The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO and rGO
2.3. Characterization
2.4. Preparation of MB Solutions
2.5. The Experiment of MB Adsorption on rGO
3. Results and Discussions
3.1. Transformation of GO into rGO
3.2. Adsorption Kinetics
3.3. Particle Diffusion
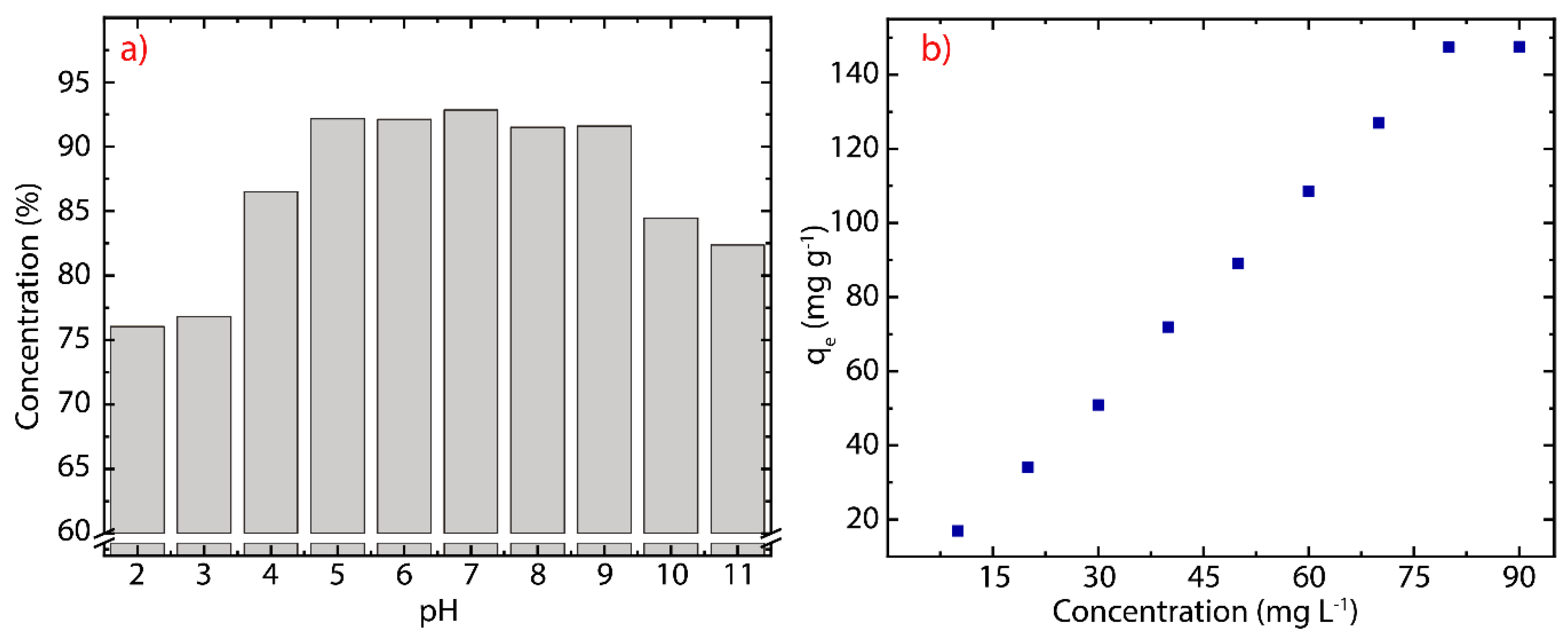
3.4. pH Effect
3.5. Adsorption Thermodynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ercin, A.E.; Hoekstra, A.Y. Water footprint scenarios for 2050: A global analysis. Environ. Int. 2014, 64, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Wang, Y.; Lin, C.; He, M.; Hao, F.; Liu, H.; Zhu, W. Heavy metal loss from agricultural watershed to aquatic system: A scientometrics review. Sci. Total Environ. 2018, 637, 208–220. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Environmental assessment of heavy metal pollution and human health risk. Am. J. Water Sci. Eng. 2016, 2, 14–19. [Google Scholar]
- Altenburger, R.; Brack, W.; Burgess, R.M.; Busch, W.; Escher, B.I.; Focks, A.; Hewitt, L.M.; Jacobsen, B.N.; de Alda, M.L.; Ait-Aissa, S.; et al. Future water quality monitoring: Improving the balance between exposure and toxicity assessments of real-world pollutant mixtures. Environ. Sci. Eur. 2019, 31, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Alharbi, O.M.L.; Tkachev, A.; Galunin, E.; Burakov, A.; Grachev, V.A. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 2018, 25, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Hao, R.; Xu, Z.; He, X.; Adeleye, A.S.; Li, Y. Removal of graphene oxide nanomaterials from aqueous media via coagulation: Effects of water chemistry and natural organic matter. Chemosphere 2017, 168, 1051–1057. [Google Scholar] [CrossRef]
- Wang, H.; Mi, X.; Li, Y.; Zhan, S. 3D graphene-based macrostructures for water treatment. Adv. Mater. 2020, 32, 1806843. [Google Scholar] [CrossRef]
- Sindona, A.; Pisarra, M.; Gravina, M.; Gomez, C.V.; Riccardi, P.; Falcone, G.; Plastina, F. Statistics of work and orthogonality catastrophe in discrete level systems: An application to fullerene molecules and ultra-cold trapped Fermi gases. Beilstein J. Nanotechnol. 2015, 6, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Gomez, C.V.; Pisarra, M.; Gravina, M.; Riccardi, P.; Sindona, A. Plasmon properties and hybridization effects in silicene. Phys. Rev. B 2017, 95, 85419. [Google Scholar] [CrossRef] [Green Version]
- Gomez, C.V.; Pisarra, M.; Gravina, M.; Pitarke, J.M.; Sindona, A. Plasmon modes of graphene nanoribbons with periodic planar arrangements. Phys. Rev. Lett. 2016, 117, 116801. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Biswas, P.; Fortner, J.D. A review of recent developments in graphene-enabled membranes for water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 915–922. [Google Scholar] [CrossRef]
- Coello-Fiallos, D.; Tene, T.; Guayllas, J.L.; Haro, D.; Haro, A.; Gomez, C.V. DFT comparison of structural and electronic properties of graphene and germanene: Monolayer and bilayer systems. Mater. Today Proc. 2017, 4, 6835–6841. [Google Scholar] [CrossRef]
- Sindona, A.; Pisarra, M.; Gomez, C.V.; Riccardi, P.; Falcone, G.; Bellucci, S. Calibration of the fine-structure constant of graphene by time-dependent density-functional theory. Phys. Rev. B 2017, 96, 201408. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Jing, N.; Xue, Q.; Ling, C.; Shan, M.; Zhang, T.; Zhou, X.; Jiao, Z. Effect of defects on Young’s modulus of graphene sheets: A molecular dynamics simulation. Rsc Adv. 2012, 2, 9124–9129. [Google Scholar] [CrossRef]
