Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Measurements
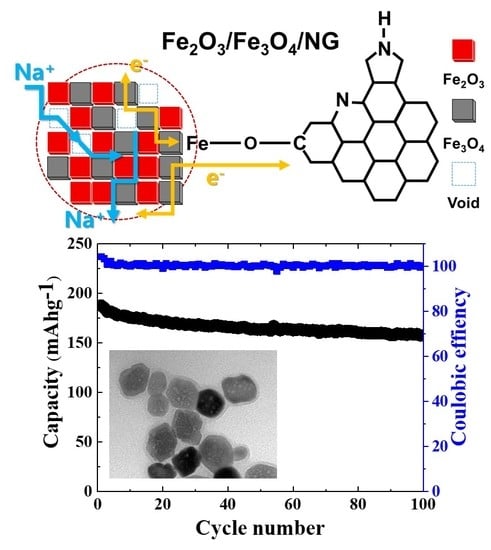
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, L.F.; Lu, H.Y.; Fang, Y.J.; Sushko, M.L.; Cao, Y.L.; Ai, X.P.; Yang, H.X.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 7. [Google Scholar] [CrossRef]
- Sun, W.P.; Rui, X.H.; Zhang, D.; Jiang, Y.Z.; Sun, Z.Q.; Liu, H.K.; Dou, S.X. Bismuth sulfide: A high-capacity anode for sodium-ion batteries. J. Power Sources 2016, 309, 135–140. [Google Scholar] [CrossRef]
- Pan, H.L.; Hu, Y.S.; Chen, L.Q. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.M.; Palacin, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.N.; Li, W.Y.; Gou, X.L. alpha-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Zhang, N.; Han, X.P.; Liu, Y.C.; Hu, X.F.; Zhao, Q.; Chen, J. 3D Porous gamma-Fe2O3@C Nanocomposite as High-Performance Anode Material of Na-Ion Batteries. Adv. Energy Mater. 2015, 5, 7. [Google Scholar] [CrossRef]
- Jian, Z.L.; Zhao, B.; Liu, P.; Li, F.J.; Zheng, M.B.; Chen, M.W.; Shi, Y.; Zhou, H.S. Fe2O3 nanocrystals anchored onto graphene nanosheets as the anode material for low-cost sodium-ion batteries. Chem. Commun. 2014, 50, 1215–1217. [Google Scholar] [CrossRef]
- Cui, Z.M.; Hang, L.Y.; Song, W.G.; Guo, Y.G. High-Yield Gas-Liquid Interfacial Synthesis of Highly Dispersed Fe3O4 Nanocrystals and Their Application in Lithium-Ion Batteries. Chem. Mater. 2009, 21, 1162–1166. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, N.; Tao, F.; Pan, Q.M. Improving the lithium storage properties of Fe2O3@C nanoparticles by superoleophilic and superhydrophobic polysiloxane coatings. J. Mater. Chem. 2012, 22, 15894–15900. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, Y.X.; Chou, S.L.; Li, H.J.; Liu, H.K.; Wang, J.Z. Rapid synthesis of alpha-Fe2O3/rGO nanocomposites by microwave autoclave as superior anodes for sodium-ion batteries. J. Power Sources 2015, 280, 107–113. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, T.Q.; Chu, H.P.; Niu, L.Y.; Sun, Z.; Pan, L.K.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]
- Zhao, C.J.; Shao, X.X.; Zhang, Y.X.; Qian, X.Z. Fe2O3/Reduced Graphene Oxide/Fe3O4 Composite in Situ Grown on Fe Foil for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30133–30142. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.J.; Li, S.X.; Xu, B.Y.; Zheng, F.Y.; Zhou, H.F.; Yu, H.W.; Lin, F.; Zhu, X.Q. Polycrystalline iron oxide nanoparticles prepared by C-dot-mediated aggregation and reduction for supercapacitor application. RSC Adv. 2016, 6, 45023–45030. [Google Scholar] [CrossRef]
- Tang, X.; Jia, R.Y.; Zhai, T.; Xia, H. Hierarchical Fe3O4@Fe2O3 Core-Shell Nanorod Arrays as High-Performance Anodes for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 27518–27525. [Google Scholar] [CrossRef]
- Mallick, S.; Jana, P.P.; Raj, C.R. Asymmetric Supercapacitor Based on Chemically Coupled Hybrid Material of Fe2O3-Fe3O4 Heterostructure and Nitrogen-Doped Reduced Graphene Oxide. Chemelectrochem 2018, 5, 2348–2356. [Google Scholar] [CrossRef]
