Systemic Review of Biodegradable Nanomaterials in Nanomedicine
Abstract
1. Introduction
2. Literature Search Methods
3. Types of Biodegradable Nanoparticles
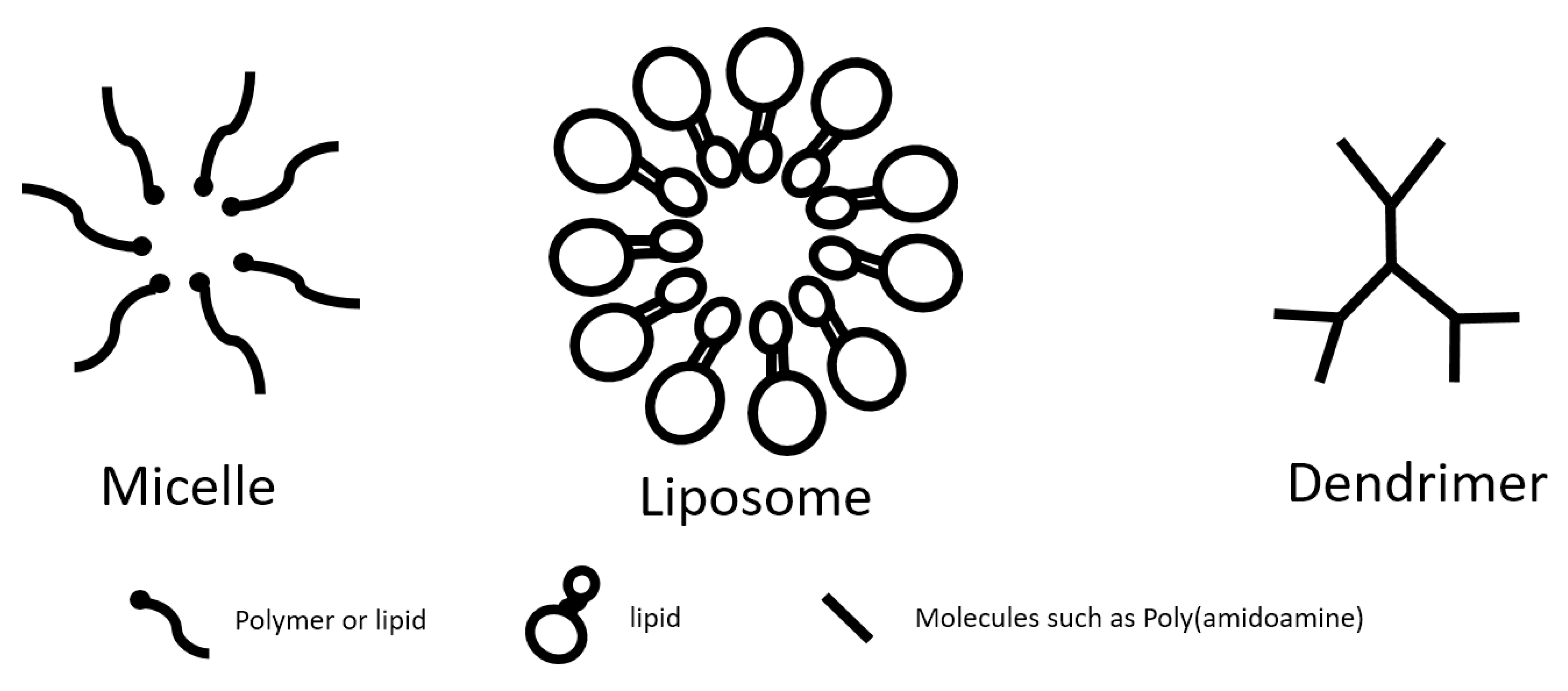
3.1. Micelles
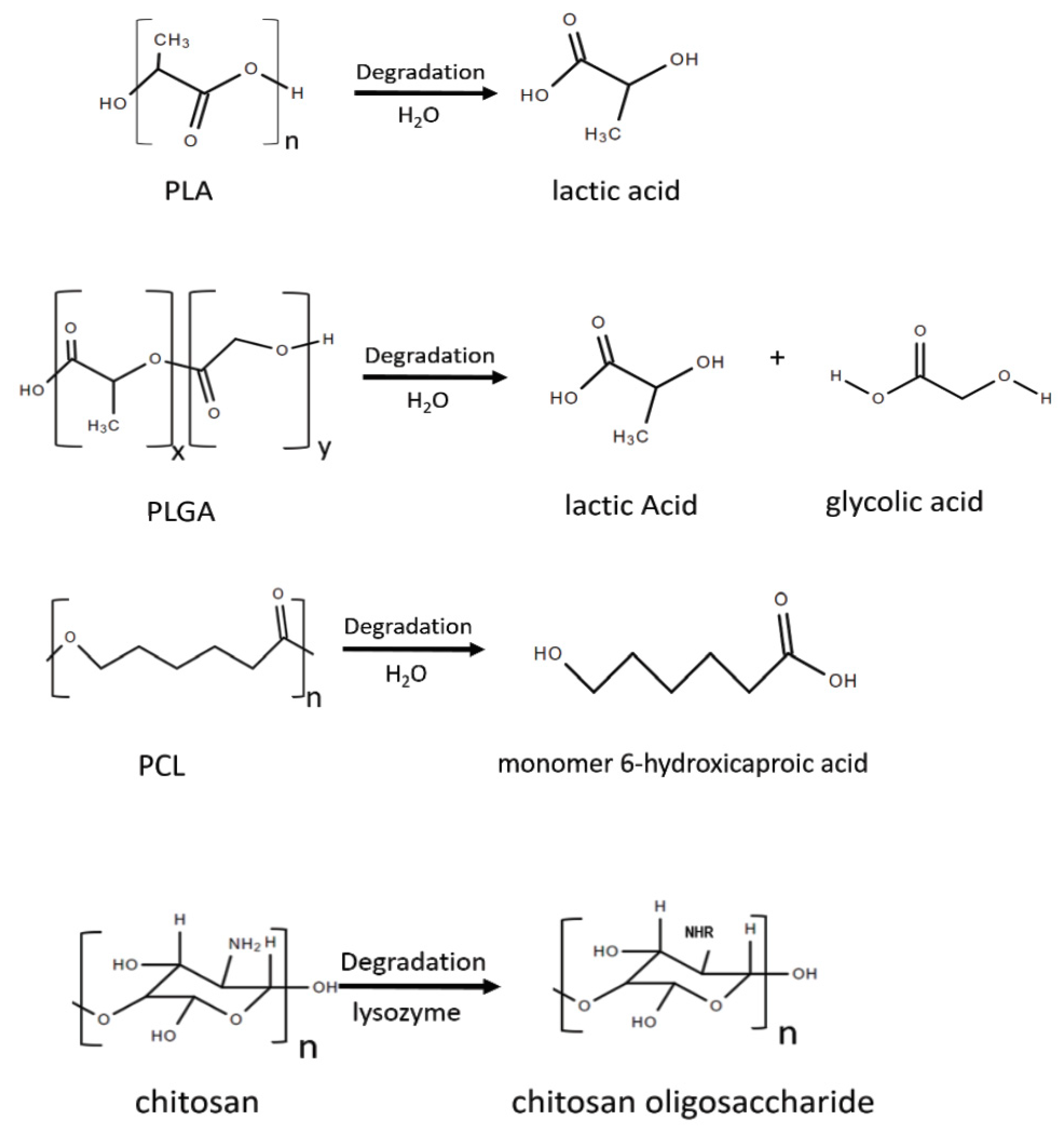
3.1.1. Polylactic Acid (PLA) Micelles
3.1.2. Polylactic-Co-Glycolic Acid (PLGA) Micelles
3.1.3. Modification of Micelles
3.2. Poly-ε-Caprolactone (PCL) Nanoparticles
3.3. Chitosan Nanoparticles
3.4. Dendrimers
3.5. Lipid-Based Nanoparticles
3.6. Other Natural Materials
4. Applications of Biodegradable Nanoparticles
4.1. Imaging
4.2. Theranostics
4.3. Targeted Delivery System
4.3.1. Antioxidant Delivery
4.3.2. Gene Therapy
4.3.3. Oral Drug Delivery
4.4. Implantable Device with Biodegradable Materials
5. Current Status of Biodegradable Nanomaterials and Challenges Ahead
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rossi, M.; Cubadda, F.; Dini, L.; Terranova, M.L.; Aureli, F.; Sorbo, A.; Passeri, D. Scientific Basis of Nanotechnology, Implications for the Food Sector and Future Trends. Trends Food Sci. Technol. 2014, 40, 127–148. [Google Scholar] [CrossRef]
- Salinas, F.M.; Smith, D.M.; Viswanathan, S. Nanotechnology: Ethical and Social Issues. Nanotechnol. Ethical Soc. Implic. 2012, 125–153. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The Present and Future of Nanotechnology in Human Health Care. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The New Perspective in Precision Agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C.; Mirkin, C.A.; Hersam, M.C. Nanotechnology Research Directions for Societal Needs in 2020: Summary of International Study. J. Nanopart. Res. 2011, 13, 897–919. [Google Scholar] [CrossRef]
- Mehta, D.; Guvva, S.; Patil, M. Future Impact of Nanotechnology on Medicine and Dentistry. J. Indian Soc. Periodontol. 2008, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Biodegradable Nanoparticles for Drug Delivery and Targeting. Surf. Modif. Nanopart. Target. Drug Deliv. 2019, 167–181. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA Micro- and Nano-Particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhu, Q. Recent Advances of PLGA Micro/Nanoparticles for the Delivery of Biomacromolecular Therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-Caprolactone (PCL), a Promising Polymer for Pharmaceutical and Biomedical Applications: Focus on Nanomedicine in Cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers (Basel) 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable Nanoparticles Are Excellent Vehicle for Site Directed In-Vivo Delivery of Drugs and Vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
