Carbon Paper as Current Collectors in Graphene Hydrogel Electrodes for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of GHE
2.2. Electrochemical Measurement
2.3. Characterization
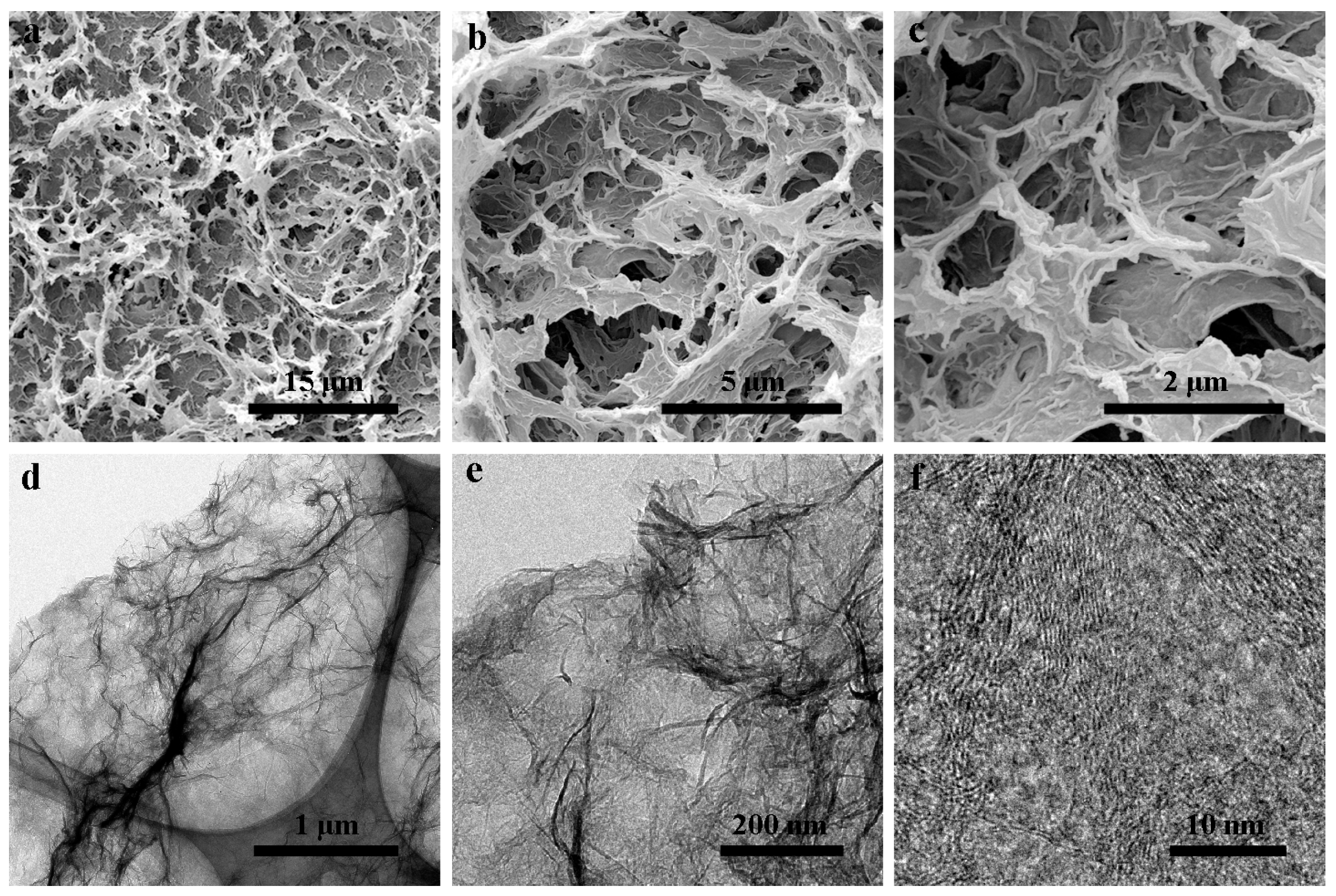
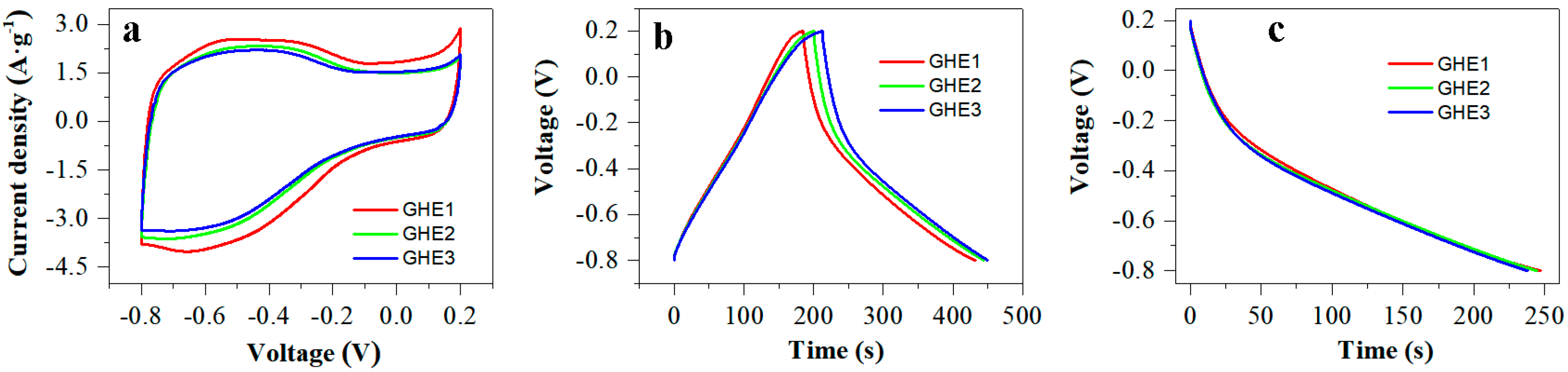
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lai, E.; Xue, X.; Ning, W.; Huang, J.; Ling, X.; Lin, H. Three-dimensional graphene-based composite hydrogel materials for flexible supercapacitor electrodes. Front. Chem. 2019, 7, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, C.; Li, B.; Lin, X.; Liu, H.; Xu, Y.; Mao, J.; Duan, C.; Li, T.; Ni, Y. The recent progress on three-dimensional porous graphene-based hybrid structure for supercapacitor. Compos. Part B Eng. 2019, 165, 10–46. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Jalili, R.; Esrafilzadeh, D.; Salari, M.; Gholamvand, Z.; Aminorroaya Yamini, S.; Konstantinov, K.; Shepherd, R.L.; Chen, J.; Moulton, S.E. High-performance multifunctional graphene yarns: Toward wearable all-carbon energy storage textiles. ACS Nano 2014, 8, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Zequine, C.; Ranaweera, C.; Wang, Z.; Singh, S.; Tripathi, P.; Srivastava, O.; Gupta, B.K.; Ramasamy, K.; Kahol, P.; Dvornic, P. High performance and flexible supercapacitors based on carbonized bamboo fibers for wide temperature applications. Sci. Rep. 2016, 6, 31704. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, L.; Hao, W.; Ren, W.; Cheng, H.M. Synthesis and applications of three-dimensional graphene network structures. Mater. Today Nano 2019, 5, 100027. [Google Scholar] [CrossRef]
- Horn, M.; Gupta, B.; MacLeod, J.; Liu, J.; Motta, N. Graphene-based supercapacitor electrodes: Addressing challenges in mechanisms and materials. Curr. Opin. Green Sust. Chem. 2019, 17, 42–48. [Google Scholar] [CrossRef]
- Anjali, J.; Jose, V.K.; Lee, J.M. Carbon-based hydrogels: Synthesis and their recent energy applications. J. Mater. Chem. A 2019, 7, 15491–15518. [Google Scholar] [CrossRef]
- Wu, Z.S.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhi, H.; Qiu, Y.; Yang, J.; Xing, L.; Zhang, Q.; Ding, X.; Wang, X.; Xu, G.; Yuan, H. Achieving commercial-level mass loading in ternary-doped holey graphene hydrogel electrodes for ultra-high energy density supercapacitors. Nano Energy 2018, 46, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sheng, K.; Luo, P.; Li, C.; Shi, G. Graphene hydrogels deposited in nickel foams for high-rate electrochemical capacitors. Adv. Mater. 2012, 24, 4569–4573. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, W. 3D graphene nanomaterials for binder-free supercapacitors: Scientific design for enhanced performance. Nanoscale 2015, 7, 6957–6990. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, L.; Tang, Y.; Tang, S.; Du, Y. Facile synthesis of nickel network supported three-dimensional graphene gel as a lightweight and binder-free electrode for high rate performance supercapacitor application. Nanoscale 2014, 6, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Tian, X.; Yao, J.; Sun, Y.; Jin, J.; Zhang, Y.; Liu, Y. Controlled synthesis of three-dimensional reduced graphene oxide networks for application in electrode of supercapacitor. Diam. Relat. Mater. 2016, 70, 186–193. [Google Scholar] [CrossRef]
- Chang, J.H.; Hung, Y.H.; Luo, X.F.; Huang, C.H.; Jung, S.; Chang, J.K.; Kong, J.; Su, C.Y. The hierarchical porosity of a three-dimensional graphene electrode for binder-free and high performance supercapacitors. RSC Adv. 2016, 6, 8384–8394. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G. Preparation of highly conductive graphene hydrogels for fabricating supercapacitors with high rate capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Q.; Wu, M.; Zhang, M.; Yao, B.; Gao, T.; Wang, H.; Li, C.; Sui, D.; Chen, Y.; et al. Tailoring the oxygenated groups of graphene hydrogels for high-performance supercapacitors with large areal mass loadings. J. Mater. Chem. A 2018, 6, 6587–6594. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Huang, X.; Liu, Y.; Huang, Y.; Duan, X. Flexible solid-state supercapacitor based on three-dimensional graphene hydrogel films. ACS Nano 2013, 7, 4042–4049. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.Y.; Zhao, Z.; Lin, Z.; Lee, C.; Xu, X.; Wang, C.; Huang, Y.; Shakir, M.I.; Duan, X. Solution processable holey graphene oxide and its derived macrostructures for high-performance supercapacitors. Nano Lett. 2015, 15, 4605–4610. [Google Scholar] [CrossRef]
- Sheng, K.X.; Sun, Y.Q.; Li, C.; Yuan, W.J.; Shi, G.Q. Ultrahigh-rate supercapacitors based on electrochemically reduced graphene oxide for ac line-filtering. Sci. Rep. 2012, 2, 247. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hansen, R.V.; Zhang, L.; Li, B.; Poh, C.K.; Lim, S.H.; Chen, L.; Yang, J.; Lai, L.; Lin, J.; et al. Binary metal sulfides and polypyrrole on vertically aligned carbon nanotube arrays/carbon fiber paper as high-performance electrodes. J. Mater. Chem. A 2015, 3, 22043–22052. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Zhou, F.; Sun, Z.; Huang, F.; Yu, Y.; Chen, L.; Pan, M. Simple and fast synthesis of polyaniline nanofibers/carbon paper composites as supercapacitor electrodes. J. Energy Storage 2016, 7, 99–103. [Google Scholar] [CrossRef]
