1. Introduction
Stretchable electrode films (SEF) have increasingly attracted attention owing to their potential uses for many devices such as flexible displays, sensors, actuators and energy storage devices [
1,
2,
3,
4]. In particular, these SEFs, which have recently begun to be widely used as sensor-films, respond to mechanical deformations by the changes in electrical characteristics, such as resistance, owing to their stretchability and reproducibility. Nowadays, SEF applications, as a gas sensing film that responds to environmental stimuli (i.e., chemical, electric, magnetic, piezo stimuli) to produce a dynamic and reversible change in their properties [
5], have attracted considerable interest owing to their promising applicability to sensors [
6,
7]. In particular, these stretchable sensor films (SSF) have great significance in being applicable to various wearable electronics such as soft robotics [
8], artificial e-skins [
9], human skin-attached film sensors, portable chem-resistive gas sensors and health care monitoring/diagnostic devices [
10]. To make skin-mountable devices or wearable, stretchable, defined by the failure strain, is essential as well as flexibility. For this purpose, several types of stretchable sensors have been developed through the combination of nanomaterials with excellent electrical and mechanical properties and elastic polymers [
11,
12,
13]. However, flexible and stretchable sensors of thin film type have become increasingly attractive due to the number of advantages such as miniaturization and portable or wearable properties.
In this regard, nanomaterials such as silver nanowires (Ag NWs) [
4], single-walled carbon nanotubes (SWCNTs) [
14,
15,
16] and graphene sheets [
17,
18,
19] and their composite structures, have been reported to be widely used as sensitive strain sensors, which have an electrical conductor [
20,
21,
22,
23]. Recently, strain sensors based on a nanocomposite of an Ag NWs network embedded between two layers of a polydimethylsiloxane (PDMS) elastomer has been reported to exhibit high stretchability up to 70% with strong piezoresistivity [
4]. In addition, conducting polymers such as polypyrrole and polyaniline and their metal oxide nanocomposites have been reported to demonstrate considerable potential as an efficient chemical gas [
24,
25,
26,
27,
28] or pH-responding materials [
29,
30] owing to their unique electrical, optical and mechanical transduction mechanism [
31,
32]. Our group [
27] has also reported that the sensor films comprising polypyrrole nanoparticles (PPy NPs) complexed with phenylalanine (PA) sensitively react to ammonia gas and exhibit significantly improved reproducibility and reversibility upon gas exposure. Fu et al. [
30] have been reported an electrical switch for smart pH sensor films based on Ag NW-polyaniline nanocomposite. However, despite of the several studies being conducted on transparency, extensibility and sensitivity, smart sensing materials or structures still do not completely satisfy the requirement as sensor with controllable chemicals, pH, piezo stimuli and rapid response at a low voltage.
Herein, we report the highly sensitive, selective, extensible and reliable “smart SSF” based on a composite of functionalized PPy NPs with SWCNT–Ag NW hybrid networks embedded into cross-linked PDMS elastomer, as shown in
Figure 1. In particular, the hybrid conductor comprising an Ag NW network interconnected with SWCNTs embedded into PDMS exhibited high elasticity, cycling stability and excellent sensitive at low voltage. In addition, the SWCNT–Ag NW hybrid network structured conductor films were confirmed to exhibit good response to the stretch/release for ≥100 cycles and hysteresis tests without the loss of conductivity under stretching conditions of 25% were also conducted. PA-complexed PPy NPs as effective chemical sensing materials were also prepared by incorporating PA into the conductive PPy backbone, which was hybridized with the SWCNT–Ag NW hybrid networked conductor. The PPy-PA complex NPs exhibited effective reproducibility and reversibility when exposed to chemicals such as ammonia, which were characterized by the ability to increase the sensitivity by 2-fold to chemicals via hybridization with SWCNT-Ag NW hybrid networked conductors.
