Electric Field Induced Dewetting of Hydrophobic Nanocavities at Ambient Temperature
Abstract
:1. Introduction
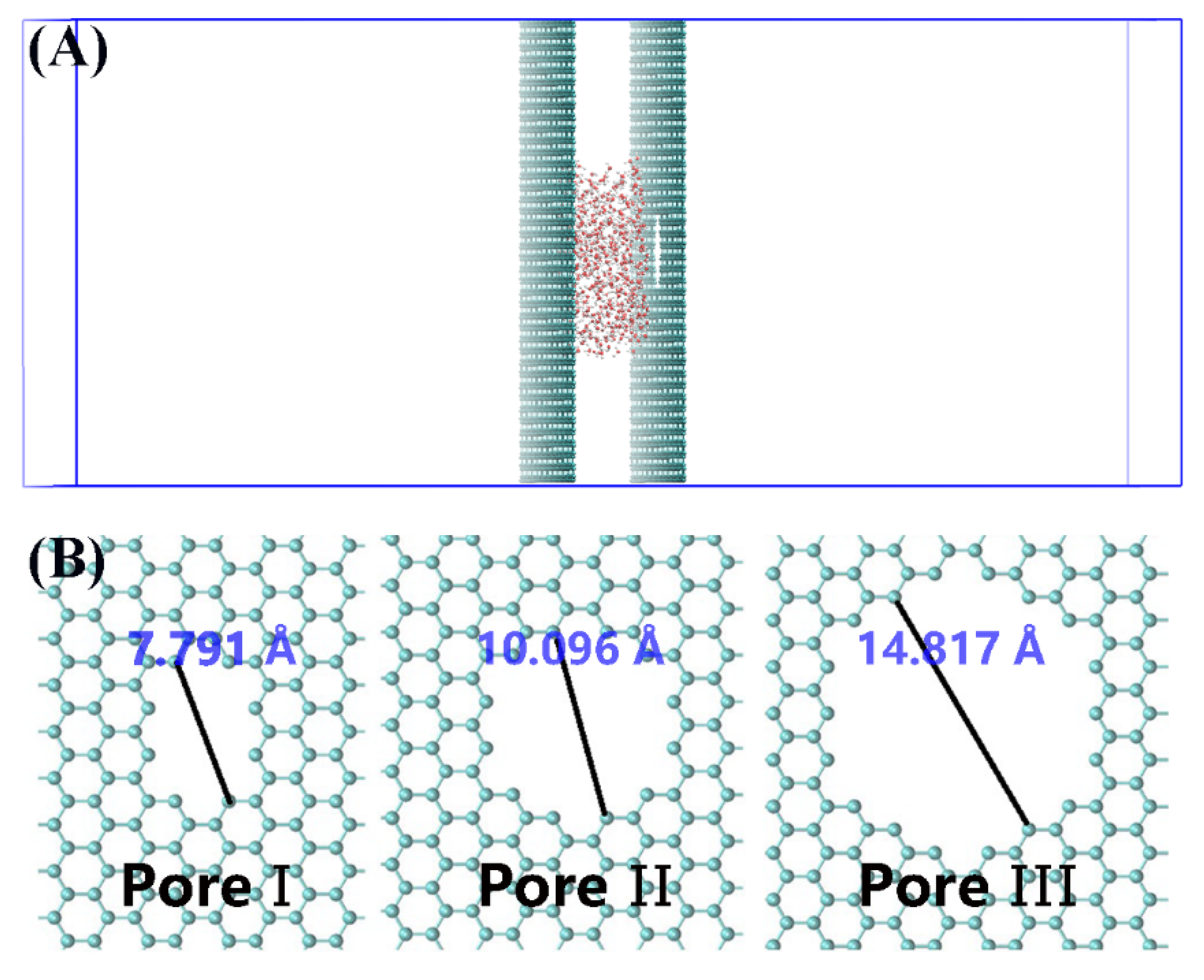
2. Materials and Methods
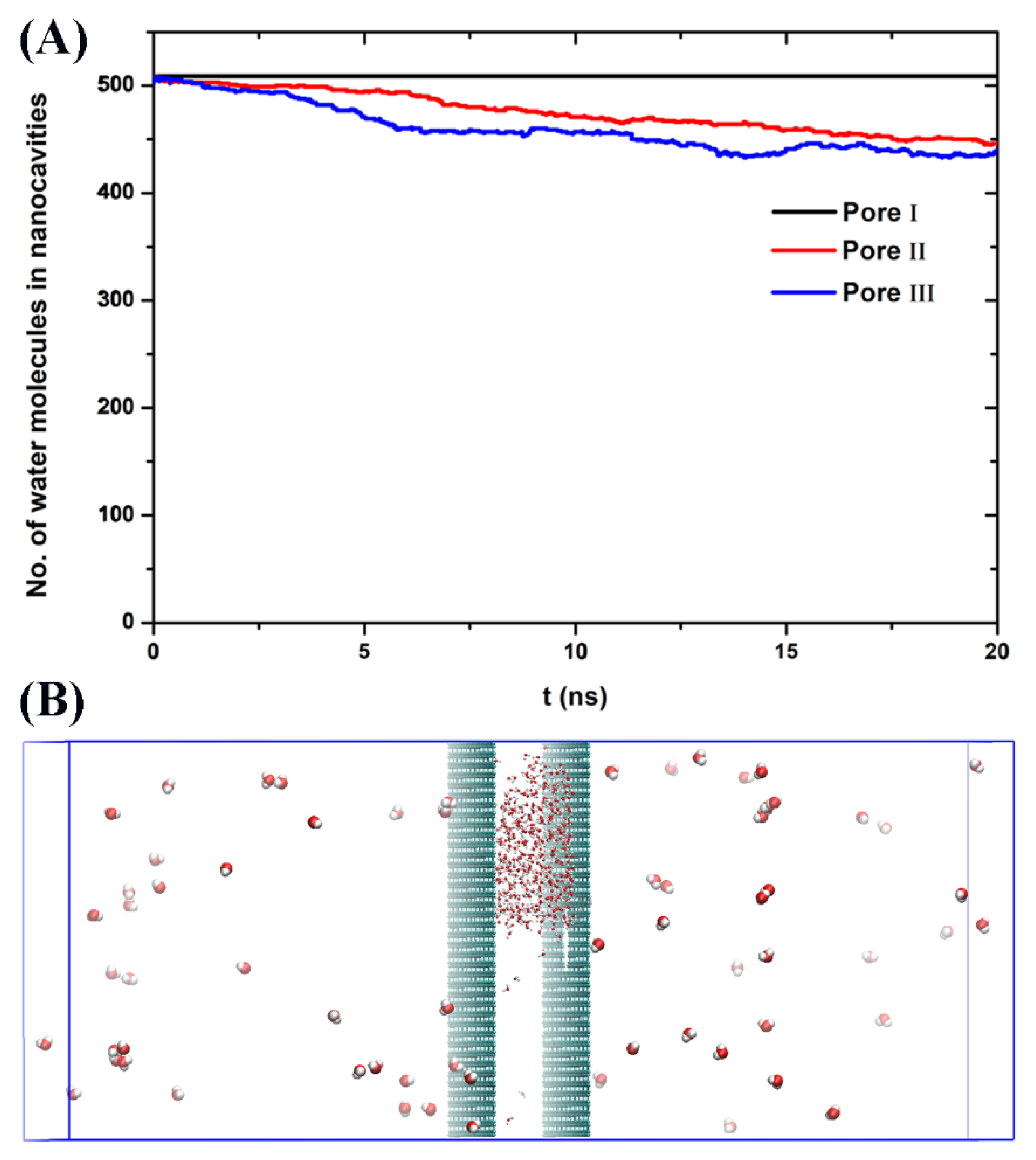
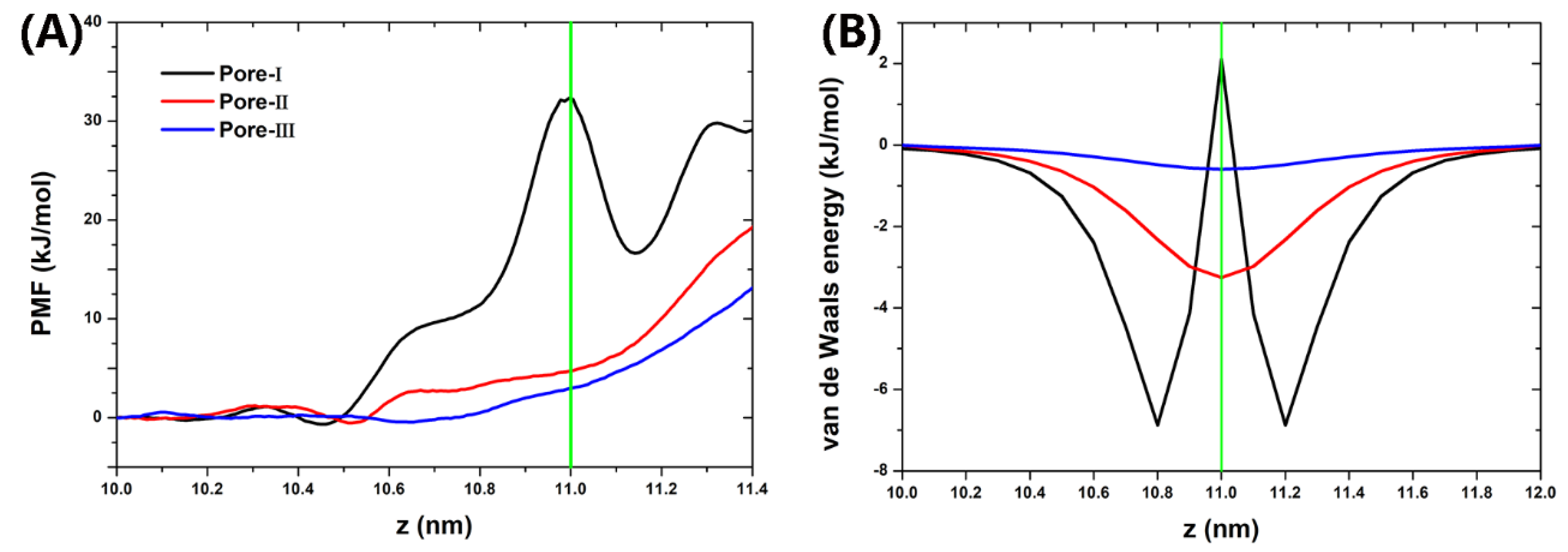
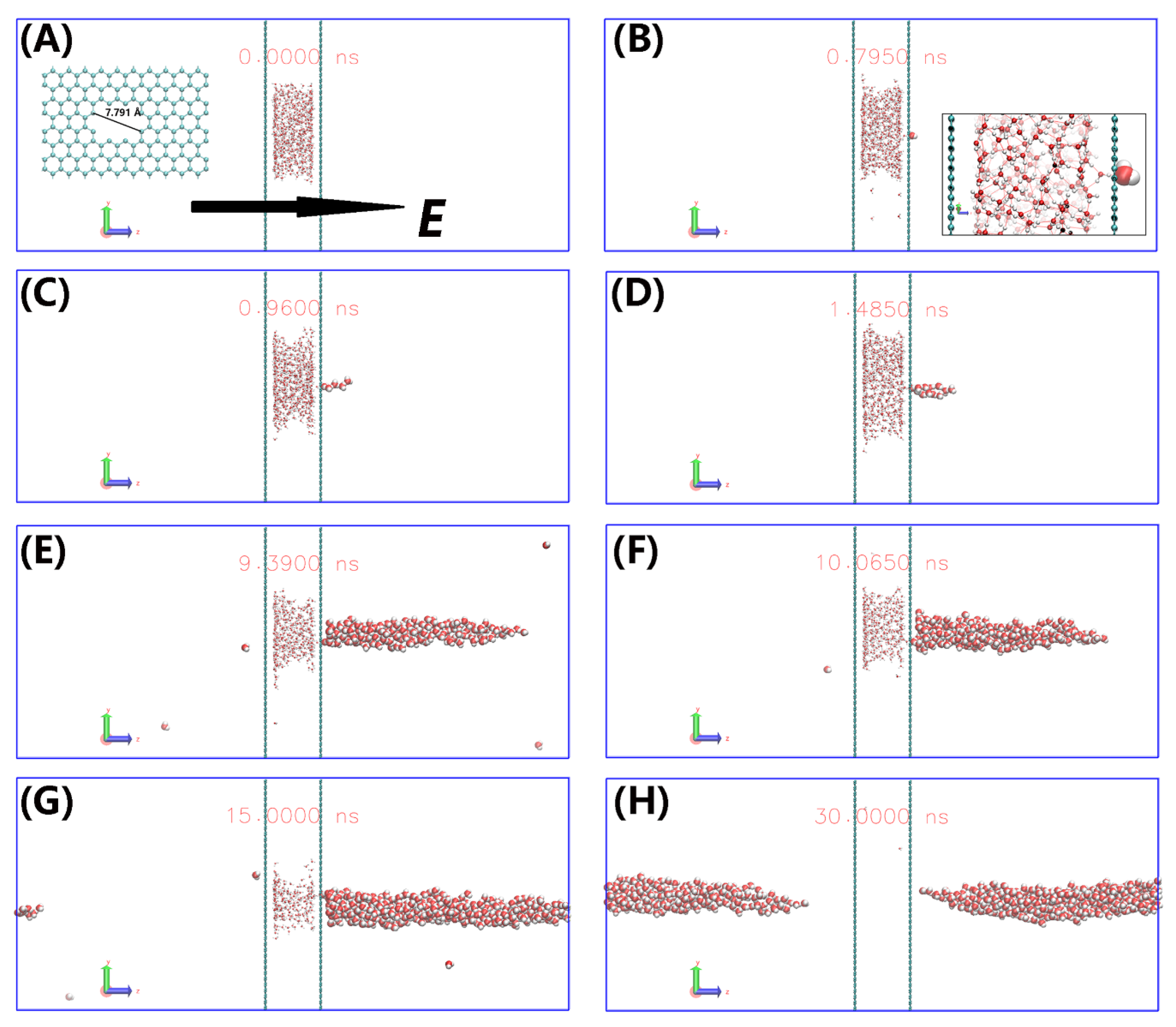
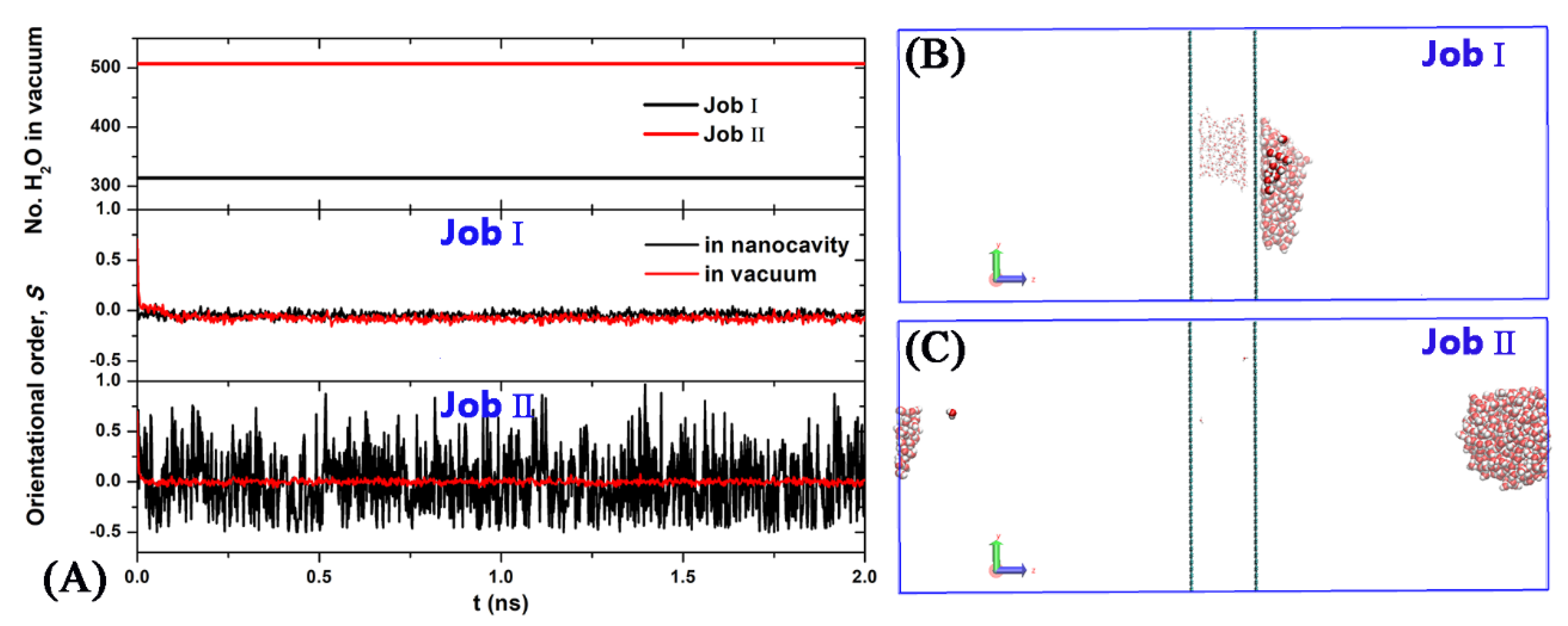
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, Y.; Kataura, H.; Masaroshi, A.; Udaka, A.; Suzuki, S.; Achiba, Y.; Kira, H.; Matsuda, K.; Kadowaki, H.; Okabe, Y. Ordered water inside carbon nanotubes: Formation of pentagonal to octagonal ice-nanotubes. Chem. Phys. Lett. 2005, 401, 534–538. [Google Scholar] [CrossRef]
- Maniwa, Y.; Matsuda, K.; Kyakuno, H.; Ogasawara, S.; Hibi, T.; Kadowaki, H.; Suzuki, S.; Achiba, Y.; Kataura, H. Water-filled single-wall carbon nanotubes as molecular nanovalves. Nat. Mater. 2007, 6, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Gopinadhan, K.; Hu, S.; Esfandiar, A.; Lozada-Hidalgo, M.; Wang, F.C.; Yang, Q.; Tyurnina, A.V.; Keerthi, A.; Radha, B.; Geim, A.K. Complete steric exclusion of ions and proton transport through confined monolayer water. Science 2019, 363, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikami, F.; Matsuda, K.; Kataura, H.; Maniwa, Y. Dielectric properties of water inside single-walled carbon nanotubes. ACS Nano 2009, 3, 1279–1287. [Google Scholar] [CrossRef]
- Koga, K.; Gao, G.T.; Tanaka, H.; Zeng, X.C. Formation of ordered ice nanotubes inside carbon nanotubes. Nature 2001, 412, 802–805. [Google Scholar] [CrossRef]
