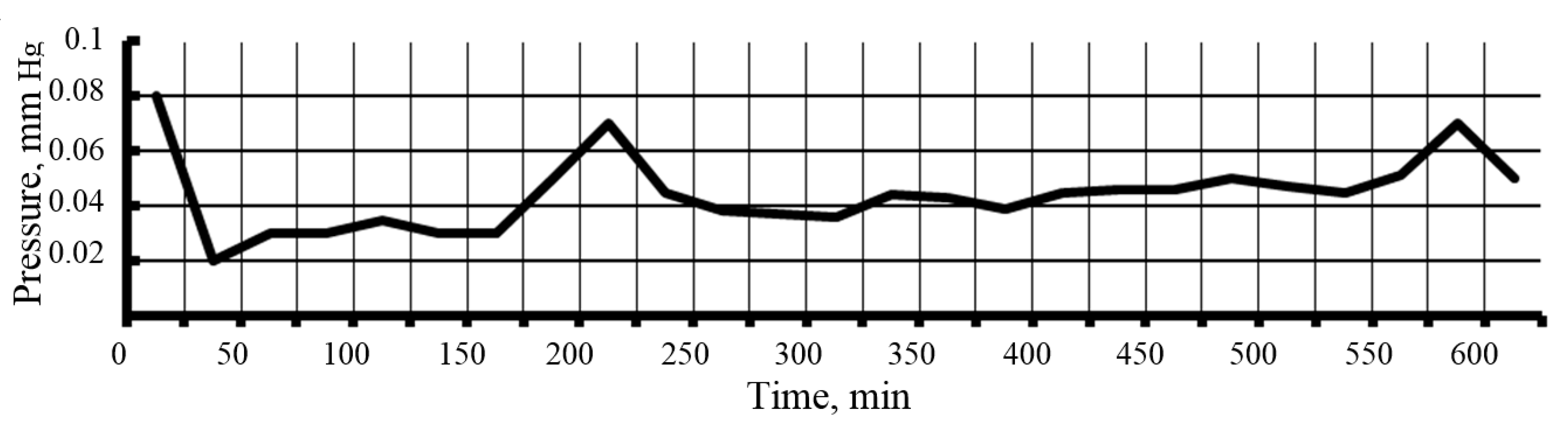
2.2. Research Methods
3. Results
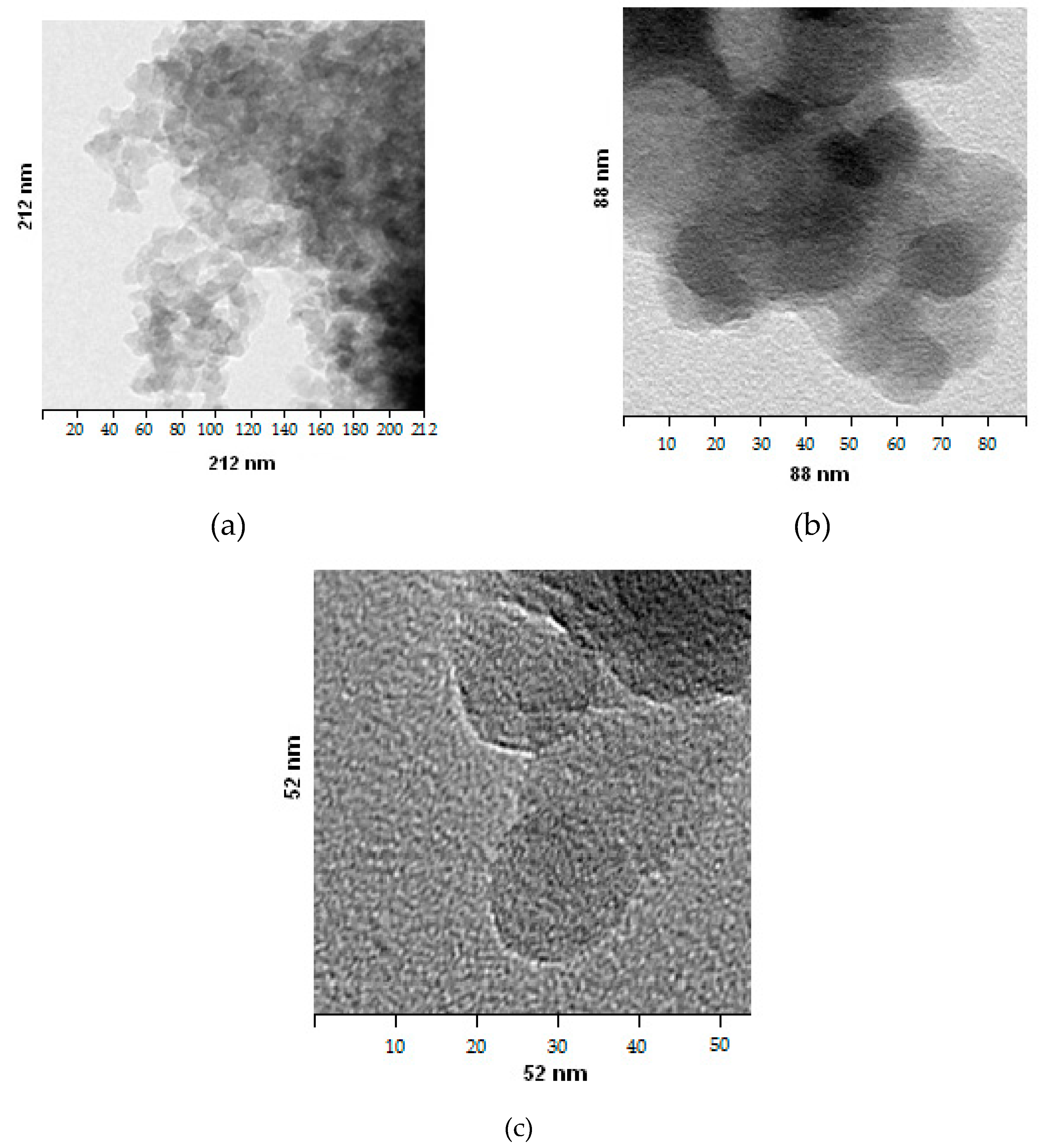
3.1. SEM Images
3.2. Pour Density of SiO2 Nanopowders
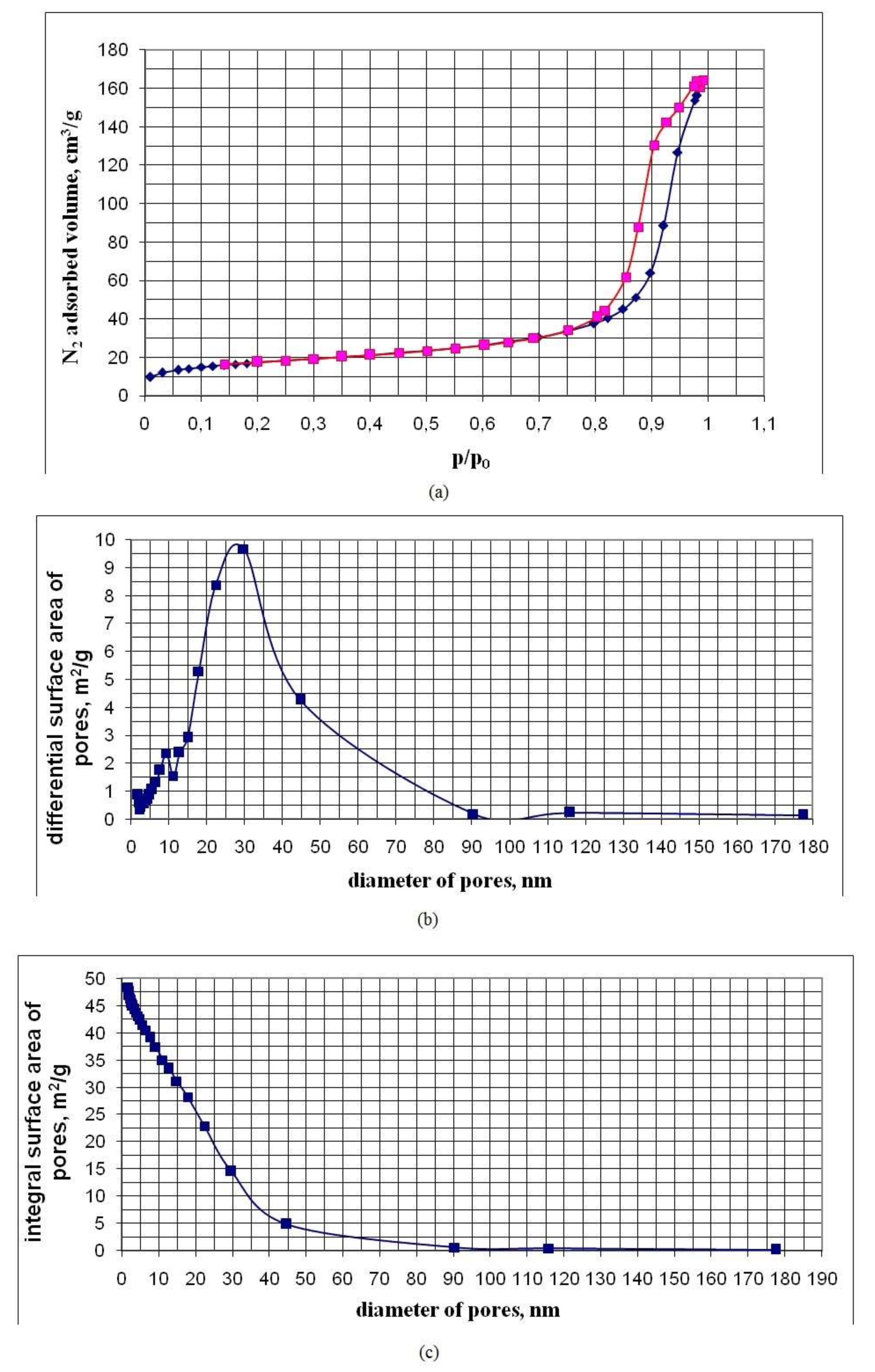
3.3. Pore Characteristics of Nanopowders Obtained by Cryochemical Vacuum Sublimation of SiO2 Sols
3.4. The XRD Data and Small Angle X-ray Scattering
3.5. The Limits of the Content of Impurity Components in Nanopowders
3.6. Evaluation of the Density of Surface Silanol Groups of Si-OH
3.7. Experiments with Compacted SiO2 Nanopowders
4. Prospects for Research and Applications of Hydrothermal Nanopowders SiO2
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Synthetic Amorphous Silicon Dioxide (NM-200, NM-201, NM-202, NM-203, NM-204): Characterisation and Physico-Chemical Properties. JRC Repository: NM-series of Representative Manufactured Nanomaterials. Joint Research Centre: Ispra, Italy. Available online: http:www.jrc.ec.europa.eu (accessed on 27 March 2020).
- Smith, D.M.; Scherer, G.W.; Anderson, J.M. Shrinkage during drying of silica gel. Non-Cryst. Solids 1995, 188, 191–206. [Google Scholar] [CrossRef]
- Ru, Y.; Guangyan, Z.; Min, L.; Nan, J. Obtaining and properties of powdered SiO2 in the form of nanoparticles prepared by drying under supercritical conditions. Chin. Ceram. Soc. 2005, 33, 281–286. [Google Scholar]
- Titulaer, M.K.; Jansen JB, H.; Geus, J.W. Fluid composition on silica gel aging. Non-Cryst. Solids 1994, 170, 11–20. [Google Scholar] [CrossRef]
- Wijnen, P.W.; Beelen, T.P.; Rummens, K.P.; Saeijs, H.C.; De Haan, J.W.; Van De Ven, L.J.; A Van Santen, R. The molecular basis of aging of aqueous silica gel. J. Colloid Interface Sci. 1991, 145, 17–32. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Costa, R.; Leite CA, P.; Galembeck, F. Size dependence of Stober silica nanoparticle microchemistry. Phys. Chem. B 2003, 107, 4747–4755. [Google Scholar] [CrossRef]
- Grun, M.; Unger, K.K.; Matsumoto, A.; Tatsumi, K. Novel pathways for the preparation of mesoporous MCM-41 materials: Control of porosity and morphology. Micropor. Mesopor. Mater. 1999, 27, 207–216. [Google Scholar] [CrossRef]
- Schumacher, K.; Grun, M.; Unger, K.K. Novel synthesis of spherical MCM-48. Micropor. Mesopor. Mater. 1999, 27, 201–206. [Google Scholar] [CrossRef]
- Scholz, S.; Bare, S.R.; Kelly, S.D.; Lercher, J.A. Controlled one-step synthesis of hierarchically structured macroscopic silica spheres. Microporous Mesoporous Mater. 2011, 146, 18–27. [Google Scholar] [CrossRef]
- Edler, K.; White, W. Further improvements in the long-range order of MCM-41 materials. Chem. Mater. 1997, 9, 1226–1233. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Ohkubo, T.; Ogura, T.; Sakai, H.; Abe, M. Synthesis of highly-ordered mesoporous silica particles using mixed cationic and anionic surfactants as templates. J. Colloid Interface Sci. 2007, 312, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, S.B.; Kim, J.Y.; Chae, Y.B.; Yu, J.S. Synthhesis of monodisperse silica spheres with solid core and mesoporous shell: Morphological control of mesopores. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 77–81. [Google Scholar] [CrossRef]