- Gomez, C.V.; Pisarra, M.; Gravina, M.; Sindona, A. Tunable plasmons in regular planar arrays of graphene nanoribbons with armchair and zigzag-shaped edges. Beilstein J. Nanotechnol. 2017, 8, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Sindona, A.; Pisarra, M.; Bellucci, S.; Tene, T.; Guevara, M.; Gomez, C.V. Plasmon oscillations in two-dimensional arrays of ultranarrow graphene nanoribbons. Phys. Rev. B 2019, 100, 235422. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563. [Google Scholar] [CrossRef] [Green Version]
- Vacacela Gomez, C.; Tene, T.; Guevara, M.; Tubon Usca, G.; Colcha, D.; Brito, H.; Molina, R.; Bellucci, S.; Tavolaro, A. Preparation of Few-Layer Graphene Dispersions from Hydrothermally Expanded Graphite. Appl. Sci. 2019, 9, 2539. [Google Scholar] [CrossRef] [Green Version]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Tubon Usca, G.; Vacacela Gomez, C.; Guevara, M.; Tene, T.; Hernandez, J.; Molina, R.; Tavolaro, A.; Miriello, D.; Caputi, L.S. Zeolite-Assisted Shear Exfoliation of Graphite into Few-Layer Graphene. Crystals 2019, 9, 377. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Zhuo, Q.; Gao, J.; Peng, M.; Bai, L.; Deng, J.; Xia, Y.; Ma, Y.; Zhong, J.; Sun, X. Large-scale synthesis of graphene by the reduction of graphene oxide at room temperature using metal nanoparticles as catalyst. Carbon N. Y. 2013, 52, 559–564. [Google Scholar] [CrossRef]
- Yu, H.; He, Y.; Xiao, G.; Fan, Y.; Ma, J.; Gao, Y.; Hou, R.; Yin, X.; Wang, Y.; Mei, X. The roles of oxygen-containing functional groups in modulating water purification performance of graphene oxide-based membrane. Chem. Eng. J. 2020, 389, 124375. [Google Scholar] [CrossRef]
- Imtiazuddin, S.M.; Mumtaz, M.; Mallick, K.A. Pollutants of wastewater characteristics in textile industries. J. Basic Appl. Sci. 2012, 8, 554–556. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Garg, V.K.; Amita, M.; Kumar, R.; Gupta, R. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste. Dye. Pigment. 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Özal, E.; Kuralay, E.; Yildirim, V.; Kilic, S.; Bolcal, C.; Kücükarslan, N.; Günay, C.; Demirkilic, U.; Tatar, H. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann. Thorac. Surg. 2005, 79, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.M.; Street, M.K. Risk of aspiration with the laryngeal mask. BJA Br. J. Anaesth. 1994, 72, 447–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, P.J.; Weinstein, R. Treatment of high-risk, refractory acquired methemoglobinemia with automated red blood cell exchange. J. Clin. Apher. Off. J. Am. Soc. Apher. 1998, 13, 28–31. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Yu, M.; Huang, J.; Xu, H.; Zhou, Y.; Song, P.; Xu, R. A novel approach for methylene blue removal by calcium dodecyl sulfate enhanced precipitation and microbial flocculant GA1 flocculation. Chem. Eng. J. 2016, 303, 1–13. [Google Scholar] [CrossRef]
- Zaghbani, N.; Hafiane, A.; Dhahbi, M. Separation of methylene blue from aqueous solution by micellar enhanced ultrafiltration. Sep. Purif. Technol. 2007, 55, 117–124. [Google Scholar] [CrossRef]