- Villemin, E.; Ong, Y.C.; Thomas, C.M.; Gasser, G. Polymer Encapsulation of Ruthenium Complexes for Biological and Medicinal Applications. Nat. Rev. Chem. 2019, 3, 261–282. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Amaral, C.; Veiga, F.; Figueiras, A. Polymeric Micelles as a Versatile Tool in Oral Chemotherapy. Des. Dev. New Nanocarr. 2018, 293–329. [Google Scholar] [CrossRef]
- Chandarana, M.; Curtis, A.; Hoskins, C. The Use of Nanotechnology in Cardiovascular Disease. Appl. Nanosci. 2018, 8, 1607–1619. [Google Scholar] [CrossRef]
- Nabar, G.M.; Mahajan, K.D.; Calhoun, M.A.; Duong, A.D.; Souva, M.S.; Xu, J.; Czeisler, C.; Puduvalli, V.K.; Otero, J.J.; Wyslouzil, B.E.; et al. Micelle-Templated, Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Hydrophobic Drug Delivery. Int. J. Nanomed. 2018, 13, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Yadav, T.P.; Pandey, B.; Gupta, V.; Singh, S.P. Engineering Nanomaterials for Smart Drug Release. Appl. Target. Nano Drugs Deliv. Syst. 2019, 411–449. [Google Scholar] [CrossRef]
- Husseini, G.A.; Pitt, W.G. Micelles and Nanoparticles for Ultrasonic Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Synthesis and Production of Poly(Lactic Acid). Polylactic Acid 2013, 71–107. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle Arrays as Transdermal and Intradermal Drug Delivery Systems: Materials Science, Manufacture and Commercial Development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(Lactic Acid) Blends in Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Da Silva, J.; Jesus, S.; Bernardi, N.; Colaço, M.; Borges, O. Poly(D, L-Lactic Acid) Nanoparticle Size Reduction Increases Its Immunotoxicity. Front. Bioeng. Biotechnol. 2019, 7, 137. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Bronich, T.K.; Vetro, J.A.; Kabanov, A.V. Polymer Micelles as Drug Carriers. Nanopart. Drug Carr. 2006, 57–93. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, S.; Matoba, T.; Koga, J.; Nakano, K.; Egashira, K. Anti-Inflammatory Nanomedicine for Cardiovascular Disease. Front. Cardiovasc. Med. 2017, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.A.; Adhikari, R.; Gadegaard, N. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, Long-Acting PLGA Formulations: Analyzing PLGA and Understanding Microparticle Formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA Micelles and PLGA-b-PEG-b-PLGA Sol–Gels for Drug Delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA Copolymers: Their Structure and Structure-Influenced Drug Delivery Applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated Nanoparticles for Biological and Pharmaceutical Applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Trivedi, R.; Kompella, U.B. Nanomicellar Formulations for Sustained Drug Delivery: Strategies and Underlying Principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, W.; Yu, J.; Li, Y.; Zhao, C. Recent