- Zhao, W.H.; Wang, L.; Bai, J.; Francisco, J.S.; Zeng, X.C. Spontaneous formation of one-dimensional hydrogen gas hydrate in carbon nanotubes. J. Am. Chem. Soc. 2014, 136, 10661–10668. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.H.; Francisco, J.S.; Zeng, X.C. CO separation from H2 via hydrate formation in single-walled carbon nanotubes. J. Phys. Chem. Lett. 2016, 7, 4911–4915. [Google Scholar] [CrossRef]
- Nomura, K.; Kaneko, T.; Bai, J.; Francisco, J.S.; Yasuoka, K.; Zeng, X.C. Evidence of low-density and high-density liquid phases and isochore end point for water confined to carbon nanotube. Proc. Natl. Acad. Sci. USA 2017, 114, 4066–4071. [Google Scholar] [CrossRef] [Green Version]
- Koga, K.; Zeng, X.C.; Tanaka, H. Freezing of confined water: A bilayer ice phase in hydrophobic nanopores. Phys. Rev. Lett. 1997, 79, 5262–5265. [Google Scholar] [CrossRef] [Green Version]
- Koga, K.; Tanaka, H.; Zeng, X.C. First-order transition in confined water between high-density liquid and low-density amorphous phases. Nature 2000, 408, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, M.Y.; Kumar, P.; Stanley, H.E. Phase transitions in confined water nanofilms. Nature Phys. 2010, 6, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zeng, X.C. Polymorphism and polyamorphism in bilayer water confined to slit nanopore under high pressure. Proc. Natl. Acad. Sci. USA 2012, 109, 21240–21245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.-H.; Wang, L.; Bai, J.; Yuan, L.-F.; Yang, J.; Zeng, X.C. Highly confined water: Two-dimensional ice, amorphous ice, and clathrate hydrates. Acc. Chem. Res. 2014, 47, 2505–2513. [Google Scholar] [CrossRef]
