Facile In Situ Photochemical Synthesis of Silver Nanoaggregates for Surface-Enhanced Raman Scattering Applications
Abstract
:1. Introduction
2. Experimental
2.1. Photochemical Deposition
2.2. SERS Measurement
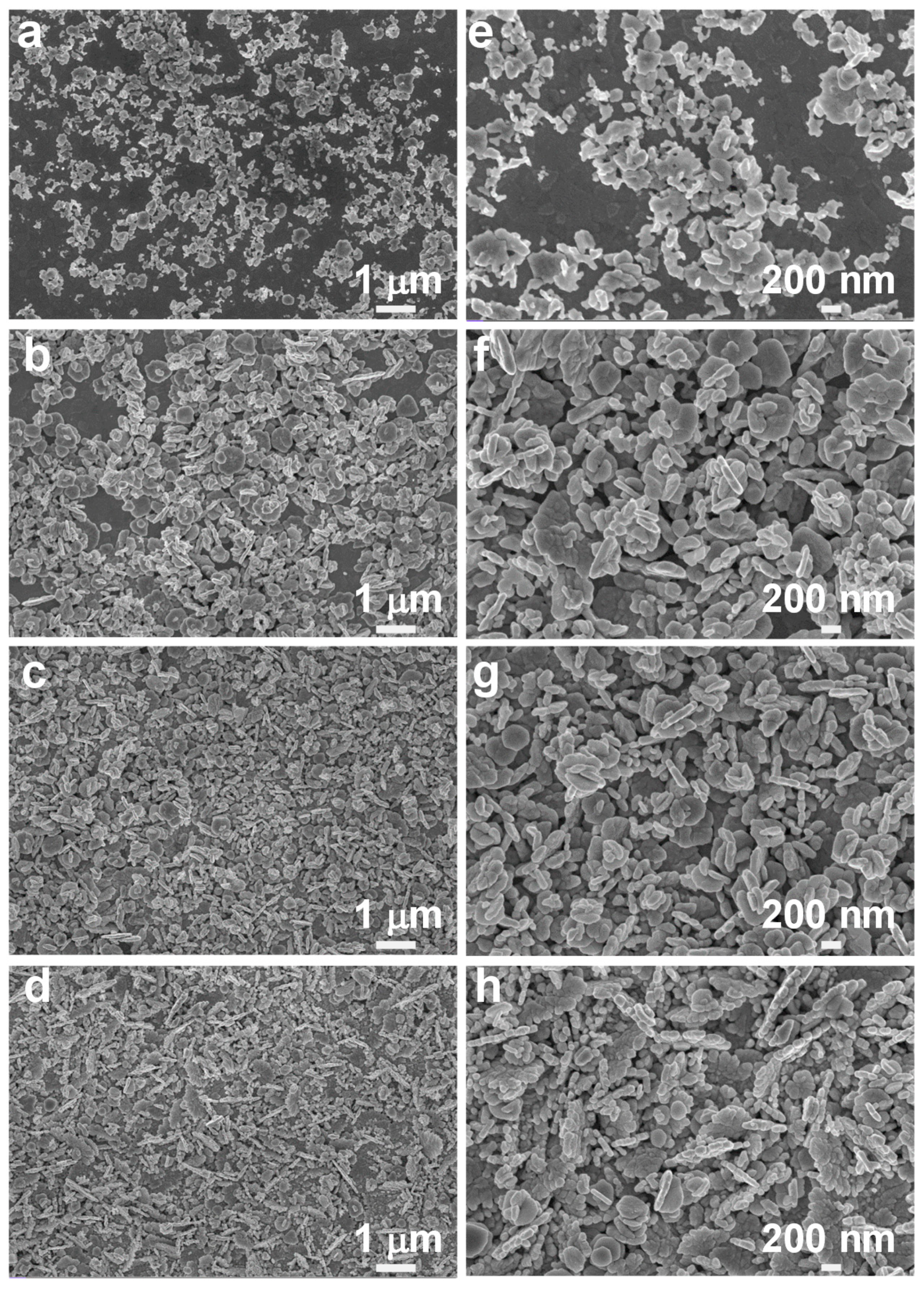
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moskovitz, M. Surface-Enhanced Spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Le Ru, E.; Etchegoin, P. Principles of Surface Enhanced Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Yang, M.; Alvarez-Puebla, R.; Kim, H.S.; Aldeanueva-Potel, P.; Liz-Marzan, L.M.; Kotov, N.A. SERS-active gold lace nanoshells with built-in hotspots. Nano Lett. 2010, 10, 4013–4019. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Li, G.Y.; Li, D.H.; Dodson, S.; Zhang, Q.; Zhang, J.; Lee, Y.H.; Demir, H.V.; Ling, X.Y.; Xiong, Q.H. Vertically aligned gold nanorod monolayer on arbitrary substrates: Self-Assembly and femtomolar detection of food contaminants. ACS Nano 2013, 7, 5993–6000. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jaber, S.; Peveler, W.J.; Quesada-Cabrera, R.; Cortes, E.; Sotelo-Vazquez, C.; Abdul-Karim, N.; Maier, S.A.; Parkin, I.P. Photo-induced enhanced Raman spectroscopy for universal ultra-trace detection of explosives, pollutants and biomolecules. Nat. Commun. 2016, 7, 12189. [Google Scholar] [CrossRef] [Green Version]
- Cialla-May, D.; Zheng, X.-S.; Weber, K.; Popp, J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: From cells to clinics. Chem. Soc. Rev. 2017, 46, 3945–3961. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Gia, C.P.-Q.; Han, X.M.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.-C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.C.; Zhou, R.; Takei, K.; Hong, M.H. Toward flexible surface-enhanced Raman scattering (SERS) sensors for point-of-care diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Reguera, J.; Langer, J.; de Aberasturi, D.J.; Liz-Marzan, L.M. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zheng, C.; Yin, Z.; Wang, Z.; Wang, J.; Liu, J.; Luo, D.; Liu, Y.J. Hierarchically ordered silicon metastructures from improved self-assembly-based nanosphere lithography. ACS Appl. Mater. Interfaces 2020, 12, 12345–12352. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent advances of plasmonic nanoparticles and their applications. Materials 2018, 11, 1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R.; Cao, Y.C.; Hao, E.; Métraux, G.S.; Schatz, G.C.; Mirkin, C.A. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 2003, 425, 487–490. [Google Scholar] [CrossRef]
- Zeng, J.; Xia, X.; Rycenga, M.; Henneghan, P.; Li, Q.; Xia, Y. Successive deposition of silver on silver nanoplates: Lateral versus vertical growth. Angew. Chem. Int. Ed. 2011, 50, 244–249. [Google Scholar] [CrossRef]
- Xu, B.B.; Wang, L.; Ma, Z.-C.; Zhang, R.; Chen, Q.-D.; Lv, C.; Han, B.; Xiao, X.-Z.; Zhang, X.-L.; Zhang, Y.-L.; et al. Surface-plasmon-mediated programmable optical nanofabrication of an oriented silver nanoplate. ACS Nano 2014, 8, 6682–6692. [Google Scholar] [CrossRef]