Development of PH-Responsive Polymers for Cancer Nanomedicine. Molecules 2019, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, S.; Diban, N.; Urtiaga, A. Hydrolytic Degradation and Mechanical Stability of Poly(ε-Caprolactone)/Reduced Graphene Oxide Membranes as Scaffolds for in Vitro Neural Tissue Regeneration. Membranes (Basel) 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Heimowska, A.; Morawska, M.; Bocho-Janiszewska, A. Biodegradation of Poly(ϵ-Caprolactone) in Natural Water Environments. Polish J. Chem. Technol. 2017, 19, 120–126. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, S.Y.; Zhang, X.Z.; Cheng, S.X.; Zhuo, R.X.; Li, H. Novel Solvent-Free Methods for Fabrication of Nano- And Microsphere Drug Delivery Systems from Functional Biodegradable Polymers. J. Phys. Chem. C 2007, 111, 12681–12685. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-Based Nanomedicines: A Biodegradable Drug Delivery System and Its Application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of Chitosan Micro/Nanoparticles for Pulmonary Drug Delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Mandal, K.; Jana, D.; Ghorai, B.K.; Jana, N.R. Functionalized Chitosan with Self-Assembly Induced and Subcellular Localization-Dependent Fluorescence “switch on” Property. New J. Chem. 2018, 42, 5774–5784. [Google Scholar] [CrossRef]
- Singh, B.; Garg, T.; Goyal, A.K.; Rath, G. Recent Advancements in the Cardiovascular Drug Carriers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 216–225. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials (Basel) 2019, 13, 65. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Izzo, L. Dendrimer Biocompatibility and Toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Li, C.; Li, L.; She, W.; Wang, G.; Gu, Z. Arginine Functionalized Peptide Dendrimers as Potential Gene Delivery Vehicles. Biomaterials 2012, 33, 4917–4927. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer Drug Delivery in the Nano Era: An Overview and Perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Dong, C.; Ma, A.; Shang, L. Nanoparticles for Postinfarct Ventricular Remodeling. Nanomedicine 2018, 13, 3037–3050. [Google Scholar] [CrossRef]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and Analytical Applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Mazur, F.; Bally, M.; Städler, B.; Chandrawati, R. Liposomes and Lipid Bilayers in Biosensors. Adv. Colloid Interface Sci. 2017, 249, 88–99. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Q.; Clulow, A.J.; Li, T.; Manohar, M.; Gilbert, E.P.; de Campo, L.; Hawley, A.; Boyd, B.J. PEGylation and Surface Functionalization of Liposomes Containing Drug Nanocrystals for Cell-Targeted Delivery. Colloids Surf. B Biointerfaces 2019, 182, 110362. [Google Scholar] [CrossRef]