- Panizza, M.; Barbucci, A.; Ricotti, R.; Cerisola, G. Electrochemical degradation of methylene blue. Sep. Purif. Technol. 2007, 54, 382–387. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Sari, F.; Gülce, H.; Gülce, A.; Avci, A. Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B Environ. 2012, 119, 197–206. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Min, J.; Li, S.; Deng, X.; Yuan, S.; Zuo, X. Adsorption Characteristics of Phenolic Compounds on Graphene Oxide and Reduced Graphene Oxide: A Batch Experiment Combined Theory Calculation. Appl. Sci. 2018, 8, 1950. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Immersion enthalpy of benzene/cyclohexane and toluene/cyclohexane binary mixtures into modified activated carbons. J. Therm. Anal. Calorim. 2019, 138, 2565–2575. [Google Scholar] [CrossRef]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the Mechanism of Benzene and Toluene Adsorption in Single-Compound and Binary Systems: Energetic Interactions and Competitive Effects. 2017. Available online: http://hdl.handle.net/11591/386633 (accessed on 3 April 2020).
- Santonastaso, G.F.; Erto, A.; Bortone, I.; Chianese, S.; Di Nardo, A.; Musmarra, D. Experimental and simulation study of the restoration of a thallium (I)-contaminated aquifer by Permeable Adsorptive Barriers (PABs). Sci. Total Environ. 2018, 630, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvestrini, S.; Vanore, P.; Bogush, A.; Mayadevi, S.; Campos, L.C. Sorption of metaldehyde using granular activated carbon. J. Water Reuse Desalin. 2017, 7, 280–287. [Google Scholar] [CrossRef]
- Arias, F.E.A.; Beneduci, A.; Chidichimo, F.; Furia, E.; Straface, S. Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017, 180, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Tubón Usca, G.; Vacacela Gomez, C.; Fiallos, D.C.; Tavolaro, P.; Martino, G.; Caputi, L.S.; Tavolaro, A. Preparation of graphene oxide as biomaterials for drug adsorption. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2015; Volume 1646, pp. 79–86. [Google Scholar]
- Fiallos, D.C.; Gómez, C.V.; Tubon Usca, G.; Pérez, D.C.; Tavolaro, P.; Martino, G.; Caputi, L.S.; Tavolaro, A. Removal of acridine orange from water by graphene oxide. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2015; Volume 1646, pp. 38–45. [Google Scholar]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon N. Y. 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Tene, T.; Tubon Usca, G.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Vacacela Gomez, C. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Hussain, N.; Borah, D.J.; Das, M.R. Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet–water interface: A comparative study. J. Chem. Eng. Data 2013, 58, 3477–3488. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y.-P. Magnetic reduced graphene oxide functionalized with $β$-cyclodextrin as magnetic solid-phase extraction adsorbents for the determination of phytohormones in tomatoes coupled with high performance liquid chromatography. J. Chromatogr. A 2016, 1441, 24–33. [Google Scholar] [CrossRef]
- Wijaya, R.; Andersan, G.; Santoso, S.P.; Irawaty, W. Green reduction of graphene oxide using kaffir lime peel extract (Citrus hystrix) and its application as adsorbent for methylene blue. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.V.; Robalino, E.; Haro, D.; Tene, T.; Escudero, P.; Haro, A.; Orbe, J. Structural and electronic properties of graphene oxide for different degree of oxidation. Mater. Today Proc. 2016, 3, 796–802. [Google Scholar] [CrossRef]