- Mao, A.Q.; Jin, X.; Gu, X.L.; Wei, X.Q.; Yang, G.J. Rapid, green synthesis and surface-enhanced Raman scattering effect of single-crystal silver nanocubes. J. Mol. Struct. 2012, 1021, 158–161. [Google Scholar] [CrossRef]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Xue, C.; Mirkin, C.A. pH-switchable silver nanoprism growth pathways. Angew. Chem. Int. Ed. 2007, 46, 2036–2038. [Google Scholar] [CrossRef]
- Tang, B.; Xu, S.; An, J.; Zhao, B.; Xu, W. Photoinduced shape conversion and reconstruction of silver nanoprisms. J. Phys. Chem. C 2009, 113, 7025–7030. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hu, A.; Zhang, T.; Lei, W.; Xue, X.J.; Zhou, Y.; Duley, W.W. Self-assembly of large-scale and ultrathin silver nanoplate films with tunable plasmon resonance properties. ACS Nano 2011, 5, 9082–9092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Langille, M.R.; Mirkin, C.A. Photomediated synthesis of silver triangular bipyramids and prisms: The effect of pH and BSPP. J. Am. Chem. Soc. 2010, 132, 12502–12510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henzie, J.; Grünwald, M.; Widmer-Cooper, A.; Geissler, P.L.; Yang, P. Self-assembly of uniform polyhedral silver nanocrystals into densest packings and exotic superlattices. Nat. Mater. 2012, 11, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.T.; Liu, Y.J.; Xu, S.C.; Li, Z.; Guo, J.; Gao, S.S.; Lu, Z.Y.; Si, H.P.; Jiang, S.Z.; Wang, S.Y. Facile and low-cost fabrication of Ag-Cu substrates via replacement reaction for highly sensitive SERS applications. Chem. Phys. Lett. 2017, 667, 351–356. [Google Scholar] [CrossRef]
- Hu, L.T.; Liu, Y.J.; Han, Y.S.; Chen, P.X.; Zhang, C.; Li, C.H.; Lu, Z.Y.; Luo, D.; Jiang, S.Z. Graphene oxide-decorated silver dendrites for high-performance surface-enhanced Raman scattering applications. J. Mater. Chem. C 2017, 5, 3908–3915. [Google Scholar] [CrossRef]
- Maillard, M.; Huang, P.; Brus, L. Silver nanodisk growth by surface plasmon enhanced photoreduction of adsorbed [Ag+]. Nano. Lett. 2003, 3, 1611–1615. [Google Scholar] [CrossRef]
- Pillai, Z.S.; Kamat, P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Al Gharib, S.; Marignier, J.-L.; EI Omar, A.K.; Naja, A.; Le Caer, S.; Mostafavi, M.; Belloni, J. Key role of the oxidized citrate-free radical in the nucleation mechanism of the metal nanoparticle Turkevich synthesis. J. Phys. Chem. C 2019, 123, 22624–22633. [Google Scholar] [CrossRef] [Green Version]
- Remita, S.; Mostafavi, M.; Delcourt, M.O. Stabilization, growth and reactivity of silver aggregates produced by radiolysis in the presence of EDTA. New J. Chem. 1994, 18, 581–588. [Google Scholar]
- Xue, C.; Métraux, G.S.; Millstone, J.E.; Mirkin, C.A. Mechanistic study of photomediated triangular silver nanoprism growth. J. Am. Chem. Soc. 2008, 130, 8337–8344. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.H.; Yu, J.; Jiang, S.Z.; Xu, S.C.; Yang, C.; Liu, Y.J.; Gao, X.G.; Liu, A.H.; Man, B.Y. SERS activated platform with three-dimensional hot spots and tunable nanometer gap. Sens. Actuators B Chem. 2018, 258, 163–171. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Liu, Y.J.; Wang, M.H.; Zhang, C.; Li, Z.; Huo, Y.Y.; Li, Z.; Xu, S.C.; Man, B.Y.; Jiang, S.Z. A novel natural surface-enhanced Raman spectroscopy (SERS) substrate based on graphene oxide-Ag nanoparticles-Mytilus coruscus hybrid system. Sens. Actuators B Chem. 2018, 261, 1–10. [Google Scholar] [CrossRef]