- Feng, L.; Mumper, R.J. A Critical Review of Lipid-Based Nanoparticles for Taxane Delivery. Cancer Lett. 2013, 334, 157–175. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-Polymer Hybrid Nanoparticles: Synthesis Strategies and Biomedical Applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Lyu, S.Y.; Wen, Y.H.; Lo, C.L. Preparation and Characterization of Electrostatically Crosslinked Polymer–Liposomes in Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 1615. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent Advancement of Gelatin Nanoparticles in Drug and Vaccine Delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin Nanoparticles and Their Biofunctionalization. Nanotechnol. Life Sci. 2007. [Google Scholar] [CrossRef]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-Induced Kidney Injury: Mechanisms, Risk Factors, and Prevention. Eur. Heart J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef]
- Tam, J.M.; Tam, J.O.; Murthy, A.; Ingram, D.R.; Ma, L.L.; Travis, K.; Johnston, K.P.; Sokolov, K.V. Controlled Assembly of Biodegradable Plasmonic Nanoclusters for Near-Infrared Imaging and Therapeutic Applications. ACS Nano 2010, 4, 2178–2184. [Google Scholar] [CrossRef]
- Huang, C.H.; Nwe, K.; Al Zaki, A.; Brechbiel, M.W.; Tsourkas, A. Biodegradable Polydisulfide Dendrimer Nanoclusters as MRI Contrast Agents. ACS Nano 2012, 6, 9416–9424. [Google Scholar] [CrossRef]
- Fathi, P.; Knox, H.J.; Sar, D.; Tripathi, I.; Ostadhossein, F.; Misra, S.K.; Esch, M.B.; Chan, J.; Pan, D. Biodegradable Biliverdin Nanoparticles for Efficient Photoacoustic Imaging. ACS Nano 2019, 13, 7690–7704. [Google Scholar] [CrossRef]
- Beard, P. Biomedical Photoacoustic Imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, Biodegradable Gold Nanoparticles as Contrast Agents for Computed Tomography and Photoacoustic Imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Wei, R.; Jiang, G.; Lv, M.Q.; Tan, S.; Wang, X.; Zhou, Y.; Cheng, T.; Gao, X.; Chen, X.; Wang, W.; et al. TMTP1-Modified Indocyanine Green-Loaded Polymeric Micelles for Targeted Imaging of Cervical Cancer and Metastasis Sentinel Lymph Node in Vivo. Theranostics 2019, 9, 7325–7344. [Google Scholar] [CrossRef]
- Bonnard, T.; Jayapadman, A.; Putri, J.A.; Cui, J.; Ju, Y.; Carmichael, C.; Angelovich, T.A.; Cody, S.H.; French, S.; Pascaud, K.; et al. Low-Fouling and Biodegradable Protein-Based Particles for Thrombus Imaging. ACS Nano 2018, 12, 6988–6996. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; Jaffer, F.A.; Fayad, Z.A.; Nahrendorf, M. Imaging and Nanomedicine in Inflammatory Atherosclerosis. Sci. Transl. Med. 2014, 6, 239sr1. [Google Scholar] [CrossRef]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J.M. Perspectives and Opportunities for Nanomedicine in the Management of Atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852. [Google Scholar] [CrossRef]