- Algara-Siller, G.; Lehtinen, O.; Wang, F.C.; Nair, R.R.; Kaiser, U.; Wu, H.A.; Geim, A.K.; Grigorieva, I.V. Square ice in graphene nanocapillaries. Nature 2015, 519, 443–445. [Google Scholar] [CrossRef]
- Cao, B.; Xu, E.; Li, T. Anomalous stability of two-dimensional ice confined in hydrophobic nanopores. ACS Nano 2019, 13, 4712–4719. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhao, Y.; Gao, Y.Q. Ice nucleation of confined monolayer water conforms to classical nucleation theory. J. Phys. Chem. Lett. 2019, 10, 3115–3121. [Google Scholar] [CrossRef]
- Jani, D.B.; Mishra, M.; Sahoo, P.K. Solid desiccant air conditioning—A state of the art review. Renew. Sustain. Energy Rev. 2016, 60, 1451–1469. [Google Scholar] [CrossRef]
- Altabet, Y.E.; Haji-Akbari, A.; Debenedetti, P.G. Effect of material flexibility on the thermodynamics and kinetics of hydrophobically induced evaporation of water. Proc. Natl. Acad. Sci. USA 2017, 114, E2548–E2555. [Google Scholar] [CrossRef] [Green Version]
- Wallqvist, A.; Berne, B.J. Computer simulation of hydrophobic hydration forces on stacked plates at short range. J. Phys. Chem. 1995, 99, 2893–2899. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Margulis, C.J.; Berne, B.J. Dewetting-induced collapse of hydrophobic particles. Proc. Natl. Acad. Sci. USA 2003, 100, 11953–11958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, J.; Eleftherious, M.; Zhou, R. Hydration and dewetting near fluorinated superhydrophobic plates. J. Am. Chem. Soc. 2006, 128, 12439–12447. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, L.; Biben, T.; Galarneau, A.; Vigier, G.; Charlaix, É. Activated drying in hydrophobic nanopores and the line tension of water. Proc. Natl. Acad. Sci. USA 2012, 109, 19557–19562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amabili, M.; Grosu, Y.; Giacomello, A.; Meloni, S.; Zaki, A.; Bonilla, F.; Faik, A.; Casciola, C.M. Pore morphology determines spontaneous liquid extrusion from nanopores. ACS Nano 2019, 13, 1728–1738. [Google Scholar] [CrossRef]
- Li, W.; Zuo, X.; Zhou, X.; Lu, H. Effect of aggregated gas molecules on dewetting transition of water between nanoscale hydrophobic plates. J. Chem. Phys. 2019, 150, 104702. [Google Scholar] [CrossRef]
- Zhao, W.H.; Shang, B.; Du, S.P.; Yuan, L.F.; Yang, J.; Zeng, X.C. Highly selective adsorption of methanol in carbon nanotubes immersed in methanol-water solution. J. Chem. Phys. 2012, 137, 034501. [Google Scholar] [CrossRef]
- Ren, X.; Wang, C.; Zhou, B.; Fang, H.; Hu, J.; Zhou, R. Ethanol promotes dewetting transition at low concentrations. Soft Matter 2013, 9, 4655. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.Q. Effects of cosolvents on the hydration of carbon nanotubes. J. Am. Chem. Soc. 2010, 132, 842–848. [Google Scholar] [CrossRef]
- Xiu, P.; Yang, Z.; Zhou, B.; Das, P.; Fang, H.; Zhou, R. Urea-induced drying of hydrophobic nanotubes: Comparison of different urea models. J. Phys. Chem. B 2011, 115, 2988–2994. [Google Scholar] [CrossRef]