- Firouzi, A.; Kumar, D.; Bull, L.; Besier, T.; Sieger, P.; Huo, Q.; Walker, S.; Zasadzinski, J.; Glinka, C.; Nicol, J.; et al. Cooperative organization of inorganic-surfactant and biomimetic assemblies. Science 1995, 267, 1138–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Ikari, K.; Imai, H. Synthesis of Silica Nanoparticles Having a Well-Ordered Mesostructure Using a Double Surfactant System. J. Am. Chem. Soc. 2004, 126, 462–463. [Google Scholar] [CrossRef]
- Grün, M.; Lauer, I.; Unger, K.K. The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv. Mater. 1997, 9, 254–257. [Google Scholar] [CrossRef]
- Fu, X.; He, X.; Wang, Y. Facile preparation of silica hollow microspheres by precipitation-phase separation method. Colloids Surf. A Phys. Eng. Asp. 2011, 380, 241–249. [Google Scholar] [CrossRef]
- Guo, W.; Luo, G.; Wang, Y. A new emulsion method to synthesize well-defined mesoporous particles. J. Colloid Interface Sci. 2004, 271, 400–406. [Google Scholar] [CrossRef]
- Chen, F.; Song, F.; Li, Q. Mixed cationic–anionic templating route to Al-MCM-48. Micropor. Mesopor. Mater. 1999, 29, 305–310. [Google Scholar] [CrossRef]
- Yang, H.; Vovk, G.; Coombs, N.; Sokolov, I.; Ozin, G.A. Synthesis of mesoporous silica spheres under quiescent aqueous acidic conditions. J. Mater. Chem. 1998, 8, 743–750. [Google Scholar] [CrossRef]
- Guo, W.; Goh, D.-C.; Zhao, X.S. Synthesis of super-microporous organosilica microspheres through in situ self-assembly of nanoparticles. J. Mater. Chem. 2005, 15, 4112. [Google Scholar] [CrossRef]
- Qi, L.; Ma, J.; Cheng, H.; Zhao, Z. Micrometer-Sized Mesoporous Silica Spheres Grown under Static Conditions. Chem. Mater. 1998, 10, 1623–1626. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, J.; Li, Q.; Stucky, G.D. Morphological Control of Highly Ordered Mesoporous Silica SBA-15. Chem. Mater. 2000, 12, 275–279. [Google Scholar] [CrossRef]
- Nemoto, N.; Kuwahara, M. Dynamic light scattering of CTAB/sodium salicylate long threadlike micelles in the semidilute regime: Applicability of the dynamic scaling law. Langmuir 1993, 9, 419–423. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, L.; Ma, J.; Wu, Y.; Liu, O.; Cheng, H. Large-pore mesoporous silica spheres: Synthesis and application in HPLC. Colloids Surf. A Phys. Eng. Asp. 2003, 229, 1–8. [Google Scholar] [CrossRef]
- Eiden-Assmann, S.; Lindlar, B.; Maret, G. Synthesis and characterization of colloidal fluorescent mesoporous silica particles. J. Colloid Interface Sci. 2004, 271, 120–123. [Google Scholar] [CrossRef]
- Allouche, J.; Dupin, J.-C.; Gonbeau, D. Generation of a mesoporous silica MSU shell onto solid core silica nanoparticles using a simple two-step sol–gel process. Chem. Commun. 2011, 47, 7476. [Google Scholar] [CrossRef]