- Villamagua, L.; Carini, M.; Stashans, A.; Gomez, C.V. Band gap engineering of graphene through quantum confinement and edge distortions. Ric. di Mat. 2016, 65, 579–584. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.-J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene oxide-based nanocomposites decorated with silver nanoparticles as an antibacterial agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Wu, S.; Wang, Y.; Wu, L.; Yuan, P.; Wang, X. Self-assembly of mildly reduced graphene oxide monolayer for enhanced Raman scattering. J. Solid State Chem. 2016, 237, 57–63. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.W.; Dallmeyer, I.; Johnson, T.J.; Brauer, C.S.; McEwen, J.-S.; Espinal, J.F.; Garcia-Perez, M. Structural analysis of char by Raman spectroscopy: Improving band assignments through computational calculations from first principles. Carbon N. Y. 2016, 100, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of several metal ions onto a low-cost biosorbent: Kinetic and equilibrium studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advances in water pollution research. In Proceedings of the First International Conference on Water Pollution Research, London, UK, 3–7 September 1962; Pergamon Press: Oxford, UK, 1962; Volume 2, p. 231. [Google Scholar]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Alcaraz, L.; López Fernández, A.; García-Díaz, I.; López, F.A. Preparation and characterization of activated carbons from winemaking wastes and their adsorption of methylene blue. Adsorpt. Sci. Technol. 2018, 36, 1331–1351. [Google Scholar] [CrossRef] [Green Version]
- Oliva, J.; Martinez, A.I.; Oliva, A.I.; Garcia, C.R.; Martinez-Luevanos, A.; Garcia-Lobato, M.; Ochoa-Valiente, R.; Berlanga, A. Flexible graphene composites for removal of methylene blue dye-contaminant from water. Appl. Surf. Sci. 2018, 436, 739–746. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Shao, W.-J.; Sun, W.; Kou, Y.-L.; Li, X.; Yang, H.-P. One-step fabrication of $β$-cyclodextrin modified magnetic graphene oxide nanohybrids for adsorption of Pb (II), Cu (II) and methylene blue in aqueous solutions. Appl. Surf. Sci. 2018, 459, 544–553. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Liu, H.; Yu, D.; Sun, T.; Du, H.; Jiang, W.; Muhammad, Y.; Huang, L. Fabrication of surface alkalinized g-C3N4 and TiO2 composite for the synergistic adsorption-photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2019, 473, 855–863. [Google Scholar] [CrossRef]
- Su, H.; Li, W.; Han, Y.; Liu, N. Magnetic carboxyl functional nanoporous polymer: Synthesis, characterization and its application for methylene blue adsorption. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wang, Y.; Feng, Y.; Xie, X.; Li, X.; Yang, S. Different adsorption-degradation behavior of methylene blue and Congo red in nanoceria/H2O2 system under alkaline conditions. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, W.; Huang, J.; Zhou, Y.; Zhu, Y.; Li, C. Rapid degradation of methylene blue in a novel heterogeneous Fe3O4-rGO-TiO2-catalyzed photo-Fenton system. Sci. Rep. 2015, 5, 10632. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Yue, R.; Zhang, X.; Huang, Y. Removal of silver nanoparticles by mussel-inspired Fe3O4@polydopamine core-shell microspheres and its use as efficient catalyst for methylene blue reduction. Sci. Rep. 2017, 7, 42773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef]