- Peters, D.; Kastantin, M.; Kotamraju, V.R.; Karmali, P.P.; Gujraty, K.; Tirrell, M.; Ruoslahti, E. Targeting Atherosclerosis by Using Modular, Multifunctional Micelles. Proc. Natl. Acad. Sci. USA 2009, 106, 9815–9819. [Google Scholar] [CrossRef]
- Zhou, Z.; Qutaish, M.; Han, Z.; Schur, R.M.; Liu, Y.; Wilson, D.L.; Lu, Z.R. MRI Detection of Breast Cancer Micrometastases with a Fibronectin-Targeting Contrast Agent. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing Poly(Lactic-Co-Glycolic Acid) Particles for Biomedical Applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- Wang, S.; Kim, G.; Lee, Y.E.K.; Hah, H.J.; Ethirajan, M.; Pandey, R.K.; Kopelman, R. Multifunctional Biodegradable Polyacrylamide Nanocarriers for Cancer Theranostics-A “see and Treat” Strategy. ACS Nano 2012, 6, 6843–6851. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; ur Rehman, A. Recent Applications of PLGA Based Nanostructures in Drug Delivery. Colloids Surfaces B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Chan, J.M.W.; Wojtecki, R.J.; Sardon, H.; Lee, A.L.Z.; Smith, C.E.; Shkumatov, A.; Gao, S.; Kong, H.; Yang, Y.Y.; Hedrick, J.L. Self-Assembled, Biodegradable Magnetic Resonance Imaging Agents: Organic Radical-Functionalized Diblock Copolymers. ACS Macro Lett. 2017, 6, 176–180. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable Gold Nanovesicles with an Ultrastrong Plasmonic Coupling Effect for Photoacoustic Imaging and Photothermal Therapy. Angew. Chem.-Int. Ed. 2013, 52, 13958–13964. [Google Scholar] [CrossRef]
- Lyu, Y.; Zeng, J.; Jiang, Y.; Zhen, X.; Wang, T.; Qiu, S.; Lou, X.; Gao, M.; Pu, K. Enhancing Both Biodegradability and Efficacy of Semiconducting Polymer Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Nano 2018, 12, 1801–1810. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, C.; Jung, H.S.; Jeon, M.; Kim, K.S.; Yun, S.H.; Kim, C.; Hahn, S.K. Biodegradable Photonic Melanoidin for Theranostic Applications. ACS Nano 2016, 10, 822–831. [Google Scholar] [CrossRef]
- Fu, L.H.; Hu, Y.R.; Qi, C.; He, T.; Jiang, S.; Jiang, C.; He, J.; Qu, J.; Lin, J.; Huang, P. Biodegradable Manganese-Doped Calcium Phosphate Nanotheranostics for Traceable Cascade Reaction-Enhanced Anti-Tumor Therapy. ACS Nano 2019, 13, 13985–13994. [Google Scholar] [CrossRef]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-Printed Biodegradable Microswimmer for Theranostic Cargo Delivery and Release. ACS Nano 2019, 13, 3353–3362. [Google Scholar] [CrossRef]