- Ho, J.; Zhu, W.; Wang, H.; Forde, G.M. Mesoporous silica spheres from colloids. J. Colloid Interface Sci. 2007, 308, 374–380. [Google Scholar] [CrossRef]
- Yuying, P.U.; Jianzhang FA, N.G.; Feng PE, N.G.; Baojian, L.I.; Huang, L. Microemulsion synthesis of nanosized SiO2/TiO2 particles and their photocatalytic activity. Chin. J. Catal. 2007, 28, 251–256. [Google Scholar]
- Yao, T.; Lin, Q.; Zhang, K.; Zhao, D.; Lv, H.; Zhang, J.; Yang, B. Preparation of SiO2 polystyrene–polypyrrole sandwich composites and hollow polypyrrole capsules with movable SiO2 spheres inside. J. Colloid Interface Sci. 2007, 315, 434–438. [Google Scholar] [CrossRef]
- Yoldas, B.E. Monolithic glass formation by chemical polymerization. J. Mater. Sci. 1979, 14, 1843–1849. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhengyi, F.; Wang, H.; Wang, W.; Zhang, J.; Lee, S.W.; Niihara, K. Transparent mullite ceramic from single-phase gel by Spark Plasma Sintering. J. Eur. Ceram. Soc. 2009, 29, 2705–2711. [Google Scholar] [CrossRef]
- Innocenzi, P.; Martucci, A.; Guglielmi, M.; Bearzotti, A.; Traversa, E.; Pivin, J.C. Mesoporous silica thin films for alcohol sensors. J. Eur. Ceram. Soc. 2001, 21, 1985–1988. [Google Scholar] [CrossRef]
- Paul, J.; Romeis, S.; Mačković, M.; Marthala, V.R.R.; Herre, P.; Przybilla, T.; Hartmann, M.; Spiecker, E.; Schmidt, J.; Peukert, W. In situ cracking of silica beads in the SEM and TEM—Effect of particle size on structure–property correlations. Powder Technol. 2015, 270, 337–347. [Google Scholar] [CrossRef]
- Bagheri, E.; Ansari, L.; Abnous, K.; Taghdisi, S.M.; Charbgoo, F.; Ramezani, M.; Alibolandi, M. Silica based hybrid materials for drug delivery and bioimaging. J. Control. Release 2018, 277, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yoon, H. Novel Fabrication of Size-Tunable Silica Nanotubes Using a Reverse- Microemulsion-Mediated Sol–Gel Method. Adv. Mater. 2004, 16, 799–802. [Google Scholar] [CrossRef]
- Loganina, V.I.; Kislitsyna, S.N.; Mazhitov, Y.B.; Ivanovna, L.V.; Nikolaevna, K.S.; Bisengalievich, M.Y. Development of sol-silicate composition for decoration of building walls. Case Stud. Constr. Mater. 2018, 9. [Google Scholar] [CrossRef]
- Kao, K.-C.; Lin, C.-H.; Chen, T.-Y.; Liu, Y.-H.; Mou, C.-Y. A General Method for Growing Large Area Mesoporous Silica Thin Films on Flat Substrates with Perpendicular Nanochannels. J. Am. Chem. Soc. 2015, 137, 3779–3782. [Google Scholar] [CrossRef]
- Chęcmanowski, J.; Szczygieł, I.; Mazur, A.; Szczygiel, B. Protective properties of SiO2 with SiO2 and Al2O3 nanoparticles sol-gel coatings deposited on FeCrAl alloys. Ceram. Int. 2019, 45, 2811–2819. [Google Scholar] [CrossRef]
