Yolk–Shell Nanostructures: Syntheses and Applications for Lithium-Ion Battery Anodes
Abstract
:1. Introduction
2. Building Yolk–Shell Nanostructure
2.1. Templating Methods
2.2. Self-Templating Methods
3. Yolk–Shell Nanostructured Anodes
3.1. Alloy-Type Materials
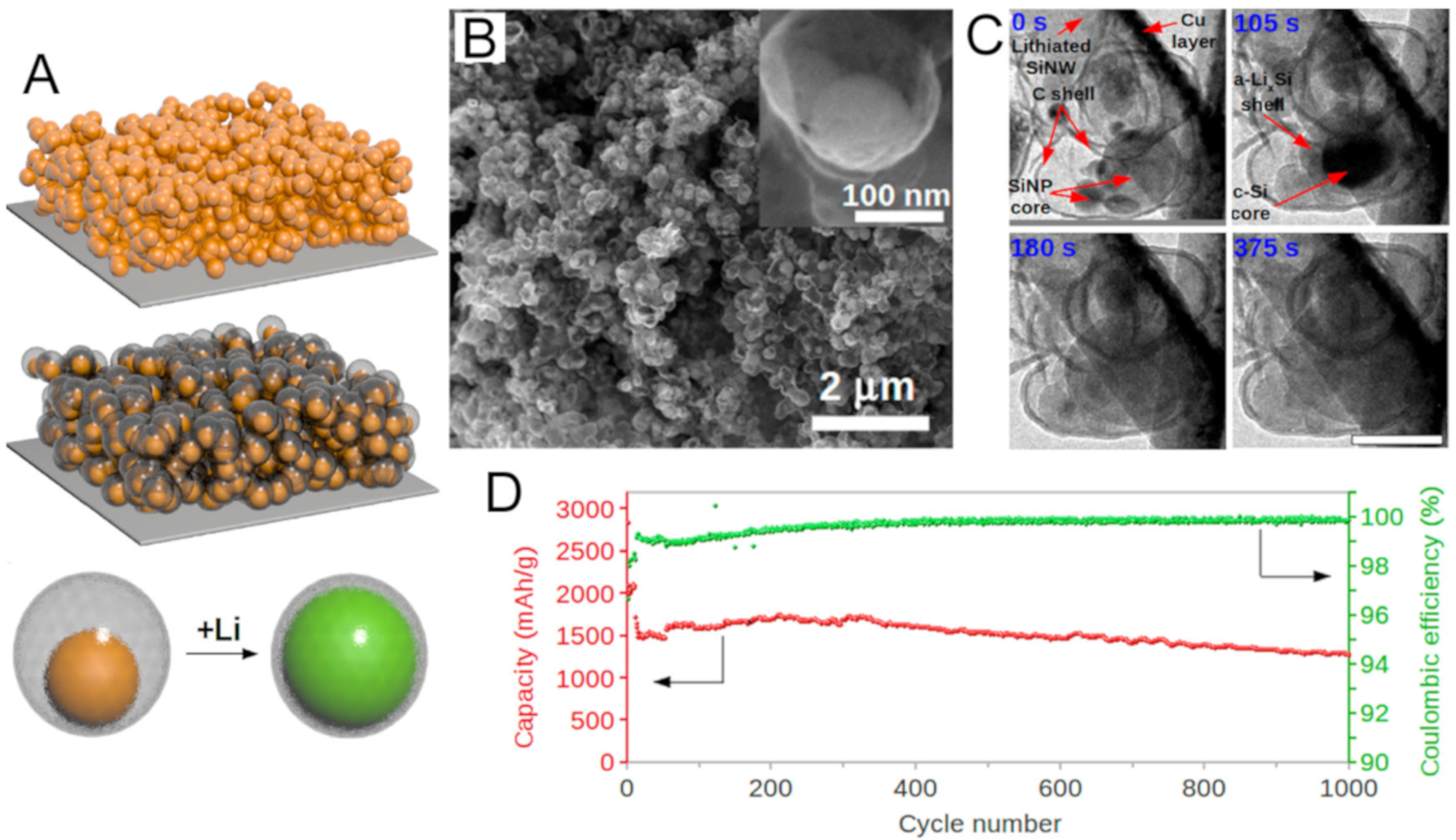
3.1.1. Silicon
3.1.2. Tin and Tin Oxide
3.1.3. Aluminum
3.2. Conversion Materials
4. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Fang, F.; Zhao, Y.; Liu, Y.; Li, J.; Huang, G.; Sun, H. A sandwich structure of mesoporous anatase TiO2 sheets and reduced graphene oxide and its application as lithium-ion battery electrodes. RSC Adv. 2014, 4, 43039–43046. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Oumellal, Y.; Delpuech, N.; Mazouzi, D.; Dupre, N.; Gaubicher, J.; Moreau, P.; Soudan, P.; Lestriez, B.; Guyomard, D. The failure mechanism of nano-sized Si-based negative electrodes for lithium ion batteries. J. Mater. Chem. 2011, 21, 6201–6208. [Google Scholar] [CrossRef]
- Dang, H.X.; Klavetter, K.C.; Meyerson, M.L.; Heller, A.; Mullins, C.B. Tin microparticles for a lithium ion battery anode with enhanced cycling stability and efficiency derived from Se-doping. J. Mater. Chem. A 2015, 3, 13500–13506. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhu, T. Germanium-Based Electrode Materials for Lithium-Ion Batteries. ChemElectroChem 2014, 1, 706–713. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Wen Lou, X. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Yang, Z.; Zhang, X.; Wang, C.; Chen, J.; Zhang, X.; Xue, M. Novel Metal Chalcogenide SnSSe as a High-Capacity Anode for Sodium-Ion Batteries. Adv. Mater. 2016, 28, 8645–8650. [Google Scholar] [CrossRef]
- Wang, X.; Kim, H.-M.; Xiao, Y.; Sun, Y.-K. Nanostructured metal phosphide-based materials for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 14915–14931. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Qiu, W.; Wang, W.; Fang, P.; Lu, X.; Tong, Y. Recent advances in metal nitrides as high-performance electrode materials for energy storage devices. J. Mater. Chem. A 2015, 3, 1364–1387. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.Y.; Kang, Y.C. First Introduction of NiSe2 to Anode Material for Sodium-Ion Batteries: A Hybrid of Graphene-Wrapped NiSe2/C Porous Nanofiber. Sci. Rep. 2016, 6, 23338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.-H.; Lu, M.-Y.; Chen, L.-J. Metal sulfide nanostructures: Synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 2012, 22, 19–30. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A Review on Design Strategies for Carbon Based Metal Oxides and Sulfides Nanocomposites for High Performance Li and Na Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, W.; Kim, Y.; Ryu, I.; Gu, M.; Wang, C.; Liu, G.; Liu, Z.; Gao, H. Regulated Breathing Effect of Silicon Negative Electrode for Dramatically Enhanced Performance of Li-Ion Battery. Adv. Funct. Mater. 2015, 25, 1426–1433. [Google Scholar] [CrossRef]
- Liu, N.; Wu, H.; McDowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A Yolk-Shell Design for Stabilized and Scalable Li-Ion Battery Alloy Anodes. Nano Lett. 2012, 12, 3315–3321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Rajagopalan, R.; Guo, H.; Hu, X.; Dou, S.; Liu, H. A Green and Facile Way to Prepare Granadilla-Like Silicon-Based Anode Materials for Li-Ion Batteries. Adv. Funct. Mater. 2016, 26, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, H.; El-Safty, S.A.; Reda, A.; Elmarakbi, A.; Metawa, H.; Shenashen, M.A. Multifaceted geometric 3D mesopolytope cathodes and its directional transport gates for superscalable LIB models. Appl. Mater. Today 2020, 19, 100590. [Google Scholar] [CrossRef]
