Double Metal Oxide Electron Transport Layers for Colloidal Quantum Dot Light-Emitting Diodes
Abstract
1. Introduction
2. Experiments
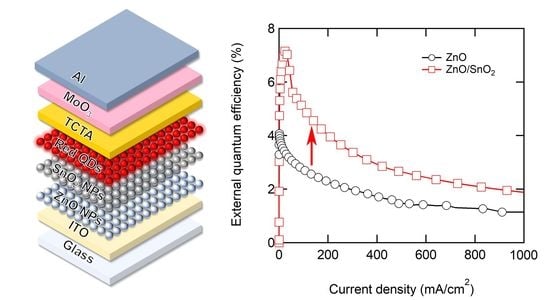
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Colvin, V.L.; Schlamp, M.C.; Alivisatos, A.P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357. [Google Scholar] [CrossRef]
- Sargent, E.H. Colloidal quantum dot solar cells. Nat. Photonics 2012, 6, 133–135. [Google Scholar] [CrossRef]
- Oertel, D.C.; Bawendi, M.G. Photodetectors based on treated CdSe quantum-dot films. Appl. Phys. Lett. 2005, 87, 213505. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Lu, M.; Ji, C.; Sun, C.; Chen, X.; Chen, H.; Colvin, V.L.; Yu, W.W. White Light-Emitting Diodes Based on AgInS2/ZnS Quantum Dots with Improved Bandwidth in Visible Light Communication. Nanomaterials 2016, 6, 13. [Google Scholar] [CrossRef]
- Yan, C.; Du, X.; Li, J.; Ding, X.; Li, Z.; Tang, Y. Effect of Excitation Wavelength on Optical Performances of Quantum-Dot-Converted Light-Emitting Diode. Nanomaterials 2019, 9, 1100. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Li, Z.; Ding, X.; Yu, S.; Yu, B. Improvement in Color-Conversion Efficiency and Stability for Quantum-Dot-Based Light-Emitting Diodes Using a Blue Anti-Transmission Film. Nanomaterials 2018, 8, 508. [Google Scholar] [CrossRef]
- Cho, K.-S.; Lee, E.K.; Joo, W.-J.; Jang, E.; Kim, T.-H.; Lee, S.J.; Kim, J.M. High-performance crosslinked colloidal quantum-dot light-emitting diodes. Nat. Photonics 2009, 3, 341–345. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Moon, A.; Kim, J. Hybrid Quantum Dot Light-Emitting Diodes for White Emission Using Blue Phosphorescent Organic Molecules and Red Quantum Dots. Micromachines 2019, 10, 609. [Google Scholar] [CrossRef]
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Lim, J.; Park, M.; Bae, W.K.; Lee, D.; Lee, S.; Lee, C.; Char, K. Highly Efficient Cadmium-Free Quantum Dot Light-Emitting Diodes Enabled by the Direct Formation of Excitons within InP@ZnSeS Quantum Dots. ACS Nano 2013, 7, 9019–9026. [Google Scholar] [CrossRef]
- Chen, S.; Tang, M.; Wu, J.; Jiang, Q.; Dorogan, V.; Benamara, M.; Mazur, Y.I.; Salamo, G.J.; Liu, H. Long-Wavelength InAs/GaAs Quantum-Dot Light Emitting Sources Monolithically Grown on Si Substrate. Photonics 2015, 2, 646–658. [Google Scholar] [CrossRef]
- Yamada, H.; Shirahata, N. Silicon Quantum Dot Light Emitting Diode at 620 nm. Micromachines 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Shirahata, N. All-Inorganic Red-Light Emitting Diodes Based on Silicon Quantum Dots. Crystals 2019, 9, 385. [Google Scholar] [CrossRef]
- Tien, C.-H.; Chen, L.-C.; Lee, K.-Y.; Tseng, Z.-L.; Dong, Y.-S.; Lin, Z.-J. High-Quality All-Inorganic Perovskite CsPbBr3 Quantum Dots Emitter Prepared by a Simple Purified Method and Applications of Light-Emitting Diodes. Energies 2019, 12, 3507. [Google Scholar] [CrossRef]
- Lao, X.; Li, X.; Ågren, H.; Chen, G. Highly Controllable Synthesis and DFT Calculations of Double/Triple-Halide CsPbX3 (X = Cl, Br, I) Perovskite Quantum Dots: Application to Light-Emitting Diodes. Nanomaterials 2019, 9, 172. [Google Scholar] [CrossRef]
- Caruge, J.M.; Halpert, J.E.; Wood, V.; Bulović, V.; Bawendi, M.G. Colloidal quantum-dot light-emitting diodes with metal-oxide charge transport layers. Nat. Photonics 2008, 2, 247–250. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Kuo, Y.P.; Chen, P.-Y.; Lu, H.H.; Lin, M.Y. Influence of Annealing Temperature on Weak-Cavity Top-Emission Red Quantum Dot Light Emitting Diode. Nanomaterials 2019, 9, 1639. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J. Correlation between the Morphology of ZnO Layers and the Electroluminescence of Quantum Dot Light-Emitting Diodes. App. Sci. 2019, 9, 4539. [Google Scholar] [CrossRef]
- Park, M.; Song, J.; An, M.; Lim, J.; Lee, C.; Roh, J.; Lee, D. Colloidal quantum dot light-emitting diodes employing solution-processable tin dioxide nanoparticles in an electron transport layer. RSC Adv. 2020, 10, 8261–8265. [Google Scholar] [CrossRef]