- Wang, X.L.; Qin, M.L.; Fang, F.; Jia, B.R.; Wu, H.Y.; Qu, X.H.; Volinsky, A.A. Solution combustion synthesis of nanostructured iron oxides with controllable morphology, composition and electrochemical performance. Ceram. Int. 2018, 44, 4237–4247. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.W.; Huang, R.S.; Yao, J.H. Ternary Fe2O3/Fe3O4/FeCO3 Composite as a High-Performance Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2019, 123, 12614–12622. [Google Scholar] [CrossRef]
- Li, Y.F.; Fu, Y.Y.; Chen, S.H.; Huang, Z.Z.; Wang, L.; Song, Y.H. Porous Fe2O3/Fe3O4@Carbon octahedron arrayed on three-dimensional graphene foam for lithium-ion battery. Compos. Part B 2019, 171, 130–137. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Vasilev, K.; Ramiasa, M.M. Nanoengineered Interfaces, Coatings, and Structures by Plasma Techniques. Nanomaterials 2017, 7, 449. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, J.; Khattak, A.M.; Cai, F.; Jiang, B.; Yang, G.; Hu, S. High-performance tin oxide-nitrogen doped graphene aerogel hybrids as anode materials for lithium-ion batteries. J. Power Sources 2014, 270, 28–33. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.S.; Chen, X.H.; Song, H.H. Amorphous Fe2O3/Graphene Composite Nanosheets with Enhanced Electrochemical Performance for Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 30899–30907. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.S.; Yang, C.; Chen, G.X.; Yan, X.Q.; Chen, S.N.; Su, B.W.; Liu, Z.B.; Tian, J.G. Preparation of high-quality graphene using triggered microwave reduction under an air atmosphere. J. Mater. Chem. C 2018, 6, 1829–1835. [Google Scholar] [CrossRef]
- Abe, T.; Miyazawa, A.; Kawanishi, Y.; Konno, H. Microwave-Assisted Synthesis of Metal Complexes. Mini-Rev. Org. Chem. 2011, 8, 315–333. [Google Scholar] [CrossRef]
- Zhou, K.; Zhen, Y.; Hong, Z.; Guo, J.; Huang, Z. Enhanced sodium ion batteries performance by the phase transition from hierarchical Fe2O3 to Fe3O4 hollow nanostructures. Mater. Lett. 2017, 190, 52–55. [Google Scholar] [CrossRef]
- Meng, S.; Zhao, D.-L.; Wu, L.-L.; Ding, Z.-W.; Cheng, X.-W.; Hu, T. Fe2O3/nitrogen-doped graphene nanosheet nanocomposites as anode materials for sodium-ion batteries with enhanced electrochemical performance. J. Alloy. Compd. 2018, 737, 130–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Synthesis of Core-Shell Carbon Encapsulated Fe2O3 Composite through a Facile Hydrothermal Approach and Their Application as Anode Materials for Sodium-Ion Batteries. Metals 2018, 8, 461. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Wang, X.; Zhang, G.; Shu, H.; Yang, X.; Tavares, A.C.; Sun, S. A facile synthesis of Fe3O4 nanoparticles/graphene for high-performance lithium/sodium-ion batteries. RSC Adv. 2016, 6, 16624–16633. [Google Scholar] [CrossRef]
- Qi, H.; Cao, L.; Li, J.; Huang, J.; Xu, Z.; Jie, Y.; Wang, C. Thin Carbon Layer Coated Porous Fe3O4 Particles Supported by rGO Sheets for Improved Stable Sodium Storage. Chemistryselect 2019, 4, 2668–2675. [Google Scholar] [CrossRef]