- Chen, H.; Qi, B.; Moore, T.; Wang, F.; Colvin, D.C.; Sanjeewa, L.D.; Gore, J.C.; Hwu, S.-J.; Mefford, O.T.; Alexis, F.; et al. Multifunctional Yolk-in-Shell Nanoparticles for pH-triggered Drug Release and Imaging. Small 2014, 10, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, X.; Guo, H.; Wang, C.; Liu, J.; Sun, P.; Liu, F.; Lu, G. Design of Au@ZnO Yolk–Shell Nanospheres with Enhanced Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 18661–18667. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-M.; Chen, Z.; Cao, C.-Y.; Jiang, L.; Song, W.-G. A yolk–shell structured Fe2O3@mesoporous SiO2 nanoreactor for enhanced activity as a Fenton catalyst in total oxidation of dyes. Chem. Commun. 2013, 49, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.I.; Hong, S.G.; Seo, H.; Kim, J.; Moon, G.D.; Hyun, D.C. Poly(ε-caprolactone) (PCL) Hollow Nanoparticles with Surface Sealability and On-Demand Pore Generability for Easy Loading and NIR Light-Triggered Release of Drug. Pharmaceutics 2019, 11, 528. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Dzubiella, J.; Kaiser, J.; Drechsler, M.; Guo, X.; Ballauff, M.; Lu, Y. Thermosensitive Au-PNIPA Yolk–Shell Nanoparticles with Tunable Selectivity for Catalysis. Angew. Chem. Int. Ed. 2012, 51, 2229–2233. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Li, X.; Zhao, J.; Bai, S.; Liu, J.; Yang, Q. A Yolk–Shell Nanoreactor with a Basic Core and an Acidic Shell for Cascade Reactions. Angew. Chem. Int. Ed. 2012, 51, 9164–9168. [Google Scholar] [CrossRef]
- Yang, P.; Luo, X.; Wang, S.; Wang, F.; Tang, C.; Wang, C. Biodegradable yolk-shell microspheres for ultrasound/MR dual-modality imaging and controlled drug delivery. Colloids Surf. B: Biointerfaces 2017, 151, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, Y.; Bu, W.; Bu, J.; Sun, Y.; Du, J.; Shi, J. Ultrasensitive Nanosensors Based on Upconversion Nanoparticles for Selective Hypoxia Imaging in Vivo upon Near-Infrared Excitation. J. Am. Chem. Soc. 2014, 136, 9701–9709. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Akhtar, N.; Selim, M.M.; Morsy, W.M.; Yamaguchi, H.; Kawada, S.; Alhamid, A.A.; Ohashi, N.; Ichinose, I.; Alamoudi, A.S.; et al. Effective, Low-Cost Recovery of Toxic Arsenate Anions from Water by Using Hollow-Sphere Geode Traps. Chem. Asian J. 2017, 12, 1952–1964. [Google Scholar] [CrossRef]
- Soliman, A.-A.E.; Shenashen, M.A.; El-Sewify, I.M.; Taha, G.M.; El-Taher, M.A.; Yamaguchi, H.; Alamoudi, A.S.; Selim, M.M.; El-Safty, S.A. Mesoporous Organic–Inorganic Core–Shell Necklace Cages for Potentially Capturing Cd2+ Ions from Water Sources. ChemistrySelect 2017, 2, 6135–6142. [Google Scholar] [CrossRef]
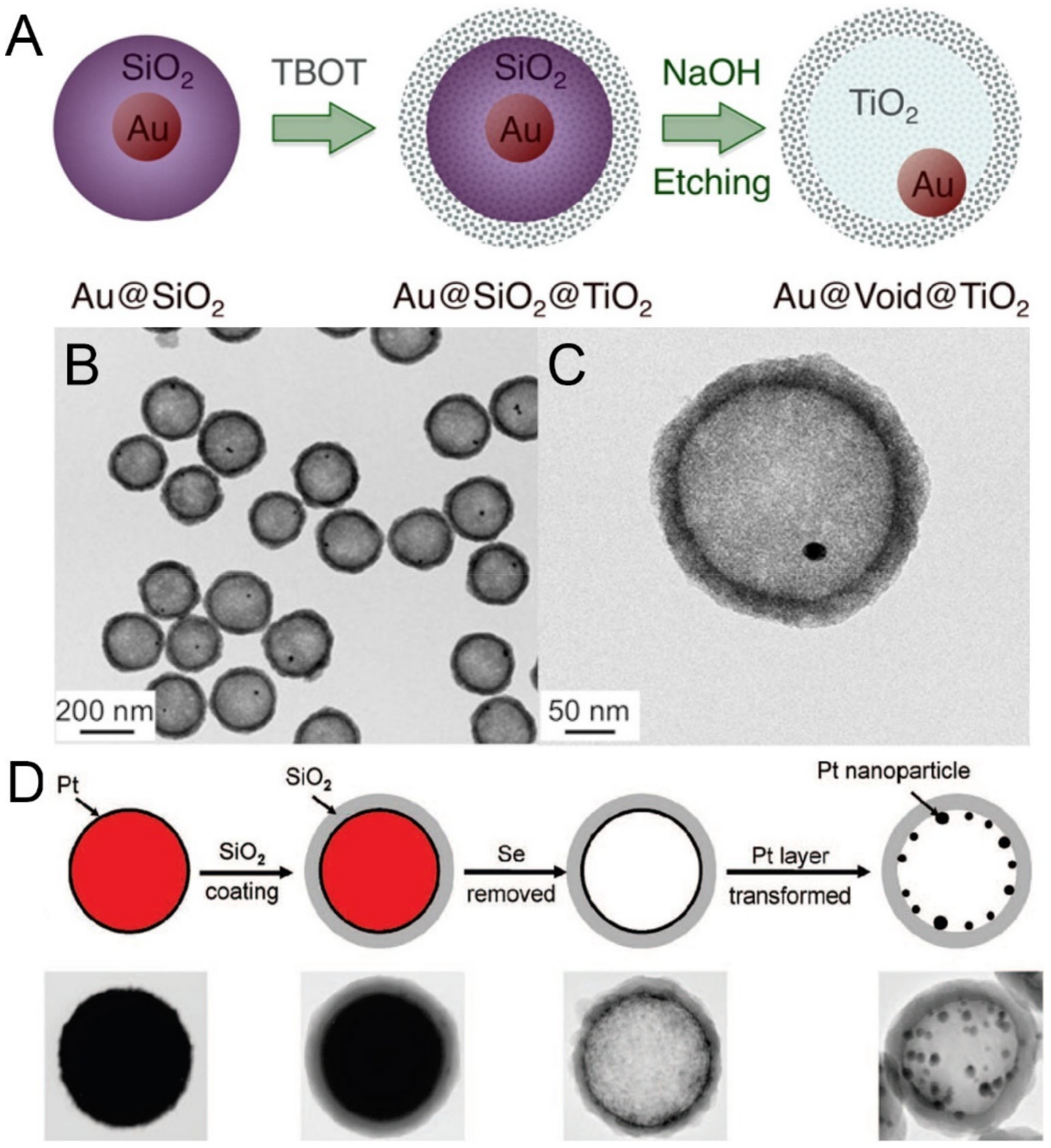
- Lee, I.; Joo, J.B.; Yin, Y.; Zaera, F. Au@Void@TiO2 yolk–shell nanostructures as catalysts for the promotion of oxidation reactions at cryogenic temperatures. Surf. Sci. 2016, 648, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-S.; Song, J.; Yang, H.-H.; Chen, X. Yolk–Shell Nanostructures: Design, Synthesis, and Biomedical Applications. Adv. Mater. 2018, 30, 1704639. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; El-Safty, S.A.; Azzam, A.M.; Shenashen, M.A.; El-Sockary, M.A.; Abo Elenien, O.M. Superhydrophobic Silicone/TiO2–SiO2 Nanorod-like Composites for Marine Fouling Release Coatings. ChemistrySelect 2019, 4, 3395–3407. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Guo, L.; He, Q.; Chen, F.; Zhou, J.; Feng, J.; Shi, J. Hollow/Rattle-Type Mesoporous Nanostructures by a Structural Difference-Based Selective Etching Strategy. ACS Nano 2010, 4, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.D.; Jeong, U. Decoration of the Interior Surface of Hollow Spherical Silica Colloids with Pt Nanoparticles. Chem. Mater. 2008, 20, 3003–3007. [Google Scholar] [CrossRef]