- Kwak, J.; Bae, W.K.; Lee, D.; Park, I.; Lim, J.; Park, M.; Cho, H.; Woo, H.; Yoon, D.Y.; Char, K.; et al. Bright and Efficient Full-Color Colloidal Quantum Dot Light-Emitting Diodes Using an Inverted Device Structure. Nano Lett. 2012, 12, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef]
- Bae, W.K.; Park, Y.-S.; Lim, J.; Lee, D.; Padilha, L.A.; McDaniel, H.; Robel, I.; Lee, C.; Pietryga, J.M.; Klimov, V.I. Controlling the influence of Auger recombination on the performance of quantum-dot light-emitting diodes. Nat. Commun. 2013, 4, 2661. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Kim, J.; Jang, H.-K.; Lee, K.J.; Seo, J.H.; Jung, B.J.; Kim, G.; Kwak, J. Controlling charge balance using non-conjugated polymer interlayer in quantum dot light-emitting diodes. Org. Electron. 2017, 50, 82–86. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, W.; Kim, D.; Lee, W.; Chae, H. Highly Efficient and Fully Solution-Processed Inverted Light-Emitting Diodes with Charge Control Interlayers. ACS Appl. Mater. Interfaces 2018, 10, 17295–17300. [Google Scholar] [CrossRef]
- Park, M.; Song, J.; Jung, H.; An, M.; Lim, J.; Lee, C.; Roh, J.; Lee, D. Improving Performance of Inverted Blue QD-LEDs by Adopting Organic/Inorganic Double Electron Transport Layer. Phys. Status Solidi RRL 2020, 1900737. [Google Scholar] [CrossRef]
- Park, M.; Lim, J.; Kwon, Y.; Jung, H.; Kwak, J.; Char, K.; Lee, S.; Lee, C. Improved Performance of Quantum Dot Light Emitting Diodes by Using Charge Blocking Layer. SID Symp. Dig. Tech. Papers 2014, 45, 1309–1311. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef]
- Lee, H.; Kang, C.-M.; Park, M.; Kwak, J.; Lee, C. Improved Efficiency of Inverted Organic Light-Emitting Diodes Using Tin Dioxide Nanoparticles as an Electron Injection Layer. ACS Appl. Mater. Interfaces 2013, 5, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Vasić, B.; Aškrabić, S.; Jakovljević, M.M.; Artemyev, M. Local electrical properties and charging/discharging of CdSe/CdS core-shell nanoplatelets. Appl. Surf. Sci. 2020, 513, 145822. [Google Scholar] [CrossRef]
- Benetti, D.; Cui, D.; Zhao, H.; Rosei, F.; Vomiero, A. Direct Measurement of Electronic Band Structure in Single Quantum Dots of Metal Chalcogenide Composites. Small 2018, 14, 1801668. [Google Scholar] [CrossRef]
- Hansen, W.N.; Hansen, G.J. Standard reference surfaces for work function measurements in air. Surf. Sci. 2001, 481, 172–184. [Google Scholar] [CrossRef]
- Lim, J.; Park, Y.-S.; Wu, K.; Yun, H.J.; Klimov, V.I. Droop-Free Colloidal Quantum Dot Light-Emitting Diodes. Nano Lett. 2018, 18, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.K.; Lim, J.; Zorn, M.; Kwak, J.; Pakr, Y.-S.; Lee, D.; Lee, S.; Char, K.; Zentel, R.; Lee, C. Reduced efficiency roll-off in light-emitting diodes enabled by quantum dot–conducting polymer nanohybrids. J. Mater. Chem. C 2014, 2, 4974–4979. [Google Scholar] [CrossRef]




| Parameter | ZnO NPs (40 nm) | SnO2 NPs/ZnO NPs (20 nm)/(20 nm) |
|---|---|---|
| J (mA/cm2) @ 5 V | 33.7 | 80.44 |
| L (cd/m2) @ 5 V | 848 | 3061 |
| CEmax (cd/A) | 3.2 | 5.31 |
| EQEmax (%) | 4.11 | 7.16 |
| PEmax (lm/W) | 3.86 | 4.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Roh, J.; Lim, J.; Lee, H.; Lee, D. Double Metal Oxide Electron Transport Layers for Colloidal Quantum Dot Light-Emitting Diodes. Nanomaterials 2020, 10, 726. https://doi.org/10.3390/nano10040726
Park M, Roh J, Lim J, Lee H, Lee D. Double Metal Oxide Electron Transport Layers for Colloidal Quantum Dot Light-Emitting Diodes. Nanomaterials. 2020; 10(4):726. https://doi.org/10.3390/nano10040726
Chicago/Turabian StylePark, Myeongjin, Jeongkyun Roh, Jaehoon Lim, Hyunkoo Lee, and Donggu Lee. 2020. "Double Metal Oxide Electron Transport Layers for Colloidal Quantum Dot Light-Emitting Diodes" Nanomaterials 10, no. 4: 726. https://doi.org/10.3390/nano10040726
APA StylePark, M., Roh, J., Lim, J., Lee, H., & Lee, D. (2020). Double Metal Oxide Electron Transport Layers for Colloidal Quantum Dot Light-Emitting Diodes. Nanomaterials, 10(4), 726. https://doi.org/10.3390/nano10040726