- Guo, T.X.; Liao, H.X.; Ge, P.; Zhang, Y.; Tian, Y.Q.; Hong, W.W.; Shi, Z.D.; Shao, C.S.; Hou, H.S.; Ji, X.B. Fe2O3 embedded in the nitrogen-doped carbon matrix with strong C-O-Fe oxygen-bridge bonds for enhanced sodium storages. Mater. Chem. Phys. 2018, 216, 58–63. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.Q.; Zhu, Q.Z.; Cao, B.; Chen, R.J.; Wang, Y.; Wu, F.; Xu, B. 3D-0D Graphene-Fe3O4 Quantum Dot Hybrids as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 26878–26885. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, Q.; Li, Y.; Chen, J.; Gu, J.; Zhang, W.; Zhang, D. Self-crosslink assisted synthesis of 3D porous branch-like Fe3O4/C hybrids for high-performance lithium/sodium-ion batteries. RSC Adv. 2017, 7, 50307–50316. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, F.X.; Wang, C.; Wang, S.; Wang, C.Y.; Zhao, Z.W.; Duan, L.L.; Liu, Y.P.; Wu, Y.P.; Li, W.; et al. Encapsulating highly crystallized mesoporous Fe3O4 in hollow N-doped carbon nanospheres for high-capacity long-life sodium-ion batteries. Nano Energy 2019, 56, 426–433. [Google Scholar] [CrossRef]








| Electrode | Rs (Ω) | Rct (Ω) | DNa+ (cm2 s−1) |
|---|---|---|---|
| Fe2O3/NG | 6 | 210.6 | 1.65 × 10−12 |
| Fe2O3/Fe3O4/NG | 8.9 | 134.7 | 1.34 × 10−11 |
| Anodes | Current Density (mA g−1) | References | |||
|---|---|---|---|---|---|
| Capacity (mAh g−1) | |||||
| Fe2O3/Fe3O4/NG | 100 | 200 | 1000 | 1200 | This work |
| 362 | 300 | 185 | 174 | ||
| Fe2O3/NG | 50 | 100 | 200 | 1000 | Meng 2017 [28] |
| 343 | 285 | 230 | 132 | ||
| Fe2O3/C | 50 | 100 | 200 | 1000 | Zhang 2018 [29] |
| 364 | 291 | 245 | 150 | ||
| Fe3O4/G | 100 | 200 | 500 | 1000 | Fu 2016 [30] |
| 310 | 225 | 180 | 140 | ||
| Fe3O4@C/G | 100 | 200 | 500 | 1000 | Qi 2019 [31] |
| 375 | 300 | 254 | 200 | ||
| Fe2O3@NC | 200 | 500 | 1000 | 4000 | Guo 2018 [32] |
| 289 | 253.7 | 221.5 | 167.8 | ||
| Fe3O4/G/QD | 100 | 200 | 500 | 1000 | Liu 2016 [33] |
| 316 | 273 | 216 | 113 | ||
| Fe3O4/C | 100 | 200 | 500 | 1000 | Wang 2017 [34] |
| 293 | 262 | 223 | 195 | ||
| Fe3O4@N–C | 80 | 240 | 400 | 800 | Zhao 2019 [35] |
| 386 | 315 | 277 | 248 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ma, Y.; Liu, L.; Yao, S.; Wu, W.; Wang, Z.; Lv, P.; Zheng, J.; Yu, K.; Wei, W.; et al. Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries. Nanomaterials 2020, 10, 782. https://doi.org/10.3390/nano10040782
Wang Q, Ma Y, Liu L, Yao S, Wu W, Wang Z, Lv P, Zheng J, Yu K, Wei W, et al. Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries. Nanomaterials. 2020; 10(4):782. https://doi.org/10.3390/nano10040782
Chicago/Turabian StyleWang, Qianqian, Yujie Ma, Li Liu, Shuyue Yao, Wenjie Wu, Zhongyue Wang, Peng Lv, Jiajin Zheng, Kehan Yu, Wei Wei, and et al. 2020. "Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries" Nanomaterials 10, no. 4: 782. https://doi.org/10.3390/nano10040782
APA StyleWang, Q., Ma, Y., Liu, L., Yao, S., Wu, W., Wang, Z., Lv, P., Zheng, J., Yu, K., Wei, W., & Ostrikov, K. (2020). Plasma Enabled Fe2O3/Fe3O4 Nano-aggregates Anchored on Nitrogen-doped Graphene as Anode for Sodium-Ion Batteries. Nanomaterials, 10(4), 782. https://doi.org/10.3390/nano10040782




_Ostrikov.png)