- Matysiak, W.; Tański, T. Analysis of the morphology, structure and optical properties of 1D SiO2 nanostructures obtained with sol-gel and electrospinning methods. Appl. Surf. Sci. 2019, 489, 34–43. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Sheng, L.; Ma, M.; Xu, Q. Influence of nanosilica on inner structure and performance of chitosan based films. Carbohydr. Polym. 2019, 212, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Sowntharya, L.; Gundakaram, R.C.; Raju, K.S.; Subasri, R. Effect of addition of surface modified nanosilica into silica–zirconia hybrid sol–gel matrix. Ceram. Int. 2013, 39, 4245–4252. [Google Scholar] [CrossRef]
- Cai, Y.; Li, X.; Dong, J. Microstructure and mechanical properties of porous Si3N4–SiO2 ceramics fabricated by a process combining carbothermal reduction and sol–gel infiltration–sintering. Mater. Sci. Eng. A 2014, 601, 111–115. [Google Scholar] [CrossRef]
- Pronin, I.; Goryacheva, M.V. Principles of structure formation and synthesis models produced by the sol–gel method SiO2–MexOy nanocomposites. Surf. Coat. Technol. 2013, 235, 835–840. [Google Scholar] [CrossRef]
- Asadi, Z.; Norouzbeigi, R. Synthesis of colloidal nanosilica from water glass powder as a low cost precursor. Ceram. Int. 2018, 44, 22692–22697. [Google Scholar] [CrossRef]
- Bakar, R.A.; Yahya, R.; Gan, S.N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Weres, O.; Yee, A.; Tsao, L. Kinetics of silica polymerization. J. Colloid Interface Sci. 1981, 84, 379–402. [Google Scholar] [CrossRef] [Green Version]
- Potapov, V.V.; Cerdan, A.A.; Kashutina, I.A. Numerical simulation of orthosilicic acid polycondensation and silica particles formation inhydrothermal solutions. Вулканoлoгия и сейсмoлoгия 2019, 4, 18–28. [Google Scholar] [CrossRef]
- Potapov, V.V.; Kamashev, D.V. Synthesis of precious opal in a hydrothermal solution. Glas. Phys. Chem. 2006, 32, 89–98. [Google Scholar] [CrossRef]
- Beaucage, G. Small-Angle Scattering from Polymeric Mass Fractals of Arbitrary Mass-Fractal Dimension. J. Appl. Cryst. 1996, 29, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Kammler, H.K.; Beaucage, G.; Mueller, R.; Pratsinis, S.E. Structure of Flame-Made Silica Nanoparticles by Ultra-Small-Angle X-ray Scattering. Langmuir 2004, 20, 1915–1921. [Google Scholar] [CrossRef]
- Kammler, H.K.; Beaucage, G.; Kohls, D.J.; Agashe, N.; Ilavsky, J. Monitoring simultaneously the growth of nanoparticles and aggregates by in situ ultra-smallangle X-ray scattering. Appl. Phys. 2005, 97, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Hyeon-Lee, J.; Beaucage, G.; Pratsinis, S.E.; Vemury, S. Fractal Analysis of Flame-Synthesized Nanostructured Silica and Titania Powders Using Small-Angle X-ray Scattering. Langmuir 1998, 14, 5751–5756. [Google Scholar] [CrossRef]
- Bushell, G.; Yan, Y.; Woodfield, D.; Raper, J.; Amal, R. On techniques for the measurement of the mass fractal dimension of aggregates. Adv. Colloid Interface Sci. 2002, 95, 1–50. [Google Scholar] [CrossRef]
- Brasil, A.M.; Farias, T.L.; Carvalho, M.G. A recipe for image characterization of fractal-like aggregates. J. Aerosol Sci. 1999, 30, 1379–1389. [Google Scholar] [CrossRef]