- Ai, L.; Li, M.; Li, L. Adsorption of methylene blue from aqueous solution with activated carbon/cobalt ferrite/alginate composite beads: Kinetics, isotherms, and thermodynamics. J. Chem. Eng. Data 2011, 56, 3475–3483. [Google Scholar] [CrossRef]
- Ghasemi, S.S.; Hadavifar, M.; Maleki, B.; Mohammadnia, E. Adsorption of mercury ions from synthetic aqueous solution using polydopamine decorated SWCNTs. J. Water Process Eng. 2019, 32, 100965. [Google Scholar] [CrossRef]







| Temperature | |||
|---|---|---|---|
| Parameters | 298 K | 313 K | 333 K |
| Experimental | |||
| qe(exp) (mg g−1) | 68.21 | 71.78 | 72.24 |
| Pseudo-first-order model | |||
| qe(cal) (mg g−1) | 69.82 | 72.90 | 74.36 |
| k1 (min−1) | 2.166 | 2.727 | 1.714 |
| SSE | 13.38 | 27.07 | 7.75 |
| R2 | 0.997 | 0.994 | 0.998 |
| RMSE | 1.157 | 1.645 | 0.884 |
| Pseudo-second-order model | |||
| qe(cal) (mg g−1) | 70.72 | 73.43 | 75.09 |
| k2 (g mg−1 min−1) | 0.075 | 0.175 | 0.056 |
| SSE | 5.239 | 2.552 | 4.497 |
| R2 | 0.999 | 0.999 | 0.999 |
| RMSE | 0.724 | 0.505 | 0.671 |
| Temperature | |||
|---|---|---|---|
| Parameters | 298 K | 313 K | 333 K |
| IPD model | |||
| Ri | 0.132 | 0.073 | 0.207 |
| Kp (mg g−1 min−1/2) | 2.95 ± 0.67 | 1.62 ± 0.14 | 5.65 ± 1.26 |
| C (mg g−1) | 59.17 ± 1.75 | 66.53 ± 0.37 | 57.27 ± 2.86 |
| R2 | 0.829 | 0.971 | 0.871 |
| T (K) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL (L g−1) | qm(cal) (mg g−1) | R2 | KF (mg(1−n) g−1 L1/n) | n | R2 | |
| 298 | 0.079 ± 0.0003 | 121.95 ± 4.11 | 0.982 | 7.956 ± 0.084 | 0.671 ± 5.5 × 10−5 | 0.945 |
| 313 | 0.081 ± 0.0004 | 116.28 ± 5.71 | 0.980 | 7.568 ± 0.101 | 0.661 ± 0.006 | 0.936 |
| 333 | 0.082 ± 0.0002 | 107.53 ± 2.85 | 0.984 | 6.869 ± 0.075 | 0.646 ± 0.004 | 0.955 |
| Adsorbents | Adsorption Capacity (mg g−1) | References |
|---|---|---|
| Graphene/SrAl2O3:Bi3+ | 42.92 | [69] |
| ß-cyclodextrin/MGO | 93.97 | [70] |
| g-C3N4 (Urea) | 2.51 | [71] |
| TiO2/Na-g-C3N4 | 1.80 | [72] |
| Magnetic carboxyl functional nanoporous polymer | 57.74 | [73] |
| CeO2 | 4.37 | [74] |
| Fe3O4—rGO-TiO2 | 1.67 | [75] |
| Ag-Fe3O4—polydopamine | 45.00 | [76] |
| Citrus hystrix—rGO | 276.06 | [54] |
| HT—activated carbons | 714–847 | [68] |
| Eco-friendly rGO | 121.95 | Present work |
| T (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (kJ mol−1 K−1) |
|---|---|---|---|
| 298 | −22.75 | −2.20 ± 0.04 | 0.069 ± 1.46 × 10−5 |
| 313 | −23.81 | ||
| 333 | −25.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias Arias, F.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Vacacela Gomez, C.; Arias, M.; Caputi, L.S. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials 2020, 10, 681. https://doi.org/10.3390/nano10040681
Arias Arias F, Guevara M, Tene T, Angamarca P, Molina R, Valarezo A, Salguero O, Vacacela Gomez C, Arias M, Caputi LS. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials. 2020; 10(4):681. https://doi.org/10.3390/nano10040681
Chicago/Turabian StyleArias Arias, Fabian, Marco Guevara, Talia Tene, Paola Angamarca, Raul Molina, Andrea Valarezo, Orlando Salguero, Cristian Vacacela Gomez, Melvin Arias, and Lorenzo S. Caputi. 2020. "The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide" Nanomaterials 10, no. 4: 681. https://doi.org/10.3390/nano10040681
APA StyleArias Arias, F., Guevara, M., Tene, T., Angamarca, P., Molina, R., Valarezo, A., Salguero, O., Vacacela Gomez, C., Arias, M., & Caputi, L. S. (2020). The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials, 10(4), 681. https://doi.org/10.3390/nano10040681