- Sun, Y.; Wiley, B.; Li, Z.-Y.; Xia, Y. Synthesis and Optical Properties of Nanorattles and Multiple-Walled Nanoshells/Nanotubes Made of Metal Alloys. J. Am. Chem. Soc. 2004, 126, 9399–9406. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.G.M.; Rodrigues, T.S.; Correia, V.G.; Alves, T.V.; Alves, R.S.; Ando, R.A.; Ornellas, F.R.; Wang, J.; Andrade, L.H.; Camargo, P.H.C. Plasmonic Nanorattles as Next-Generation Catalysts for Surface Plasmon Resonance-Mediated Oxidations Promoted by Activated Oxygen. Angew. Chem. Int. Ed. 2016, 55, 7111–7115. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Bodnarchuk, M.I.; Kovalenko, M.V.; Talapin, D.V.; Smith, R.K.; Aloni, S.; Heiss, W.; Alivisatos, A.P. Gold/Iron Oxide Core/Hollow-Shell Nanoparticles. Adv. Mater. 2008, 20, 4323–4329. [Google Scholar] [CrossRef]
- Cao, L.; Chen, D.; Caruso, R.A. Surface-Metastable Phase-Initiated Seeding and Ostwald Ripening: A Facile Fluorine-Free Process towards Spherical Fluffy Core/Shell, Yolk/Shell, and Hollow Anatase Nanostructures. Angew. Chem. Int. Ed. 2013, 52, 10986–10991. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.-Y.; Paik, U. Etching-in-a-Box: A Novel Strategy to Synthesize Unique Yolk-Shelled Fe3O4@Carbon with an Ultralong Cycling Life for Lithium Storage. Adv. Energy Mater. 2016, 6, 1502318. [Google Scholar] [CrossRef]
- Wei Seh, Z.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.-C.; Cui, Y. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Chen, J.S.; Qi, G.; Duan, X.; Wang, Z.; Giannelis, E.P.; Archer, L.A.; Lou, X.W. Formation of SnO2 Hollow Nanospheres inside Mesoporous Silica Nanoreactors. J. Am. Chem. Soc. 2011, 133, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Tu, D.; Li, X.; Li, R.; Chen, X. A facile “ship-in-a-bottle” approach to construct nanorattles based on upconverting lanthanide-doped fluorides. Nano Res. 2016, 9, 187–197. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Qi, J.; Wang, J.; Luo, R.; Shen, J.; Sun, X.; Han, W.; Wang, L. Yolk–Shell Fe0@SiO2 Nanoparticles as Nanoreactors for Fenton-like Catalytic Reaction. ACS Appl. Mater. Interfaces 2014, 6, 13167–13173. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, S.N.; Pinkhassik, E. Simultaneous templating of polymer nanocapsules and entrapped silver nanoparticles. Chem. Commun. 2010, 46, 7346–7348. [Google Scholar] [CrossRef]
- Shmakov, S.N.; Jia, Y.; Pinkhassik, E. Selectively Initiated Ship-In-A-Bottle Assembly of Yolk–Shell Nanostructures. Chem. Mater. 2014, 26, 1126–1132. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, K.; Xu, Q.; Zhou, Y.; Cheng, F.; Guo, S. Beyond Yolk–Shell Nanoparticles: Fe3O4@Fe3C Core@Shell Nanoparticles as Yolks and Carbon Nanospindles as Shells for Efficient Lithium Ion Storage. ACS Nano 2015, 9, 3369–3376. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, S.; Su, X.; Chen, G.; Liu, Y.; Luo, Z.; Luo, W.; Tang, Y.; Ju, H.; Zhao, D.; et al. Facile Synthesis of Yolk–Shell Structured Inorganic–Organic Hybrid Spheres with Ordered Radial Mesochannels. Adv. Mater. 2014, 26, 3741–3747. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Ge, J.; Yin, Y. Permeable Silica Shell through Surface-Protected Etching. Nano Lett. 2008, 8, 2867–2871. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, G.; Kim, Y.-U.; Kim, J.-H.; Park, C.-M.; Sohn, H.-J. Metallic anodes for next generation secondary batteries. Chem. Soc. Rev. 2013, 42, 9011–9034. [Google Scholar] [CrossRef] [PubMed]