- Luo, P.; Guan, X.; Yu, Y.; Li, X.; Yan, F. Hydrothermal synthesis of graphene quantum dots supported on three-dimensional graphene for supercapacitors. Nanomaterials 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Ma, H.; Kong, D.; Xu, Y.; Xie, X.; Tao, Y.; Xiao, Z.; Lv, W.; Jang, H.D.; Huang, J.; Yang, Q.H. Energy storage: Disassembly-reassembly approach to RuO2/graphene composites for ultrahigh volumetric capacitance supercapacitor. Small 2017, 13, 1701026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Li, C.; Zhou, Q.; Zhang, M.; Chen, J.; Yu, X.; Shi, G. Graphene-based organic electrochemical capacitors for AC line filtering. Adv. Energy Mater. 2017, 7, 1700591. [Google Scholar] [CrossRef]
- Xu, Y.X.; Lin, Z.Y.; Zhong, X.; Huang, X.Q.; Weiss, N.O.; Huang, Y.; Duan, X.F. Holey graphene frameworks for highly efficient capacitive energy storage. Nat. Commun. 2014, 5, 4554. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, Y.; Hu, C.; Cheng, H.; Zhang, Z.; Shao, H.; Qu, L. Graphene quantum dots-three-dimensional graphene composites for high-performance supercapacitors. Phys. Chem. Chem. Phys. 2014, 16, 19307–19313. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, X.; Liang, Y.; Peng, L.; Dong, H.; Xiao, Y.; Hu, C.; Hu, H.; Liu, Y.; Zheng, M. Small nitrogen-doped carbon dots as efficient nanoenhancer for boosting the electrochemical performance of three-dimensional graphene. J. Colloid Interf. Sci. 2018, 536, 628–637. [Google Scholar] [CrossRef] [PubMed]




| Samples | Capacitance | Current Collectors | Electrolyte | References |
|---|---|---|---|---|
| GH | 160 F·g−1 at 1 A·g−1 | Platinum foil | 5 M KOH | [25] |
| GH | 222 F·g−1 at 1 A·g−1 | Platinum foil | 5 M KOH | [17] |
| Holey GH | 310 F·g−1 at 1 A·g−1 | Platinum or aluminum foils | 6 M KOH | [29] |
| GH | 136 F·g−1 at 1.25 A·g−1 | Gold foil | 1 M H2SO4 | [30] |
| Hydroxyl-rich GH | 260 F·g−1 at 1 A·g−1 | Gold foil | 1 M H2SO4 | [18] |
| GH | Ca. 180 F·g−1 at 1 A·g−1 | Nickel foam | 6 M KOH | [31] |
| GH | 294 F·g−1 at 1.18 A·g−1 | Carbon paper | 1 M KOH | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, P.; Huang, L. Carbon Paper as Current Collectors in Graphene Hydrogel Electrodes for High-Performance Supercapacitors. Nanomaterials 2020, 10, 746. https://doi.org/10.3390/nano10040746
Luo P, Huang L. Carbon Paper as Current Collectors in Graphene Hydrogel Electrodes for High-Performance Supercapacitors. Nanomaterials. 2020; 10(4):746. https://doi.org/10.3390/nano10040746
Chicago/Turabian StyleLuo, Peihui, and Lili Huang. 2020. "Carbon Paper as Current Collectors in Graphene Hydrogel Electrodes for High-Performance Supercapacitors" Nanomaterials 10, no. 4: 746. https://doi.org/10.3390/nano10040746