- De Temmerman, P.-J.; Van Doren, E.; Verleysen, E.; Van der Stede, Y.; Francisco, M.A.D.; Mast, J. Quantitative characterization of agglomerates and aggregates of pyrogenic and pprecipitated amorphous silica nanomaterials by transmission electron microscopy. J. Nanobiotechnol. 2012, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Boldridge, D. Morphological Characterization of Fumed Silica Aggregates. Aerosol Sci. Technol. 2010, 44, 182–186. [Google Scholar] [CrossRef]
- Nel, A.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Chu, Z.; Huang, Y.; Tao, Q.; Li, Q. Cellular uptake, evolution, and excretion of silica nanoparticles in human cells. Nanoscale 2011, 3, 3291–3299. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Powers, K.W.; Brown, S.C.; Krishna, V.B.; Wasdo, S.C.; Moudgil, B.M.; Roberts, S.M. Research Strategies for Safety Evaluation of Nanomaterials. Part VI. Characterization of Nanoscale Particles for Toxicological Evaluation. Toxicol. Sci. 2006, 90, 296–303. [Google Scholar] [CrossRef]
- Roebben, G.; Rasmussen, K.; Kestens, V.; Linsinger, T.P.J.; Rauscher, H.; Emons, H.; Stamm, H. Reference materials and representative test materials: The nanotechnology case. J. Nanoparticle Res. 2013, 15, 1455–1468. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.V.; Zhuravlev, L.T. Temperature Dependence of the Concentration of Silanol Groups in Silica Precipitated from a Hydrothermal Solution. Glas. Phys. Chem. 2005, 31, 661–670. [Google Scholar] [CrossRef]
- Zhuravlev, L. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Phys. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Potapov, V.V. Physical and chemical processes of nanosilica precipitation from hydrothermal solution. Theor. Found. Chem. Technol. 2003, 37, 1–9. [Google Scholar]
- Potapov, V.V. Method of Utilization of Geothermal Silica for Liquid Glass Production. Patent of Russian Federation on Invention No. 2186025, 25 December 2000. [Google Scholar]
- Potapov, V.V. Method of Precipitaion of Nanosilica from Hydrothermal Heat Carrier with Production of Metal’s Silicates. Patent of Russian Federation on Invention No. 2259318, 8 August 2003. [Google Scholar]
- Sobolev, K.; Gutiérrez, M.F.; Society, T.A.C. How Nanotechnology Can Change the Concrete World. Prog. Nanotechnol. 2014, 10, 117–120. [Google Scholar]
- Sanchez, F.; Sobolev, K. Nanotechnology in Concrete—A Review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Flores-Vivian, I.; Pradoto, R.G.K.; Moini, M.; Kozhukhova, M.; Potapov, V.; Sobolev, K. The effect of SiO2 nanoparticles derived from hydrothermal solutions on the performance of portland cement based materials. Front. Struct. Civ. Eng. 2017, 11, 436–445. [Google Scholar] [CrossRef]
- Fediuk, R. Composite Binders for Concretes with Improved Shock Resistance. Inorg. Mater. 2019, 10, 1177–1184. [Google Scholar] [CrossRef]