- Mogi, R.; Inaba, M.; Jeong, S.-K.; Iriyama, Y.; Abe, T.; Ogumi, Z. Effects of Some Organic Additives on Lithium Deposition in Propylene Carbonate. J. Electrochem. Soc. 2002, 149, A1578–A1583. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, D.; Zhao, J.; Lu, Z.; Liu, Y.; Liu, C.; Lu, Y.; Wang, H.; Yan, K.; Tao, X.; et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl. Acad. Sci. USA 2016, 113, 2862–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th Anniversary Article: Understanding the Lithiation of Silicon and Other Alloying Anodes for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, P.; Ahn, D. Ultrathin Multifunctional Oxide Coatings for Lithium Ion Batteries. Adv. Mater. 2011, 23, 3911–3915. [Google Scholar] [CrossRef]
- Meng, X.; Yang, X.-Q.; Sun, X. Emerging Applications of Atomic Layer Deposition for Lithium-Ion Battery Studies. Adv. Mater. 2012, 24, 3589–3615. [Google Scholar] [CrossRef]
- Khalifa, H.; El-Safty, S.A.; Reda, A.; Shenashen, M.A.; Eid, A.I. Anisotropic alignments of hierarchical Li2SiO3/TiO2 @nano-C anode//LiMnPO4@nano-C cathode architectures for full-cell lithium-ion battery. Natl. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Li, H.-H.; Wang, J.-W.; Wu, X.-L.; Sun, H.-Z.; Yang, F.-M.; Wang, K.; Zhang, L.-L.; Fan, C.-Y.; Zhang, J.-P. A novel approach to prepare Si/C nanocomposites with yolk-shell structures for lithium ion batteries. RSC Adv. 2014, 4, 36218–36225. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gao, D.; Chen, S.; Tan, L.; Li, L. Facile synthesis of yolk-shell structured Si-C nanocomposites as anodes for lithium-ion batteries. Chem. Commun. 2014, 50, 5878–5880. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Xie, J.; Xu, Y.; Wang, L.; Wang, Y.; Ren, M. Preparation and lithium storage performance of yolk-shell Si@void@C nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 17562–17565. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Evans, D.G.; Zhu, H.; Yang, W. Facile fabrication of yolk-shell structured porous Si-C microspheres as effective anode materials for Li-ion batteries. RSC Adv. 2014, 4, 71–75. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.-X.; Chou, S.-L.; Zhang, R.; Xu, Y.; Fan, J.; Zhang, W.-X.; Kun Liu, H.; Zhao, D.; Xue Dou, S. Yolk-shell silicon-mesoporous carbon anode with compact solid electrolyte interphase film for superior lithium-ion batteries. Nano Energy 2015, 18, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Kwok, T.; Mangolini, L. Spray pyrolysis of yolk–shell particles and their use for anodes in lithium-ion batteries. Electrochem. Commun. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Yang, L.Y.; Li, H.Z.; Liu, J.; Sun, Z.Q.; Tang, S.S.; Lei, M. Dual yolk-shell structure of carbon and silica-coated silicon for high-performance lithium-ion batteries. Sci. Rep. 2015, 5, 10908. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.J.; Kang, Y.C. General Formation of Tin Nanoparticles Encapsulated in Hollow Carbon Spheres for Enhanced Lithium Storage Capability. Small 2015, 11, 2157–2163. [Google Scholar] [CrossRef]
- Chen, P.; Wu, F.; Wang, Y. Four-Layer Tin–Carbon Nanotube Yolk–Shell Materials for High-Performance Lithium-Ion Batteries. ChemSusChem 2014, 7, 1407–1414. [Google Scholar] [CrossRef]
- Ni, W.; Cheng, J.; Shi, L.; Li, X.; Wang, B.; Guan, Q.; Huang, L.; Gu, G.; Li, H. Integration of Sn/C yolk-shell nanostructures into free-standing conductive networks as hierarchical composite 3D electrodes and the Li-ion insertion/extraction properties in a gel-type lithium-ion battery thereof. J. Mater. Chem. A 2014, 2, 19122–19130. [Google Scholar] [CrossRef]