- Banik, B.; Surnar, B.; Askins, B.W.; Banerjee, M.; Dhar, S. Dual-Targeted Synthetic Nanoparticles for Cardiovascular Diseases. ACS Appl. Mater. Interfaces 2020, 12, 6852–6862. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Derakhshandeh, K.; Erfan, M.; Dadashzadeh, S. Encapsulation of 9-Nitrocamptothecin, a Novel Anticancer Drug, in Biodegradable Nanoparticles: Factorial Design, Characterization and Release Kinetics. Eur. J. Pharm. Biopharm. 2007, 66, 34–41. [Google Scholar] [CrossRef]
- Onwordi, E.N.C.; Gamal, A.; Zaman, A. Anticoagulant Therapy for Acute Coronary Syndromes. Interv. Cardiol. Rev. 2018, 13, 87–92. [Google Scholar] [CrossRef]
- Takahama, H.; Shigematsu, H.; Asai, T.; Matsuzaki, T.; Sanada, S.; Fu, H.Y.; Okuda, K.; Yamato, M.; Asanuma, H.; Asano, Y.; et al. Liposomal Amiodarone Augments Anti-Arrhythmic Effects and Reduces Hemodynamic Adverse Effects in an Ischemia/ Reperfusion Rat Model. Cardiovasc. Drugs Ther. 2013, 27, 125–132. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted Therapy in Chronic Diseases Using Nanomaterial-Based Drug Delivery Vehicles. Signal Transduct. Target. Ther. 2019, 4. [Google Scholar] [CrossRef]
- Flores, A.M.; Ye, J.; Jarr, K.U.; Hosseini-Nassab, N.; Smith, B.R.; Leeper, N.J. Nanoparticle Therapy for Vascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 635–646. [Google Scholar] [CrossRef]
- Binderup, T.; Duivenvoorden, R.; Fay, F.; Van Leent, M.M.T.; Malkus, J.; Baxter, S.; Ishino, S.; Zhao, Y.; Sanchez-Gaytan, B.; Teunissen, A.J.P.; et al. Imaging-Assisted Nanoimmunotherapy for Atherosclerosis in Multiple Species. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kagan, V.E.; Fadeel, B. Close Encounters of the Small Kind: Adverse Effects of Man-Made Materials Interfacing with the Nano-Cosmos of Biological Systems. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 63–88. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Lee, D.; Bae, S.; Hong, D.; Lim, H.; Yoon, J.H.; Hwang, O.; Park, S.; Ke, Q.; Khang, G.; Kang, P.M. H2O2-Responsive Molecularly Engineered Polymer Nanoparticles as Ischemia/Reperfusion-Targeted Nanotherapeutic Agents. Sci. Rep. 2013, 3, 2233. [Google Scholar] [CrossRef]
- Bae, S.; Park, M.; Kang, C.; Dilmen, S.; Kang, T.H.; Kang, D.G.; Ke, Q.; Lee, S.U.; Lee, D.; Kang, P.M. Hydrogen Peroxide-Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003697. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Yang, J.; Gao, Y.; Kuang, Y.; Labhasetwar, V. Nanoparticles with Antioxidant Enzymes Protect Injured Spinal Cord from Neuronal Cell Apoptosis by Attenuating Mitochondrial Dysfunction. J. Control. Release 2020, 317, 300–311. [Google Scholar] [CrossRef]
- Tapeinos, C.; Larrañaga, A.; Sarasua, J.R.; Pandit, A. Functionalised Collagen Spheres Reduce H2O2 Mediated Apoptosis by Scavenging Overexpressed ROS. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2397–2405. [Google Scholar] [CrossRef]