- Svischev, I.M.; Kusalik, P.G. Crystallization of liquid water in a molecular dynamics simulation. Phys. Rev. Lett. 1994, 73, 975. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Berkowitz, M.L. Electric-field induced restructuring of water at a platinum-water interface: A molecular dynamics computer simulation. Phys. Rev. Lett. 1995, 74, 3193. [Google Scholar] [CrossRef] [PubMed]
- Zangi, R.; Mark, A.E. Electrofreezing of confined water. J. Chem. Phys. 2004, 120, 7123–7130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.-M.; Yoon, Y.-H.; Lee, S.; Kang, H. Freezing transition of interfacial water at room temperature under electric fields. Phys. Rev. Lett. 2005, 95, 085701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.H.; Bai, J.; Yuan, L.F.; Yang, J.; Zeng, X.C. Ferroelectric hexagonal and rhombic monolayer ice phases. Chem. Sci. 2014, 5, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Guo, W. Electromelting of confined monolayer ice. Phys. Rev. Lett. 2013, 110, 195701. [Google Scholar] [CrossRef]
- Okuno, Y.; Minagawa, M.; Matsumoto, H.; Tanioka, A. Simulation study on the influence of an electric field on water evaporation. J. Mol. Struct. Theochem. 2009, 904, 83–90. [Google Scholar] [CrossRef]
- Vaitheeswaran, S.; Yin, H.; Rasaiah, J.C. Water between plates in the presence of an electric field in an open system. J. Phys. Chem. B 2005, 109, 6629–6635. [Google Scholar] [CrossRef]
- Qian, Z.; Wei, G. Electric-field-induced phase transition of confined water nanofilms between two graphene sheets. J. Phys. Chem. A 2014, 118, 8922–8928. [Google Scholar] [CrossRef]