- Ni, W.; Wang, Y.; Xu, R. Formation of Sn@C Yolk–Shell Nanospheres and Core–Sheath Nanowires for Highly Reversible Lithium Storage. Part. Part. Syst. Charact. 2013, 30, 873–880. [Google Scholar] [CrossRef]
- Zhang, W.-M.; Hu, J.-S.; Guo, Y.-G.; Zheng, S.-F.; Zhong, L.-S.; Song, W.-G.; Wan, L.-J. Tin-Nanoparticles Encapsulated in Elastic Hollow Carbon Spheres for High-Performance Anode Material in Lithium-Ion Batteries. Adv. Mater. 2008, 20, 1160–1165. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, L.; Wang, C.; Dhanabalan, A.; van Aken, P.A.; Maier, J. Encapsulation of Sn@carbon Nanoparticles in Bamboo-like Hollow Carbon Nanofibers as an Anode Material in Lithium-Based Batteries. Angew. Chem. Int. Ed. 2009, 48, 6485–6489. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Wang, F.; Xia, Y.; Asiri, A.M.; Zhao, D. Controllable synthesis of SnO2@C yolk-shell nanospheres as a high-performance anode material for lithium ion batteries. Nanoscale 2014, 6, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, P.; Ye, Y.; Wang, H.; Zhou, Y.; Tang, Y.; Lu, T. Designed synthesis of SnO2@C yolk-shell spheres for high-performance lithium storage. CrystEngComm 2014, 16, 517–521. [Google Scholar] [CrossRef]
- Song, H.; Li, N.; Cui, H.; Wen, X.; Wei, X.; Wang, C. Significantly improved high-rate Li-ion batteries anode by encapsulating tin dioxide nanocrystals into mesotunnels. CrystEngComm 2013, 15, 8537–8543. [Google Scholar] [CrossRef]
- Guo, H.; Mao, R.; Tian, D.; Wang, W.; Zhao, D.; Yang, X.; Wang, S. Morphology-controlled synthesis of SnO2/C hollow core-shell nanoparticle aggregates with improved lithium storage. J. Mater. Chem. A 2013, 1, 3652–3658. [Google Scholar] [CrossRef]
- Hong, Y.J.; Son, M.Y.; Kang, Y.C. One-Pot Facile Synthesis of Double-Shelled SnO2 Yolk-Shell-Structured Powders by Continuous Process as Anode Materials for Li-ion Batteries. Adv. Mater. 2013, 25, 2279–2283. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Niu, J.; Zhao, Y.C.; So, K.P.; Wang, C.; Wang, C.A.; Li, J. High-rate aluminium yolk-shell nanoparticle anode for Li-ion battery with long cycle life and ultrahigh capacity. Nat. Commun. 2015, 6, 7872. [Google Scholar] [CrossRef] [Green Version]
- Son, M.Y.; Hong, Y.J.; Kang, Y.C. Superior electrochemical properties of Co3O4 yolk-shell powders with a filled core and multishells prepared by a one-pot spray pyrolysis. Chem. Commun. 2013, 49, 5678–5680. [Google Scholar] [CrossRef]
- Kim, M.H.; Hong, Y.J.; Kang, Y.C. Electrochemical properties of yolk-shell and hollow CoMn2O4 powders directly prepared by continuous spray pyrolysis as negative electrode materials for lithium ion batteries. RSC Adv. 2013, 3, 13110–13114. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Z.; Xu, Z.J. Yolk-shell Fe2O3@C composites anchored on MWNTs with enhanced lithium and sodium storage. Nanoscale 2015, 7, 9520–9525. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Wu, C.; Ding, Y.; Guan, L. A Yolk–Shell Fe3O4@C Composite as an Anode Material for High-Rate Lithium Batteries. ChemPlusChem 2012, 77, 748–751. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Noonan, O.; Martin, D.J.; Whittaker, A.K.; Yu, C. Tailoring the Void Size of Iron Oxide@Carbon Yolk–Shell Structure for Optimized Lithium Storage. Adv. Funct. Mater. 2014, 24, 4337–4342. [Google Scholar] [CrossRef]