3. Results and Discussion
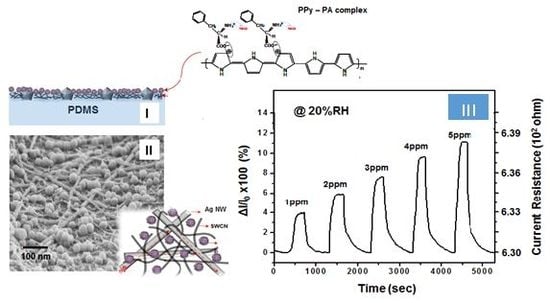
Figure 1-(I) and
Figure 3-(I) show the cross section of a sample of the fabricated SSF and their SEM image and material structures prepared using a composite of functionalized PPy as the sensing material with high-conducting SWCNT–Ag NW hybrid networks embedded into the PDMS elastomer and
Figure 1-(II) show a SEM image of the surface of the SEF. As shown in the model description in
Figure 1, PPy–PA-complexed NPs of functionalized PPy as the sensing material, comprising PA-bound PPy NPs, have been reported to be potentially applicable as good chemical sensors [
27]. Basically, they are connected via a layer-to-layer hybrid to the SWCNT–Ag NW hybrid network conductors. In this study, PPy–PA-complexed NPs were prepared via emulsion polymerization using PVP as the capping reagent. Spheroid-type well-defined PPy–PA-complexed NPs were obtained without aggregation (
Figure 3-(II)). Changes in reaction conditions such as PVP concentration and temperature did not affect the shape of the resulting polymer; however, the NP size was strongly affected by the PVP molecular weight.
Figure 3-II-(a to c) shows the structures of PPy–PA-complexed NPs obtained using different PVP molecular weights of 40000, 360,000 and 1300,000, respectively. Using a PVP molecular weight of 40,000, NPs with sizes ranging from 80 to 110 nm were obtained, whereas on using PVP molecular weights of 360,000 and 1300,000, NPs with sizes ranging from 60 to 90 nm and 40 to 60 nm were obtained, respectively. PA was incorporated into the PPy backbone via charge–charge interactions between the positively charged polaron of doped PPy and the appropriate counter ion of PA. PPy–PA NPs were found to be electrically good conducting materials, with a conductivity of ~12 S/cm and could be used for redox reactions. In addition, the doping level of PPy–PA NPs, which can determine the degree of conductivity, can be changed via oxidation or reduction, leading to the change in conductivity. With this characteristic, PPy–PA NPs can exhibit sensitivity to specific chemical species following the redox process, for example, ammonia and these NPs can demonstrate excellent sensitivity via enhanced interaction between the PPy–PA NPs and analytes. In particular, when a functional structural material (here, PA) with a carboxylic group and an amino group symmetric to each other, as shown in the chemical structure of
Figure 3, was electrically coupled to the electro-conductive PPy main chain, the positive charges of an amine group (NH
3+ region of PA) were sensitive to NH
3 [
27]. In this process, the lone pair of NH
3 selectively reacts only with the NH
3+ part of PA without affecting the positively charged (polaron) structure comprising the main chain of PPy. At this instance, the current density of the main chain of PPy increases with the flow of the external current. Conversely, after the removal of NH
3, the bond between NH
3 and NH
3+ is broken, returning to the current at the existing state. This reversible reaction is a structural feature of a PPy–PA-complexed polymer having a π-conjugated double bond system as the sensing material. However, based on the abovementioned reaction mechanism, PPy–PA NPs can react via rapid adsorption and desorption kinetics for analytes, leading to a rapid response–recovery time.
Figure 4-(I) shows a photograph of the finally produced stretchable sensor film sample and
Figure 4-(II), (III) and (IV) shows the film’s SEM surface images of approximately 50-nm PPy–PA-complexed NPs of 1, 2 and 3 wt% coated on the Ag NW–SWCNT hybrid conductive layer with a sheet resistance of 30 Ω/sq, respectively. PPy–PA-complexed NPs with diameters of 40–60 nm was uniformly dispersed on the top of the Ag NW-SWCNT network layer. Next, PPy–PA-complexed NPs were interconnected with the Ag NW–SWCNT network, affording a junction of possible mutual electron transitions and the Ag NW–SWCNT network improved the electrical conductivity owing to the electrical force and also acted on the electrical spreaders in the sensor films. This ensured good contact between the Ag NW–SWCNT network and PPy–PA-complexed NPs, leading to an advanced electrical property of the conductor. In particular, as a result of coating 1, 2 and 3 wt% PPy-PA-complexed NP solution on the Ag NW–SWCNT hybrid conductor, the sheet resistance of the conductor film itself was 30 Ω/sq, regardless of the concentration of PPy-PA-complexed NP. However, the sheet resistance did not change with the Ag NW–SWCNT network conductor before the PPy–PA-complexed NP coating but different light transmittances were observed depending on the density or concentration of the PPy–PA-complexed NPs. Herein, a highly sensitive, extensible and reliable “smart SSF” based on a bilayer hybrid structure of PPY–PA NPs and an Ag NW–SWCNT hybrid layer embedded in the surface layer of the cross-linked PDMS elastomer film, with characteristic elastic deformation is reported. PDMS completely penetrated into the Ag NW– SWCNT network and filled the gaps between Ag NWs and SWCNTs, affording an Ag NW–SWCNT hybrid network and PDMS.