- Kayal, A.; Chandra, A. Wetting and dewetting of narrow hydrophobic channels by orthogonal electric fields: Structure, free energy, and dynamics for different water models. J. Chem. Phys. 2015, 143, 224708. [Google Scholar] [CrossRef]
- Naguib, N.; Ye, H.; Gogotsi, Y.; Yazicioglu, A.G.; Megaridis, C.M.; Yoshimura, M. Observation of water confined in nanometer channels of closed carbon nanotubes. Nano Lett. 2004, 4, 2237–2243. [Google Scholar] [CrossRef]
- Zambrano, H.A.; Walther, J.H.; Koumoutsakos, P.; Sbalzarini, I.F. Thermophoretic motion of water nanodroplets confined inside carbon nanotubes. Nano Lett. 2009, 9, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaban, V.V.; Prezhdo, V.V.; Prezhdo, O.V. Confinement by carbon nanotubes drastically alters the boiling and critical behavior of water droplets. ACS Nano 2012, 6, 2766–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, W.; Fang, H.; Zhang, J. Dynamics of evaporation from confined water in an SWCNT in the presence of an external field. J. Phys. Chem. C 2016, 120, 6493–6501. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, X.; Wu, J.; Liu, C.; Wei, Y.; Liu, J. Electro-dewatering of sewage sludge: Influence of combined action of constant current and constant voltage on performance and energy consumption. Sci. Total. Environ. 2019, 667, 751–760. [Google Scholar] [CrossRef]
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F.A. Electrical field: A historical review of its application and contributions in wastewater sludge dewatering. Water Res. 2010, 44, 2381–2407. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Gao, Y.; Wang, J.; Yang, Y.; Xia, Z. Rapid water harvesting and nonthermal drying in humid air by N-doped graphene micropads. Langmuir 2019, 35, 12389–12399. [Google Scholar] [CrossRef]