- Son, M.Y.; Hong, Y.J.; Lee, J.-K.; Chan Kang, Y. One-pot synthesis of Fe2O3 yolk-shell particles with two, three, and four shells for application as an anode material in lithium-ion batteries. Nanoscale 2013, 5, 11592–11597. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Um, J.H.; Ahn, J.; Yu, S.-H.; Sung, Y.-E.; Lee, J.-K. Soft Template Strategy to Synthesize Iron Oxide–Titania Yolk–Shell Nanoparticles as High-Performance Anode Materials for Lithium-Ion Battery Applications. Chem. Eur. J. 2015, 21, 7954–7961. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, C.; Xing, Y.; Xu, H.; Zhang, S. Formation of a stable carbon framework in a MnO yolk-shell sphere to achieve exceptional performance for a Li-ion battery anode. J. Mater. Chem. A 2015, 3, 15591–15597. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, L.; Yan, M.; Han, C.; He, L.; Hercule, K.M.; Niu, C.; Yuan, Z.; Xu, W.; Qu, L.; et al. Manganese Oxide/Carbon Yolk–Shell Nanorod Anodes for High Capacity Lithium Batteries. Nano Lett. 2015, 15, 738–744. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Gao, S.; Xu, Y.; Cheng, X.; Zhao, H.; Huo, L. Facile synthesis of yolk-shell MoO2 microspheres with excellent electrochemical performance as a Li-ion battery anode. J. Mater. Chem. A 2013, 1, 6858–6864. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Liu, J.; Qian, F.; Zhang, C.; Li, T.; Chen, W.; Yang, X.; Guo, Y. Self-assembled hierarchical yolk-shell structured NiO@C from metal-organic frameworks with outstanding performance for lithium storage. Chem. Commun. 2014, 50, 9485–9488. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Cui, H.; Wang, C. Abnormal Cyclibility in Ni@Graphene Core–Shell and Yolk–Shell Nanostructures for Lithium Ion Battery Anodes. ACS Appl. Mater. Interfaces 2014, 6, 13765–13769. [Google Scholar] [CrossRef]
- Kong, S.; Dai, R.; Li, H.; Sun, W.; Wang, Y. Microwave Hydrothermal Synthesis of Ni-based Metal–Organic Frameworks and Their Derived Yolk–Shell NiO for Li-Ion Storage and Supported Ammonia Borane for Hydrogen Desorption. ACS Sustain. Chem. Eng. 2015, 3, 1830–1838. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Ultrafast Synthesis of Yolk-Shell and Cubic NiO Nanopowders and Application in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 2312–2316. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, G.D.; Kang, Y.C.; Lee, J.-H. Phase-pure β-NiMoO4 yolk-shell spheres for high-performance anode materials in lithium-ion batteries. Electrochim. Acta 2015, 174, 102–110. [Google Scholar] [CrossRef]
- Yu, L.; Guan, B.; Xiao, W.; Lou, X.W. Formation of Yolk-Shelled Ni–Co Mixed Oxide Nanoprisms with Enhanced Electrochemical Performance for Hybrid Supercapacitors and Lithium Ion Batteries. Adv. Energy Mater. 2015, 5, 1500981. [Google Scholar] [CrossRef]
- Jiang, L.; Qu, Y.; Ren, Z.; Yu, P.; Zhao, D.; Zhou, W.; Wang, L.; Fu, H. In Situ Carbon-Coated Yolk–Shell V2O3 Microspheres for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 1595–1601. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, X.; Wu, X.; Wu, H.; Liu, X.; Yue, G.; Yang, Y.; Peng, D.-L. Yolk-shell ZnO-C microspheres with enhanced electrochemical performance as anode material for lithium ion batteries. Electrochim. Acta 2014, 125, 659–665. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, Y.; Zeng, D.; Wang, L.; Yue, G.; Peng, D.-L. Facile fabrication of various zinc-nickel citrate microspheres and their transformation to ZnO-NiO hybrid microspheres with excellent lithium storage properties. Sci. Rep. 2015, 5, 8351. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Yolk–Shell, Hollow, and Single-Crystalline ZnCo2O4 Powders: Preparation Using a Simple One-Pot Process and Application in Lithium-Ion Batteries. ChemSusChem 2013, 6, 2111–2116. [Google Scholar] [CrossRef]