- Kim, K.S.; Song, C.G.; Kang, P.M. Targeting Oxidative Stress Using Nanoparticles as a Theranostic Strategy for Cardiovascular Diseases. Antioxid. Redox Signal. 2019, 30, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and Clinical Development of Nanoparticles for Gene Delivery. Mol. Ther.-Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Hu, Q.; Feng, X.; Gao, X.; Menglin, J.; Kang, T.; Jiang, D.; Song, Q.; Chen, H.; Chen, J. PEG-PLA Nanoparticles Modified with APTEDB Peptide for Enhanced Anti-Angiogenic and Anti-Glioma Therapy. Biomaterials 2014, 35, 8215–8226. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in Humans from Systemically Administered SiRNA via Targeted Nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating Animal and Human Phase Ia/Ib Clinical Data with CALAA-01, a Targeted, Polymer-Based Nanoparticle Containing SiRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef]
- Voss, M.H.; Hussain, A.; Vogelzang, N.; Lee, J.L.; Keam, B.; Rha, S.Y.; Vaishampayan, U.; Harris, W.B.; Richey, S.; Randall, J.M.; et al. A Randomized Phase II Trial of CRLX101 in Combination with Bevacizumab versus Standard of Care in Patients with Advanced Renal Cell Carcinoma. Ann. Oncol. 2017, 28, 2754–2760. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers (Basel) 2019, 11, 745. [Google Scholar] [CrossRef]
- Zhang, G.; Khan, A.A.; Wu, H.; Chen, L.; Gu, Y.; Gu, N. The Application of Nanomaterials in Stem Cell Therapy for Some Neurological Diseases. Curr. Drug Targets 2018, 19, 279–298. [Google Scholar] [CrossRef]
- Díaz, A.; David, A.; Pérez, R.; González, M.L.; Báez, A.; Wark, S.E.; Zhang, P.; Clearfield, A.; Colón, J.L. Nanoencapsulation of Insulin into Zirconium Phosphate for Oral Delivery Applications. Biomacromolecules 2010, 11, 2465–2470. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional Oral Delivery Systems for Enhanced Bioavailability of Therapeutic Peptides/Proteins. Acta Pharm. Sin. B 2019, 9, 902–922. [Google Scholar] [CrossRef]
- Safari, M.; Kamari, Y.; Ghiaci, M.; Sadeghi-aliabadi, H.; Mirian, M. Synthesis and Characterization of Insulin/Zirconium Phosphate@TiO2 Hybrid Composites for Enhanced Oral Insulin Delivery Applications. Drug Dev. Ind. Pharm. 2017, 43, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of Biodegradable, Implantable Devices towards Clinical Translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Lee, D.H.; de la Torre Hernandez, J.M. The Newest Generation of Drug-Eluting Stents and Beyond. Eur. Cardiol. Rev. 2018, 13, 54–59. [Google Scholar] [CrossRef]
- Lih, E.; Kum, C.H.; Park, W.; Chun, S.Y.; Cho, Y.; Joung, Y.K.; Park, K.S.; Hong, Y.J.; Ahn, D.J.; Kim, B.S.; et al. Modified Magnesium Hydroxide Nanoparticles Inhibit the Inflammatory Response to Biodegradable Poly(Lactide- Co-Glycolide) Implants. ACS Nano 2018, 12, 6917–6925. [Google Scholar] [CrossRef]