- Svintsov, A.P.; Shchesnyak, E.L.; Galishnikova, V.V.; Fediuk, R.; Stashevskaya, N.A. Effect of nano-modified additives on properties of concrete mixtures during winter season. Constr. Build. Mater. 2020, 237, 117527. [Google Scholar] [CrossRef]
- Artamonova, O.; Slavcheva, G.; Chernyshov, E.M. Effectiveness of combined nanoadditives for cement systems. Inorg. Mater. 2017, 53, 1080–1085. [Google Scholar] [CrossRef]
- Elistratkin, M.Y.; Minakov, S.V.; Shatalova, S.V. Composite binding mineral additive influence on the plasticizer efficiency. Constr. Mater. Prod. 2019, 2, 10–16. [Google Scholar]
- Potapov, V.; Efimenko, Y.; Gorev, D. Modification of concrete by hydrothermal nanosilica. Nanotechnologies Constr. A. 2019, 11, 248–265. [Google Scholar]
- Potapov, V.; Efimenko, Y.; Gorev, D. Determination of the amount of Ca(OH)2 bound by additive nano-SiO2 in cement matrices. Nanotechnol. Constr. A 2019, 11, 415–432. [Google Scholar] [CrossRef]
- Potapov, V.V.; Kashutin, A.N. Method of Increasing of Concrete’s Compressive Strength by Using of Nanosilica Recovered from Hydrothermal Solution. Patent of Russian Federation on Invention No. 259739, 5 August 2015. [Google Scholar]
- Potapov, V.V.; Revina, A.A.; Baranova, E.K. The optical properties of nanodisperse silica in hydrothermal solutions. Russ. J. Phys. Chem. A 2008, 82, 1002–1009. [Google Scholar] [CrossRef]
- Revina, A.A.; Potapov, V.V.; Baranova, E.K.; Smirnov, Y.V. Research of interaction of nanosilica and metall’s nanoparticles by the method of spectrophotometry. Phys. Chem. A 2013, 87, 262–269. [Google Scholar]
- Zelenkov, V.N.; Potapov, V.V. Hydrothermal nanosilica in agricultural and crop bitechnology. Nanoindustry 2020, 1, 22–33. [Google Scholar]
- Zelenkov, V.N.; Petrichenko, V.N.; Potapov, V.V.; Eliseeva, L.G.; Ivanova, M.I.; Latushkin, V.V.; Novikov, V.B. Verification of the complex preparation of hydrothermal nanosilica with krezacin for hydroponic growing of lettuce in a closed system of the ITS-1 phytotron. Curr. Biotechnol. 2018, 3, 378. [Google Scholar]
- Zelenkov, V.N.; Petrichenko, V.N.; Potapov, V.V. Method of Hydrothermal Nanosilica Using for Production of Lettuce in Agricultural Techonological Systems. Patent of Russian Federation on Invention No. 2701495, 11 December 2018. [Google Scholar]
- Zelenkov, V.N.; Ivanova, M.I.; Potapov, V.V. Hydrothermal Nanosilica in the Agrotechnology of Radish Cultivated in the Conditions of Low Positive Temperature. In Proceedings of the AIP Conference, Yekaterinburg, Russia, 15–17 November 2018. [Google Scholar]
- Lapin, A.A.; Kalayda, M.L.; Potapov, V.V.; Zelenkov, V.N.; Voropaeva, N.L. The influence of hydrothermal nanosilica powder aquaspersions on the vital capacity of Daphnia Magna Straus Crustataceans. Int. J. Nanotechnol. 2018, 15, 422–432. [Google Scholar]
- Zelenkov, V.N.; Potapov, V.V. Method of Inhibition of Mealy Dew of Tomatoes. Patent of Russian Federation on Invention No. 2646058, 6 July 2017. [Google Scholar]
- Potapov, V.V.; Sivashenko, V.N.A.; Zelenkov, V.N. Nanodispersed silicon dioxide: Plant growing and veterinary science. Nanoindustry 2013, 4, 18–25. [Google Scholar]