Figure 5 shows the elastic behavior for the sample under dynamic loading. In
Figure 5 (I), the change in R/R
0 at strain restraint for tensile strain at 15%, 20% and 25% tensile strain was observed at 25 °C room temperature. The initial sheet resistance (R
0) was almost completely recovered for a stretch/release cycle test with strains ε between 15% and 25%, revealing the outstanding stretchable property of film sensors (
Figure 5 (I)). Given that the flexible and stretchable characteristics of PPY–PA NPs/Ag NW–SWCNT-hybrid network-embedded PDMS film sensors can obtain highly reliable mechanical performance under continuous strain deformation, repeated stretch/release tests were conducted on the films. In any case, as shown in
Figure 5 (I), the resistance of the SSF sensors sharply increases at the very first stretching and then returns to its initial value in the range of 15% to 25%. With the repetition of the test for ≥100 cycles under stretching conditions of 1%, 20% and 25%, the change in the resistance was also restored to its original position without any change in the resistance, as shown in
Figure 5-(II).
Figure 2 shows the fabrication of the proposed highly sensitive strain sensor films based on the composite of functionalized PPy-NPs with the SWCNT–Ag NW hybrid networks embedded into the cross-linked PDMS elastomer surface layer owing to its high elasticity, flexibility, heat resistance and strong adhesion. After the peeling the cured PDMS from the Si wafer. Here, when liquid PDMS covers the SWCNT–Ag NW hybrid network layer, it penetrates into the interconnected pores of the two-dimensional (2D) SWCNT–Ag NW hybrid network because of its low viscosity and low surface energy. After curing, all 2D SWCNT–Ag NW hybrid networks are buried on the cross-linked PDMS surface without considerable voids, indicative of the successful transfer of SWCNT–Ag NW hybrid networks from Si wafers to PDMS. The Ag NW–SWCNT network layer comprising an Ag NW network interconnected with SWCNTs is embedded into the surface of ~50-µm-thick-PDMS films. Finally, a PPy–PA-complexed NP aqueous solution was directly coated on the upper surface of the Ag NW–SWCNT hybrid conductive layer, which exhibited optical transparency and good electrical conductivity.
Before the gas detection test, the SSF sample was dried in a vacuum chamber at 80 °C for 30 min. The SSF sample was exposed to dry argon gas through the chamber for at least 10 min to stabilize the initial dc electrical resistance. Gas detection experiments were performed at 40 °C and the interference of humidity was also examined at 20, 50 and 80% RH conditions, respectively, as presented in
Figure 6.
Figure 6 (I) shows the flow of NH
3 at 1 to 5 ppm for 30 s at 20% RH condition and the current resistance change at that time ad actual current resistance value. In addition, the flow of NH
3 gas for 30 seconds was stopped and the chamber filled with dry argon gas until a steady current was reached. This process was repeated with varying concentrations of ammonia gas. Sensor response was evaluated as Δ
R/
R0 for ammonia, where
R0 (current resistance) was evaluated after argon-gas exposure and
R was evaluated after exposure of NH
3 gas. The normalized current resistance change, S (defined as S = 100 × Δ
R/
R0, where
R0 and
R denote the current resistance in argon gas before exposure to NH
3 and current resistance during exposure to NH
3, respectively) was examined to measure the current variation.