- Wu, X.T.; Fan, X.K.; Yin, Z.; Liu, Y.J.; Zhao, J.; Quan, Z.W. Ordered mesoporous silver superstructures with SERS hot spots. Chem. Commun. 2019, 55, 7982–7985. [Google Scholar] [CrossRef] [PubMed]
- Foti, A.; D’Andrea, C.; Villari, V.; Micali, N.; Donato, M.G.; Fazio, B.; Maragò, O.M.; Gillibert, R.; de la Chapelle, M.L.; Gucciardi, P.G. Optical aggregation of gold nanoparticles for SERS detection of proteins and toxins in liquid environment: Towards ultrasensitive and selective detection. Materials 2018, 11, 440. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, C.; Irrera, A.; Fazio, B.; Foti, A.; Messina, E.; Maragò, O.M.; Kessentini, S.; Artoni, P.; David, C.; Gucciardi, P.G. Red shifted spectral dependence of the SERS enhancement in a random array of gold nanoparticles covered with a silica shell: Extinction versus scattering. J. Opt. 2015, 17, 114016. [Google Scholar] [CrossRef]
- Ke, X.; Lu, B.; Hao, J.; Zhang, J.; Qiao, H.; Zhang, Z.; Xing, C.; Yang, W.; Zhang, B.; Tang, J. Facile fabrication of SERS arrays through galvanic replacement of silver onto electrochemically deposited copper micropatterns. ChemPhysChem 2012, 13, 3786–3789. [Google Scholar] [CrossRef]
- Débarre, A.; Jaffiol, R.; Julien, C.; Tchénio, P.; Mostafavi, M. Raman scattering from single Ag aggregates in presence of EDTA. Chem. Phys. Lett. 2004, 386, 244–247. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, S.; Mao, Z.; Li, P.; Zhao, C.; Cohick, Z.; Huang, P.H.; Huang, T.J. In situ fabrication of 3D Ag@ZnO nanostructures for microfluidic surface-enhanced Raman scattering systems. ACS Nano 2014, 8, 12175–12184. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Z.; He, H.; Wang, Z.; Fang, X.; Xu, C.; Luo, D.; Jiang, S.; Liu, Y.J. Facile In Situ Photochemical Synthesis of Silver Nanoaggregates for Surface-Enhanced Raman Scattering Applications. Nanomaterials 2020, 10, 685. https://doi.org/10.3390/nano10040685
Yin Z, He H, Wang Z, Fang X, Xu C, Luo D, Jiang S, Liu YJ. Facile In Situ Photochemical Synthesis of Silver Nanoaggregates for Surface-Enhanced Raman Scattering Applications. Nanomaterials. 2020; 10(4):685. https://doi.org/10.3390/nano10040685
Chicago/Turabian StyleYin, Zhen, Huilin He, Zhenming Wang, Xiaoguo Fang, Chunxiang Xu, Dan Luo, Shouzhen Jiang, and Yan Jun Liu. 2020. "Facile In Situ Photochemical Synthesis of Silver Nanoaggregates for Surface-Enhanced Raman Scattering Applications" Nanomaterials 10, no. 4: 685. https://doi.org/10.3390/nano10040685
APA StyleYin, Z., He, H., Wang, Z., Fang, X., Xu, C., Luo, D., Jiang, S., & Liu, Y. J. (2020). Facile In Situ Photochemical Synthesis of Silver Nanoaggregates for Surface-Enhanced Raman Scattering Applications. Nanomaterials, 10(4), 685. https://doi.org/10.3390/nano10040685