- Hens, A.; Biswas, G.; De, S. Evaporation of water droplets on Pt-surface in presence of external electric field-A molecular dynamics study. J. Chem. Phys. 2015, 143, 094702. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.C. Wetting and interfacial properties of water nanodroplets in contact with graphene and monolayer boron-nitride sheets. ACS Nano 2012, 6, 2401–2409. [Google Scholar] [CrossRef]
- Raj, R.; Maroo, S.C.; Wang, E.N. Wettability of graphene. Nano Lett. 2013, 13, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Taherian, F.; Marcon, V.; van der Vegt, N. What is the contact angle of water on graphene. Langmuir 2013, 29, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Wloch, J.; Terzyk, A.P.; Kowalczyk, P. New forcefield for water nanodroplet on a graphene surface. Chem. Phys. Lett. 2017, 674, 98–102. [Google Scholar] [CrossRef]
- Qiu, H.; Guo, W. Phase diagram of nanoscale water on solid surfaces with various wettabilities. J. Phys. Chem. Lett. 2019, 10, 6316–6323. [Google Scholar] [CrossRef]
- Acosta-Gutierrez, S.; Hernandez-Rojas, J.; Breton, J.; Llirente, J.M.G.; Wales, D.J. Physical properties of small water clusters in low and moderate electric fields. J. Chem. Phys. 2011, 135, 124303. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Kulkarni, A.D.; Gejji, S.P.; Bartolotti, L.J.; Pathak, R.K. Exploring electric field induced structural evolution of water clusters, (H2O)n [n = 9–20]: Density functional approach. J. Chem. Phys. 2013, 138, 124303. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, H.; Hu, Z.; Liu, C. The impact of the electric Field on surface condensation of water vapor: Insight from molecular dynamics simulation. Nanomaterials 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Saitta, A.M.; Saija, F.; Giaquinta, P.V. Ab initio molecular dynamics study of dissociation of water under an electric field. Phys. Rev. Lett. 2012, 108, 207801. [Google Scholar] [CrossRef] [Green Version]
- Gavish, M.; Wang, J.-L.; Eisenstein, M.; Lahav, M.; Leiserowitz, L. The role of crystal polarity in alpha-amino acid crystals for induced nucleation of ice. Science 1992, 256, 815–818. [Google Scholar] [CrossRef]
- Fornés, J.A. Electrical fluctuations on the surfaces of proteins from hydrodynamic data. J. Colloid Interface Sci. 2008, 323, 255–259. [Google Scholar] [CrossRef]
- Zhou, K.; Xu, Z. Field-enhanced selectivity in nanoconfined ionic transport. Nanoscale 2020, 12, 6512–6521. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Lin, D.; Zhao, W. Electric Field Induced Dewetting of Hydrophobic Nanocavities at Ambient Temperature. Nanomaterials 2020, 10, 736. https://doi.org/10.3390/nano10040736
Li C, Lin D, Zhao W. Electric Field Induced Dewetting of Hydrophobic Nanocavities at Ambient Temperature. Nanomaterials. 2020; 10(4):736. https://doi.org/10.3390/nano10040736
Chicago/Turabian StyleLi, Chenchao, Dongdong Lin, and Wenhui Zhao. 2020. "Electric Field Induced Dewetting of Hydrophobic Nanocavities at Ambient Temperature" Nanomaterials 10, no. 4: 736. https://doi.org/10.3390/nano10040736