As can be seen from
Figure 6 sensitivity reached 90% within a short response time (defined as the time to achieve a 90% current response) of 25 s. When film sensor was exposed to argon gas, the recovery time (defined as the time of a 90% current decrease) was ~700 s (
Figure 6-(II)). However, as shown in the
Figure 6-(I), the initial resistance (
R0) before the introduction of 1 ppm NH
3 gas was 6.31E + 02 ohm but their resistance value was rapidly increased to with the influx of ammonia. At this time, the change rate of the resistance value (S = 100×Δ
R/
R0) was 4.3% and current resistances (
R) was 6.33E + 02 ohm. On the other hand, when NH
3 gas was introduced at 2, 3, 4 and 5 ppm, respectively, under the 20% RH conditions, then S increased to 6.2, 8.2, 10.4 and 11.6%. Moreover, the base line is very stable after each cycle, indicating good real-time repeatability. In a steady-state response after 1500 s of exposure to gaseous ammonia as a function of ammonia concentration of 1 ppm to 5 ppm, the response shows a generally linear increase. However, as demonstrated by this work, this SSF provide very satisfactory performance results, especially when considered in terms of the response value under low temperature. In any case, the gas detection experiment in
Figure 6 was performed on undrawn film and examined at 20% RH conditions. However, as shown in
Figure 5, when the strain is stretched from 20% to 25%, the change value of the sheet resistance of the sensor film (initial: 30 Ω/sq) was increased to 55–75 Ω/sq, which is 1.8 to 2.5 times. When the stretched state was relaxed to the initial state, it was also found that the sheet resistance value of the stretched state was restored to the initial resistance. However, as shown in
Figure 5, when the strain was stretched between 0% and 25%, even if the current resistance in the sensor film varies by 2.5 times and there was no significant difference in ammonia gas’s sensitivity to current change within this range. This means that the sensitivity to ammonia gas responsiveness does not differ significantly when the stretchable sensor dynamically expands and contracts. In addition, when the same procedure was performed at 50% RH and 80% RH with 1 ppm ammonia gas flow, then S was slightly increased to 5.8% and 5.7% but the influence of humidity was not so large, as indicated in
Figure 6-(III). That is, it showed good humidity-resistant gas sensing characteristics. It seems to be because the sensing material (PPy-PA NP/Ag NW-SWCNT) is composed of a complex of conjugated carbon compounds with high electrical conductivity and the organic sensing group (PA) has a high binding force with ammonia gas. As a result, the change of the electrical resistance value with humidity was very small as 6% below. We experimented with argon, nitrogen and dry air conditions, respectively, to recover the sensor resistance to the original state. Similar results were obtained regardless of the type of inlet gas. However, in our experiment, by using dry argon gas, it was possible to keep the steady state current of the gas sensor more constant.
On the other hand, for comparison, reversible, reproducible responses of a sensor film comprising only PPy–PA NP with the injection of 3 ppm of NH
3 were recorded as reference data (
Figure 6-(II-b)) [
21]. In this case, ~50% lower sensitivity was observed (
Figure 6-(II-a)). However, the sensor films fabricated on the basis of the composite of functionalized PPy NP with SWCNTs–Ag NW hybrid networks were more sensitive to NH
3 as compared with a sensor film comprising only PPy–PA-complexed NPs. This result is explained by the fact that SWCNTs–Ag NW hybrid networks as conductors extend the movement path of electrons and the electron movement becomes more active (the sensing mechanism of NH
3, in the case of sensor films comprising PPy NP-complexed with PA, is explained in a previous paper [
27]. Selectivity is another important parameter for a gas sensor in practical use. Our work further tested various volatile organic compounds (VOCs), such as acetone, formaldehyde, ethanol and toluene. The results were measured at the optimum operating conditions of each sensor to evaluate the selective properties. As clearly shown above, the PPY–PA NPs/Ag NW–SWCNT-hybrid network film sensor exhibits high selectivity for gaseous ammonia (S = 4 at 1 ppm) compared to other interfering analytes, to which it presents very high responses. On the other hand, in the case of acetone, formaldehyde, ethanol and toluene, the changes of resistance (S) with gas exposure were hardly observed at concentrations below 5 ppm, only when more than 100 ppm of gas was introduced could some amount of current change (S = ~4 at 100 ppm) be detected. These results suggest that the sensing material in this work can be confirmed to have a special selectivity in the ammonia gas composition. The experiments in this work are the result of sensitivity measurement under one gas condition and did not confirm the selectivity of specific gas component in two or more mixed gas environments.