- Won, J.M.; Choi, S.H.; Hong, Y.J.; Ko, Y.N.; Kang, Y.C. Electrochemical properties of yolk-shell structured ZnFe2O4 powders prepared by a simple spray drying process as anode material for lithium-ion battery. Sci. Rep. 2014, 4, 5857. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kang, Y.C. Using Simple Spray Pyrolysis to Prepare Yolk–Shell-Structured ZnO–Mn3O4 Systems with the Optimum Composition for Superior Electrochemical Properties. Chem. Asia. J. 2014, 20, 3014–3018. [Google Scholar]
- Qiu, W.; Jiao, J.; Xia, J.; Zhong, H.; Chen, L. A Self-Standing and Flexible Electrode of Yolk–Shell CoS2 Spheres Encapsulated with Nitrogen-Doped Graphene for High-Performance Lithium-Ion Batteries. Chem. Eur. J. 2015, 21, 4359–4367. [Google Scholar] [CrossRef]
- Ko, Y.N.; Choi, S.H.; Park, S.B.; Kang, Y.C. Preparation of Yolk-Shell and Filled Co9S8 Microspheres and Comparison of their Electrochemical Properties. Chem. Asian J. 2014, 9, 572–576. [Google Scholar] [CrossRef]
- Ko, Y.N.; Choi, S.H.; Park, S.B.; Kang, Y.C. Hierarchical MoSe2 yolk-shell microspheres with superior Na-ion storage properties. Nanoscale 2014, 6, 10511–10515. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Synthesis for Yolk-shell-structured Metal Sulfide Powders with Excellent Electrochemical Performances for Lithium-ion Batteries. Small 2014, 10, 474–478. [Google Scholar] [CrossRef]
- Won, J.M.; Lee, J.-H.; Kang, Y.C. Electrochemical Properties of Yolk–Shell-Structured Zn–Fe–S Multicomponent Sulfide Materials with a 1:2 Zn/Fe Molar Ratio. Chem. Eur. J. 2015, 21, 1429–1433. [Google Scholar] [CrossRef]
- Jin, J.; Huang, S.-Z.; Li, Y.; Tian, H.; Wang, H.-E.; Yu, Y.; Chen, L.-H.; Hasan, T.; Su, B.-L. Hierarchical nanosheet-constructed yolk-shell TiO2 porous microspheres for lithium batteries with high capacity, superior rate and long cycle capability. Nanoscale 2015, 7, 12979–12989. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yang, L.; Wang, K.; Lou, X.; Cai, B. Template-free synthesis of homogeneous yolk–shell TiO2 hierarchical microspheres for high performance lithium ion batteries. J. Power Sources 2014, 262, 72–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Hou, H.; Zou, G.; Ji, X. Nitrogen Doped/Carbon Tuning Yolk-Like TiO2 and Its Remarkable Impact on Sodium Storage Performances. Adv. Energy Mater. 2016, 7, 1600173. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Y.; Hong, Z.; Lü, X.; Wan, D.; Huang, F. Biomolecule-Assisted Route to Prepare Titania Mesoporous Hollow Structures. Chem. Eur. J. 2011, 17, 11535–11541. [Google Scholar] [CrossRef]
- Yang, K.M.; Ko, Y.N.; Yun, J.-Y.; Kang, Y.C. Preparation of Li4Ti5O12 Yolk–Shell Powders by Spray Pyrolysis and their Electrochemical Properties. Chem. Asian J. 2014, 9, 443–446. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Wu, X.-L.; Guo, Y.-G.; Wan, L.-J. Rational Design of Anode Materials Based on Group IVA Elements (Si, Ge, and Sn) for Lithium-Ion Batteries. Chem. Asian J. 2013, 8, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Gu, L.; Wang, C.; Tang, K.; Maier, J. Highly reversible lithium storage in Si (core)-hollow carbon nanofibers (sheath) nanocomposites. Nanoscale 2013, 5, 2647–2650. [Google Scholar] [CrossRef]
- Liu, L.; Xie, F.; Lyu, J.; Zhao, T.; Li, T.; Choi, B.G. Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries. J. Power Sources 2016, 321, 11–35. [Google Scholar] [CrossRef]
- Courtney, I.A.; Dahn, J.R. Electrochemical and In Situ X-Ray Diffraction Studies of the Reaction of Lithium with Tin Oxide Composites. J. Electrochem. Soc. 1997, 144, 2045–2052. [Google Scholar] [CrossRef]