- Potapov, V.V. Method of Hydrothermal Nanosilica Using as Food Additive. Patent RU on Invention No. 2638322, 12 December 2016. [Google Scholar]
- Potapov, V.V. Method of Amorphous Hydrothermal Nanosilica Using in Poultry-Keeping. Patent RU on Invention No. 2655739, 5 June 2017. [Google Scholar]
- Potapov, V.V.; Muradov, S.V.; Sivashenko, V.A.; Rogatyh, S.V. Nanodispersed silicon dioxide: Applications in medicine and veterinary. Nanoindustry 2012, 3, 32–36. [Google Scholar]






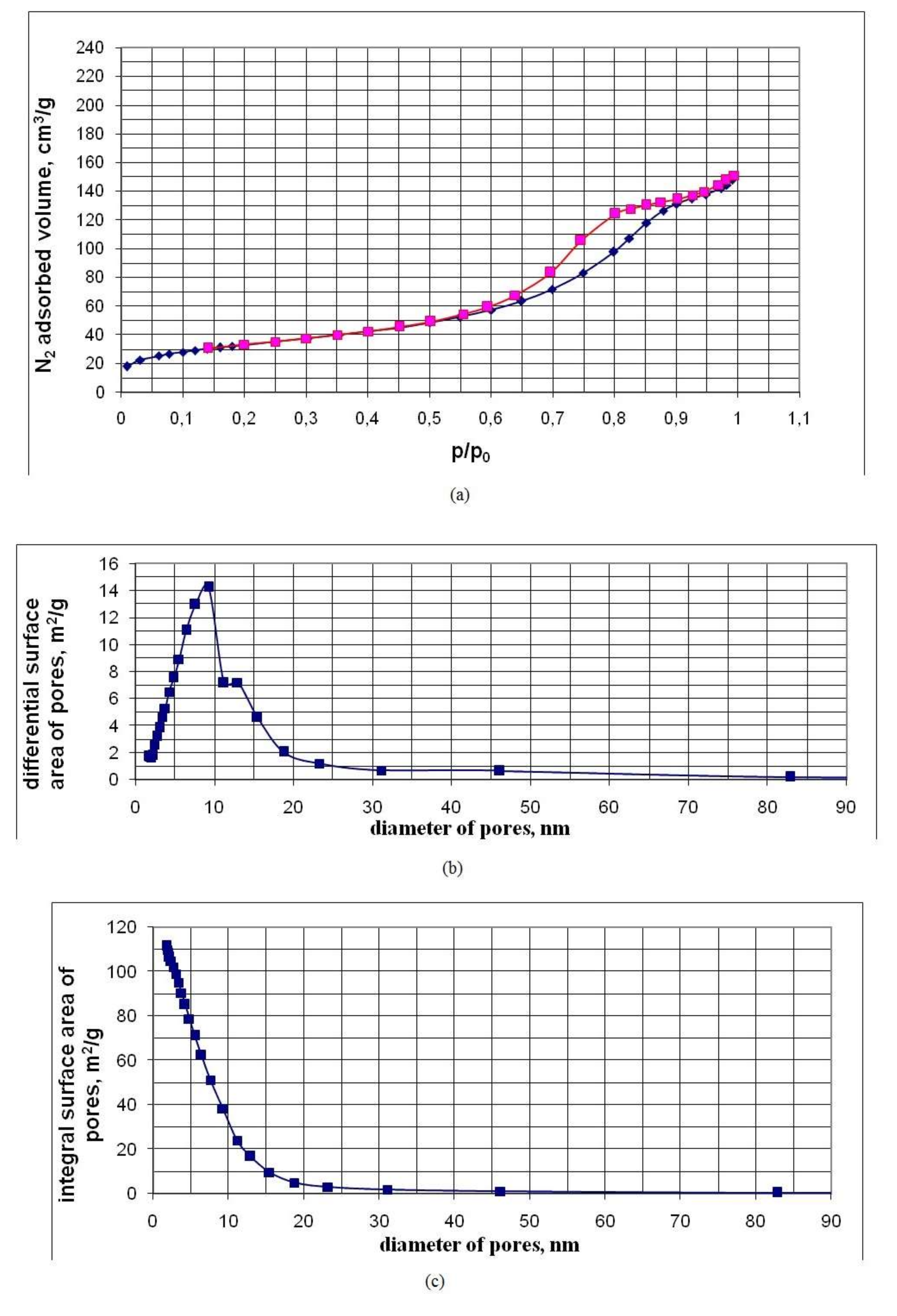



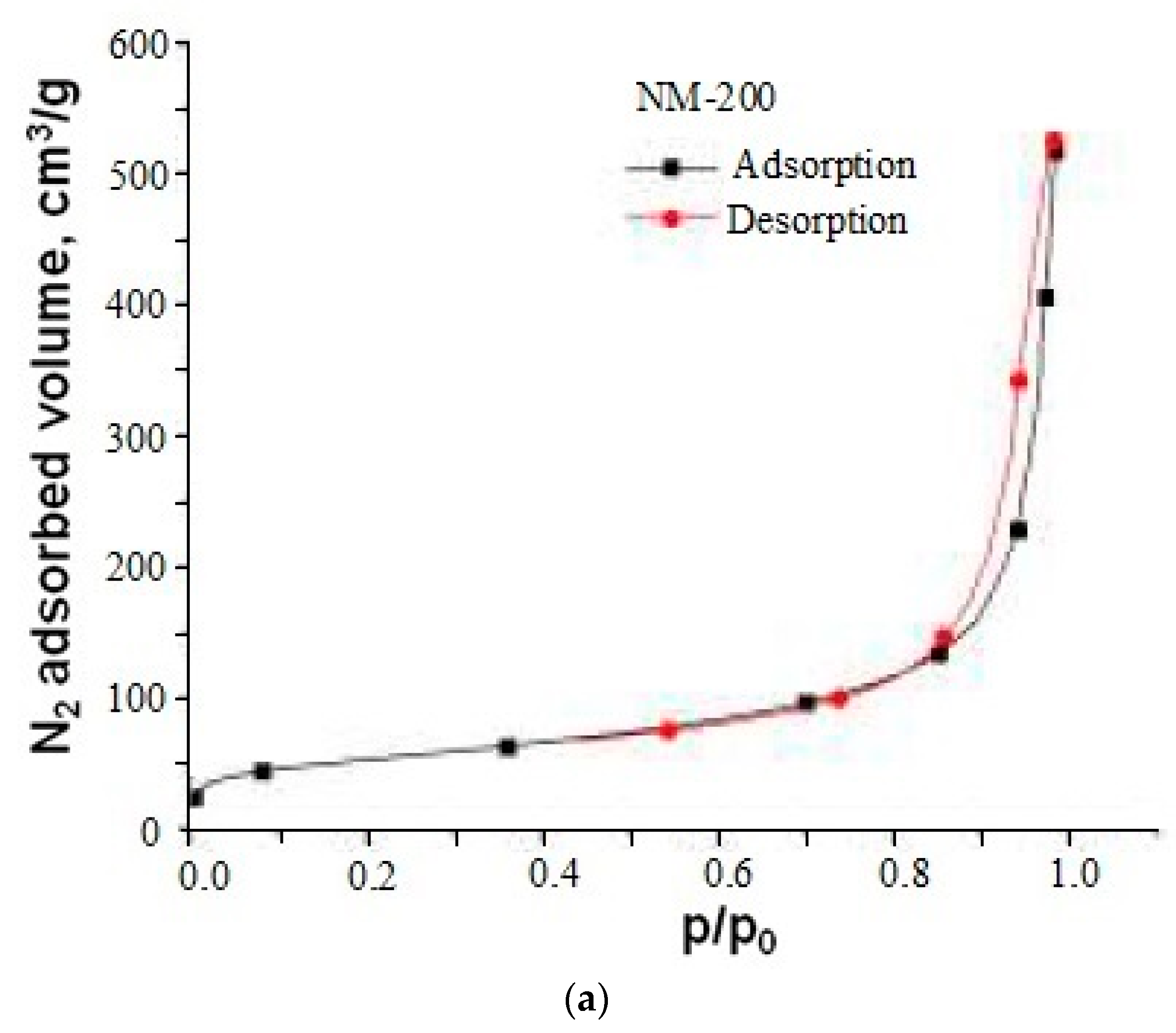

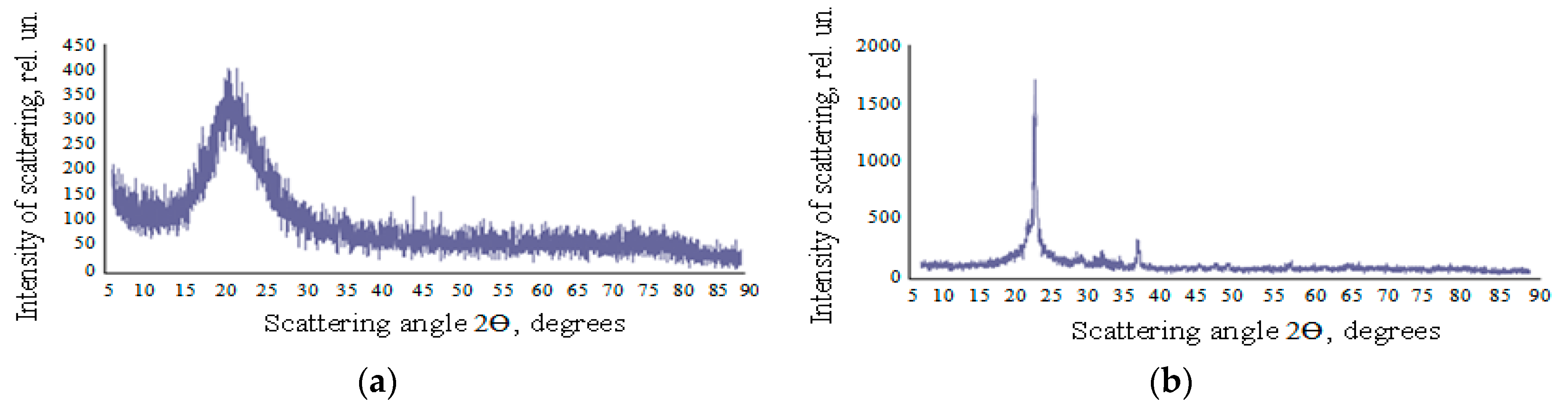


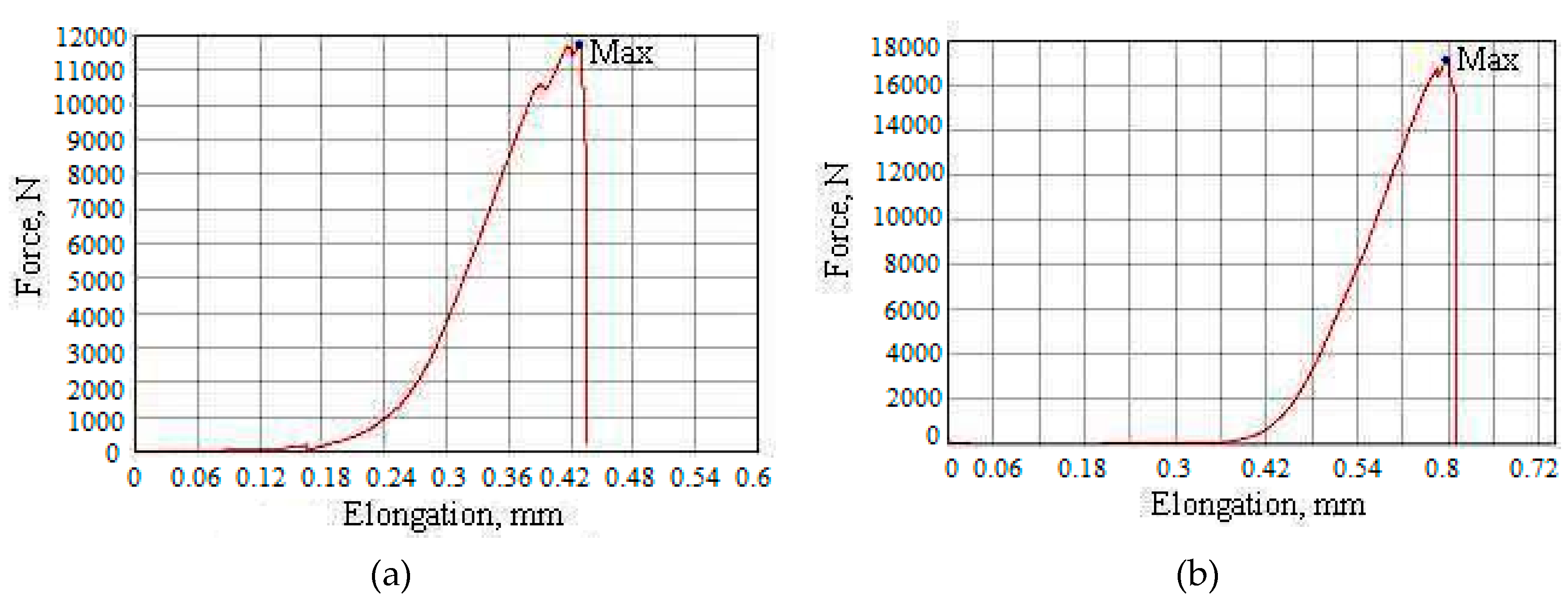
| Component | Na+ | K+ | Li+ | Ca2+ | Mg2+ | Fe2+, 3+ | Al3+ | Cl– | SO42 | HCO3– | CO32– | H3BO3 | SiO2 total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration, mg/dm3 | 282 | 48.1 | 1.5 | 2.8 | 4.7 | < 0.1 | < 0.1 | 251.8 | 220.9 | 45.2 | 61.8 | 91.8 | 780 |
| [SiO2],g/dm3 | 2.4 | 5.2 | 6.93 | 10.4 | 17.56 | 32 | 65.85 | 120 | 131.7 | 160 | 260 | 520 |
| ρp, g/dm3 | 20.5 | 29 | 35 | 43.8 | 55 | 55 | 84 | 91 | 100 | 117 | 168 | 274 |
| ρp/[SiO2] | 8.5 | 5.6 | 5.05 | 4.21 | 3.13 | 1.71 | 1.27 | 0.76 | 0.76 | 0.73 | 0.65 | 0.53 |
| Sample ID | [SiO2], g/dm3 | ρp, g/dm3 | SBET, m2/g | Pore area by adsorption curve (BJH), SBET,,m2/g | Pore area by desorption curve (BJH), m2/g | Single point pore volume, vp,cm3/g | Pore volume by adsorption curve (BJH), cm3/g | Pore volume by desorption curve (BJH), cm3/g | dBET,nm | Average pore diameter, dp, nm | Average pore diameter by adsorption curve, nm | Average pore diameter by desorption curve, nm | Area of micropores, m2/g | Volume of micropores, cm3/g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UF-1-9 | 128.0 | 86 | 45.4 | 35.6 | 37.7 | 0.10 | 0.23 | 0.237 | 60 | 9.4 | 26.4 | 25.1 | 2.18 | d.n. |
| UF-2-32 | 233.8 | 229 | 56.8 | 47.0 | 51.8 | 0.15 | 0.19 | 0.19 | 48.0 | 10.9 | 16.6 | 15.2 | 5.6 | 0.001 |
| UF-3-8 | 24.4 | 52 | 62.0 | 48.3 | 58.0 | 0.19 | 0.24 | 0.25 | 44.0 | 12.6 | 20.5 | 17.3 | 11.4 | 0.004 |
| UF-4-34 | 586.9 | 344 | 74.0 | 63.9 | 69.7 | 0.18 | 0.19 | 0.20 | 36.8 | 10.0 | 12.4 | 11.6 | 5.0 | 0.001 |
| UF-5-25 | 108.9 | 52 | 97.7 | 78.8 | 90.4 | 0.22 | 0.26 | 0.27 | 27.9 | 9.4 | 13.5 | 11.9 | 13.9 | 0.005 |
| UF-6-26 | 114.5 | 90 | 120.4 | 111.4 | 121.2 | 0.21 | 0.22 | 0.23 | 22.6 | 7.0 | 8.2 | 7.6 | 8.9 | 0.002 |
| UF-7-17 | 28.0 | 35 | 166.5 | 151.4 | 162.1 | 0.25 | 0.28 | 0.28 | 16.4 | 6.2 | 7.5 | 7.1 | 8.2 | 0.001 |
| UF-8-21 | 14.0 | 15.7 | 200.8 | 158.1 | 166.6 | 0.20 | 0.22 | 0.23 | 13.6 | 4.0 | 5.8 | 5.5 | 10.8 | 0.001 |
| UF-9-43 | 170.9 | 231.7 | 209.9 | 199.6 | 239.1 | 0.21 | 0.20 | 0.22 | 13.0 | 4.0 | 4.0 | 4.0 | 0.1 | d.n. |
| UF-10-3 | 82.5 | 90.0 | 316.0 | 272.1 | 289.9 | 0.243 | 0.216 | 0.221 | 8.6 | 3.0 | 3.2 | 3.0 | d.n. | d.n. |