- Di Mario, C.; Ferrante, G. Biodegradable Drug-Eluting Stents: Promises and Pitfalls. Lancet 2008, 371, 873–874. [Google Scholar] [CrossRef]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-Nano Approach: Enabling the Translation of Metal Nanomaterials to Clinics. Bioconjug. Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Nanoparticles and the Blood Coagulation System. Part II: Safety Concerns. Nanomedicine 2013, 8, 969–981. [Google Scholar] [CrossRef]
- Sulheim, E.; Iversen, T.G.; Nakstad, V.T.; Klinkenberg, G.; Sletta, H.; Schmid, R.; Hatletveit, A.R.; Wågbø, A.M.; Sundan, A.; Skotland, T.; et al. Cytotoxicity of Poly(Alkyl Cyanoacrylate) Nanoparticles. Int. J. Mol. Sci. 2017, 18, 2454. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, Y.M. Taxol-Loaded Block Copolymer Nanospheres Composed of Methoxy Poly(Ethylene Glycol) and Poly(ε-Caprolactone) as Novel Anticancer Drug Carriers. Biomaterials 2001, 22, 1697–1704. [Google Scholar] [CrossRef]
- Ho, D.; Wang, P.; Kee, T. Artificial Intelligence in Nanomedicine. Nanoscale Horiz. 2019, 4, 365–377. [Google Scholar] [CrossRef]
- Cai, P.; Zhang, X.; Wang, M.; Wu, Y.L.; Chen, X. Combinatorial Nano-Bio Interfaces. ACS Nano 2018, 12, 5078–5084. [Google Scholar] [CrossRef]
| Biodegradable Nanoparticles | Advantages | Limitations | |
|---|---|---|---|
| General |
|
| |
| Polymer-based | PLA micelles |
|
|
| PLGA micelles |
|
| |
| PCL nanoparticles |
|
| |
| Chitosan nanoparticles |
|
| |
| Dendrimers |
|
| |
| Lipid-based | Liposomes |
|
|
| Purpose | Application | Advantages of Biodegradable Nanomaterials | References |
|---|---|---|---|
| Imaging | MRI |
| [66,67,68,69,70,71,72,73,74,75,76] |
| photoacoustic imaging | |||
| Theranostics | photoacoustic imaging and photothermal therapy |
| [78,79,80,81,82,83,84,85,86,87] |
| Targeted Delivery(carried by liposomes, polymeric nanoparticles, dendrimers, or micelles) | Drug delivery |
| [88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
| Gene therapy |
| [102,103,104,105,106,107] | |
| Antigen delivery |
| [112] | |
| Implants | Stents |
| [112] |
| Mesh |
| [112] | |
| Suture |
| [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. https://doi.org/10.3390/nano10040656
Su S, Kang PM. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials. 2020; 10(4):656. https://doi.org/10.3390/nano10040656
Chicago/Turabian StyleSu, Shi, and Peter M. Kang. 2020. "Systemic Review of Biodegradable Nanomaterials in Nanomedicine" Nanomaterials 10, no. 4: 656. https://doi.org/10.3390/nano10040656
APA StyleSu, S., & Kang, P. M. (2020). Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials, 10(4), 656. https://doi.org/10.3390/nano10040656