- Gay, E.C.; Vissers, D.R.; Martino, F.J.; Anderson, K.E. Performance Characteristics of Solid Lithium-Aluminum Alloy Electrodes. J. Electrochem. Soc. 1976, 123, 1591–1596. [Google Scholar] [CrossRef]
- Park, J.H.; Hudaya, C.; Kim, A.Y.; Rhee, D.K.; Yeo, S.J.; Choi, W.; Yoo, P.J.; Lee, J.K. Al-C hybrid nanoclustered anodes for lithium ion batteries with high electrical capacity and cyclic stability. Chem. Commun. 2014, 50, 2837–2840. [Google Scholar] [CrossRef]
- Do, J.-S.; Weng, C.-H. Preparation and characterization of CoO used as anodic material of lithium battery. J. Power Sources 2005, 146, 482–486. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.-K.; Kang, Y.C. Controllable synthesis of yolk-shell-structured metal oxides with seven to ten components for finding materials with superior lithium storage properties. Nanoscale 2014, 6, 12421–12425. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon-Encapsulated Fe3O4 Nanoparticles as a High-Rate Lithium Ion Battery Anode Material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Lin, J.; Shen, Z.; Fan, H.J. Three-Dimensional Graphene Foam Supported Fe3O4 Lithium Battery Anodes with Long Cycle Life and High Rate Capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef]











| Yolk | Shell | Fabrication | Pros/Cons |
|---|---|---|---|
| Alloy/de-alloy materials | |||
| Si | C [17,18,19,59,60,61,62,63], NiO [64], SiO2@C [65] | Etching [17,18,19,60,61,62,63,65], Pyrolysis [59,64] | high capacity and energy density, good safety/poor cycling |
| Sn | C [66,67,68,69,70,71] | Thermal [67,68,69,70,71], Pyrolysis [66] | |
| SnO, SnO2 | C [72,73,74,75], SnO2 [76] | Etching [72,73], Thermal [74,75,76] | |
| Al | TiO2 [77] | Etching [77] | |
| Conversion materials | |||
| Co3O4, CoMn2O4 | Co3O4 [78], CoMn2O4 [79] | Pyrolysis [78,79] | high capacity, high energy, environmentally-compatibility/low coulombic efficiency, poor cycling, unstable SEI formation, large potential hysteresis |
| Cr2O3 | TiO2 [47] | Thermal [47] | |
| Fe2O3, Fe3O4, FeOx | C [47,80,81,82], grapheme [44], Fe2O3 [83], TiO2 [84] | Etching [80,81,82], Pyrolysis [83], Thermal [44,47,84] | |
| MnO, MnO2 | C [85,86] | Thermal [85], Etching [86] | |
| MoO2 | MoO2 [87] | Ripening [87] | |
| Ni, NiO, NiMoO4, NiCO2O4 | C [88], graphene [89], NiO [90,91], NiMoO4 [92], NiCO2O4 [93] | Etching [89], Pyrolysis [91,92], Thermal [88,90,93] | |
| V2O3 | V2O3@C [94] | Ripening [94] | |
| ZnO, ZnCo2O4, ZnFe2O4, ZnO–Mn3O4 | C [95], ZnO–NiO [96], ZnCo2O4 [97], ZnFe2O4 [98], ZnO/Mn3O4 [99] | Pyrolysis [97,98,99], Ripening [95,96] | |
| CoS2, Co9S8 | CoS2 [100], Co9S8 [101] | Ripening [100], Pyrolysis [101] | |
| MoSe2 | MoSe2 [102] | Pyrolysis [102] | |
| SnS | SnS [103] | Pyrolysis [103] | |
| Zn–Fe–S | Zn–Fe–S [104] | Pyrolysis [104] | |
| Insertion/de-insertion materials | |||
| TiO2, Li4Ti5O12 | TiO2 [105,106,107], TiO2–C [108], Li4Ti5O12 [109] | Ripening [105,106,107], Pyrolysis [109] | extreme safety/low capacity |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, G.D. Yolk–Shell Nanostructures: Syntheses and Applications for Lithium-Ion Battery Anodes. Nanomaterials 2020, 10, 675. https://doi.org/10.3390/nano10040675
Moon GD. Yolk–Shell Nanostructures: Syntheses and Applications for Lithium-Ion Battery Anodes. Nanomaterials. 2020; 10(4):675. https://doi.org/10.3390/nano10040675
Chicago/Turabian StyleMoon, Geon Dae. 2020. "Yolk–Shell Nanostructures: Syntheses and Applications for Lithium-Ion Battery Anodes" Nanomaterials 10, no. 4: 675. https://doi.org/10.3390/nano10040675
APA StyleMoon, G. D. (2020). Yolk–Shell Nanostructures: Syntheses and Applications for Lithium-Ion Battery Anodes. Nanomaterials, 10(4), 675. https://doi.org/10.3390/nano10040675