| UF-11-20 | 33.2 | 58 | 360.4 | 256.9 | 280.8 | 0.301 | 0.280 | 0.290 | 7.56 | 3.3 | 4.2 | 4.1 | 33.8 | 0.010 |
| UF-12-16 | 66.0 | 86 | 476.3 | 354.3 | 367.1 | 0.32 | 0.26 | 0.27 | 5.72 | 2.70 | 3.0 | 2.94 | 0.1 | d.n. |
| Sample ID | ρp, g/dm3 | SBET, m2/g | Pore Volume, cm3/g | Area of Micropores, m2/g | Volume of Micropores, cm3/g |
|---|---|---|---|---|---|
| NM-200 | 120.0 | 189.1 | 0.79 | 30.0 | 0.01181 |
| NM-201 | 280.0 | 140.4 | 0.581 | 23.1 | 0.00916 |
| NM-202 | 130.0 | 204.1 | 0.513 | 8.26 | 0.00084 |
| NM-203 | 30.0 | 203.9 | 0.499 | 5.3 | 0.0 |
| NM-204 | 160.0 | 136.6 | 0.50 | 17.48 | 0.00666 |
| Oxides | Concentration, wt.% |
|---|---|
| SiO2 | 99.7 |
| TiO2 | 0.00 |
| Al2O3 | 0.173 |
| FeO | 0.00 |
| Cr2O3 | 0.00 |
| MgO | 0.00 |
| CaO | 0.034 |
| Na2O | 0.034 |
| K2O | 0.069 |
| MnO | 0.00 |
| NiO | 0.00 |
| ZnO | 0.00 |
| Total | 100.0 |
| 22.6 °C | 100 °C | 200 °C | 300 °C | 400 °C | 500 °C | 600 °C | 700 °C | 800 °C | 900 °C | 1000 °C | 1100 °C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100% | 94.65% | 92.81% | 92.10% | 91.30% | 90.58% | 90.09% | 89.76% | 89.49% | 89.27% | 89.09% | 88.61% |
| T, °C | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 |
| δOH, OH/nm2 | 8.29 | 6.71 | 4.92 | 3.33 | 2.23 | 1.49 | 0.89 | 0.40 |
| αOH, OH/nm2 | 4.90 | 3.56 | 2.33 | 1.84 | 1.52 | 1.30 | 0.70 | 0.40 |
| γOH, OH/nm2 | 3.39 | 3.15 | 2.59 | 1.49 | 0.71 | 0.19 | 0.19 | 0.0 |
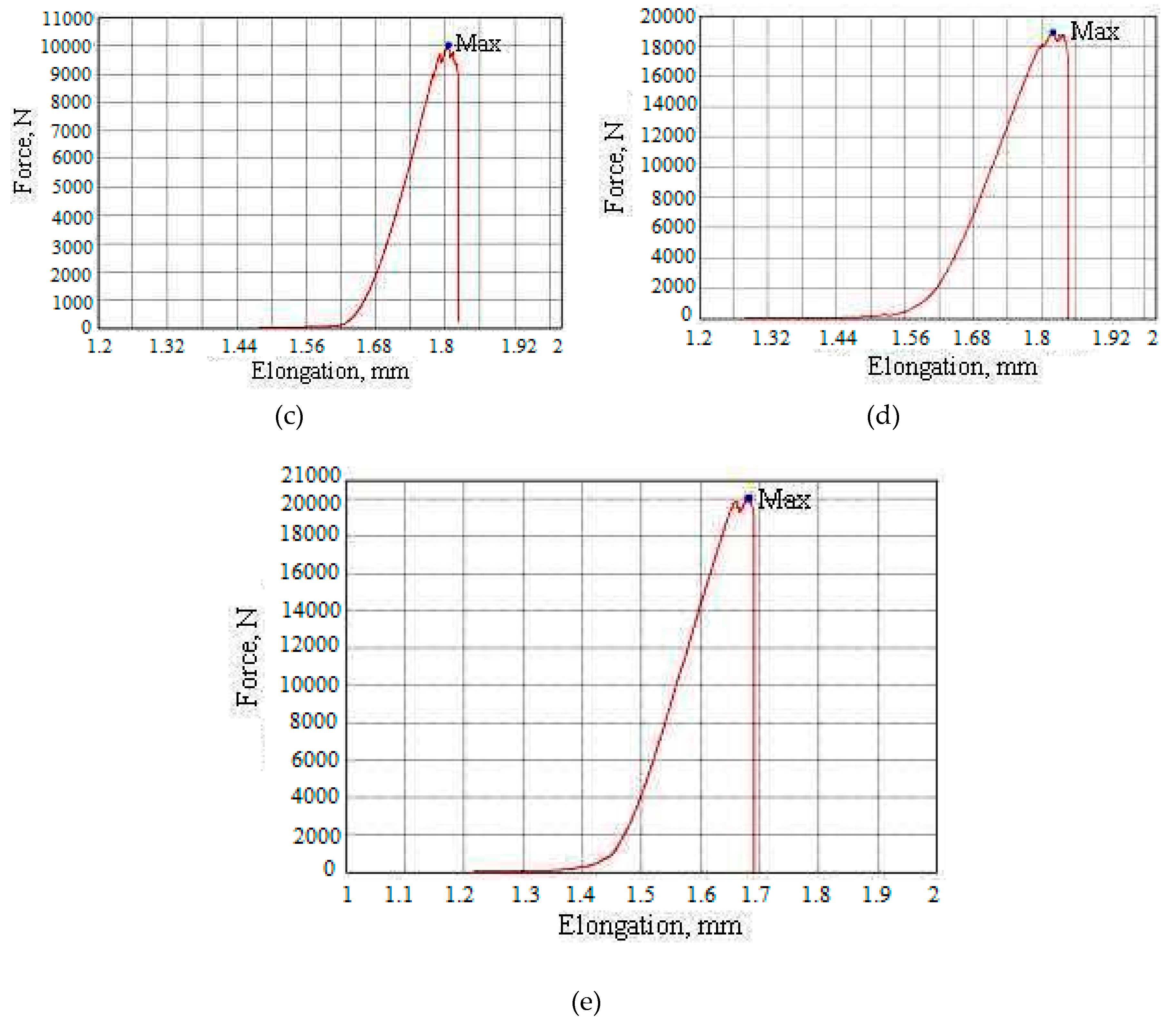
| Sample ID | Speed, mm/min | Shape | Dimensions (Thickness × Width × Height), mm | Maximum Force, N | Maximum Strain, N/mm2 | Amplitude of the Stroke, mm | Maximum Elongation, % | Maximum Elongation, mm | Maximum Time, s |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | plane | 4.9 × 11.9 × 3.3 | 11735.4 | 201.259 | 0.42831 | 12.9792 | 0.42831 | 25.7 |
| 2 | 1 | plane | 4.9 × 11.9 × 3.6 | 17145.4 | 294.040 | 0.65710 | 18.2529 | 0.65710 | 39.46 |
| 3 | 1 | plane | 5.5 × 13.5 × 3.1 | 10032.2 | 135.114 | 1.80619 | 58.2641 | 1.80619 | 108.370 |
| 4 | 1 | plane | 5.1 × 12.0 × 3.5 | 18897.6 | 308.784 | 1.81967 | 51.9905 | 1.81967 | 109.170 |
| 5 | 1 | plane | 5.0 × 11.9 × 2.9 | 20057.6 | 337.102 | 1.68196 | 57.9986 | 1.68196 | 100.950 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, V.; Fediuk, R.; Gorev, D. Hydrothermal SiO2 Nanopowders: Obtaining Them and Their Characteristics. Nanomaterials 2020, 10, 624. https://doi.org/10.3390/nano10040624
Potapov V, Fediuk R, Gorev D. Hydrothermal SiO2 Nanopowders: Obtaining Them and Their Characteristics. Nanomaterials. 2020; 10(4):624. https://doi.org/10.3390/nano10040624
Chicago/Turabian StylePotapov, Vadim, Roman Fediuk, and Denis Gorev. 2020. "Hydrothermal SiO2 Nanopowders: Obtaining Them and Their Characteristics" Nanomaterials 10, no. 4: 624. https://doi.org/10.3390/nano10040624
APA StylePotapov, V., Fediuk, R., & Gorev, D. (2020). Hydrothermal SiO2 Nanopowders: Obtaining Them and Their Characteristics. Nanomaterials, 10(4), 624. https://doi.org/10.3390/nano10040624






