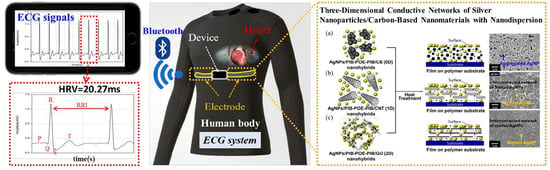
Controlling the Structures, Flexibility, Conductivity Stability of Three-Dimensional Conductive Networks of Silver Nanoparticles/Carbon-Based Nanomaterials with Nanodispersion and their Application in Wearable Electronic Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Organic Dispersant of Polyisobutylene-b-Poly(oxyethylene)-b-Polyisobutylene (PIB-POE-PIB) Triblock Copolymers
2.3. Preparation of AgNPs/PIB-POE-PIB/carbon-based Nanomaterial Hybrids
2.4. Synthesis of PI Electrode Layers Using Hybrids of AgNPs/PIB-POE-PIB/Carbon-Based Nanomaterial
2.5. Characterization and Instruments
3. Results and Discussion
3.1. Synthesis of the PIB–POE–PIB Triblock Copolymer for Use as an Organic Dispersant
3.2. Preparation of Colloidal AgNPs Dispersed in PIB-POE-PIB/Carbon-Based Nanohybrids as Organic/Inorganic Dispersants
3.3. Preparation of Highly Conductive Films
3.4. Preparation of Wearable Electronic Sensors and ECG Monitoring at Rest and during Exercise
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cho, H.; Lee, J.H. A study on the optimal positions of ECG electrodes in a garment for the design of ECG-monitoring clothing for male. J. Med. Syst. 2015, 39, 95. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Huang, T.H.; Hsu, P.C.; Ko, Y.C.; Chen, F.L.; Wang, W.C.; Kao, T.; Chan, C.T. Respiratory rate estimation by using ECG, impedance, and motion sensing in smart clothing. J. Med. Biol. Eng. 2017, 37, 826–842. [Google Scholar] [CrossRef]
- Wang, W.D.; Zhang, Z.B.; Shen, Y.H.; Wang, B.Q.; Zheng, J.W. Design and implementation of sensing shirt for ambulatory cardiopulmonary monitoring. J. Med. Biol. Eng. 2011, 31, 207–215. [Google Scholar]
- Okada, Y.; Yoto, T.Y.; Suzuki, T.-A.; Sakuragawa, S.; Sakakibara, H.; Shimoi, K.; Sugiura, T. Wearable ECG recorder with acceleration sensors for monitoring daily stress. J. Med. Biol. Eng. 2013, 33, 420–426. [Google Scholar] [CrossRef]
- Weng, J.; Guo, X.-M.; Chen, L.-S.; Yuan, Z.-H.; Ding, X.-R.; Lei, M. Study on real-time monitoring technique for cardiac arrhythmia based on smartphone. J. Med. Biol. Eng. 2013, 33, 394–399. [Google Scholar] [CrossRef]
- Domanski, M.J.; Fuster, V.; Diaz-Mitoma, F.; Grundy, S.; Lloyd-Jones, D.; Mamdani, M.; Roberts, R.; Thorpe, K.; Hall, J.; Udell, J.A. Next steps in primary prevention of coronary heart disease: Rationale for and design of the ECAD trial. J. Am. Coll. Cardiol. 2015, 66, 1828–1836. [Google Scholar] [CrossRef]
- O’Mahony, C.; Grygoryev, K.; Ciarlone, A.; Giannoni, G.; Kenthao, A.; Galvin, P. Design, fabrication and skin-electrode contact analysis of polymer microneedle-based ECG electrodes. J. Micromech. Microeng. 2016, 26, 084005. [Google Scholar] [CrossRef]
- Lou, C.; Li, R.; Li, Z.; Liang, T.; Wei, Z.; Run, M.; Yan, X.; Liu, X. Flexible graphene electrodes for prolonged dynamic ECG monitoring. Sensors 2016, 16, 1833. [Google Scholar] [CrossRef] [Green Version]
- Celik, N.; Manivannan, N.; Strudwick, A.; Balachandran, W. Graphene-enabled electrodes for electrocardiogram monitoring. Nanomaterials 2016, 6, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlach, K.; Kijonka, J.; Jurek, F.; Vavra, P.; Zonca, P. Capacitive biopotential electrode with a ceramic dielectric layer. Sens. Actuators B 2017, 245, 988–995. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiu, C.W. Immobilization and 3D hot-junction formation of gold nanoparticles on two-dimensional silicate nanoplatelets as substrates for high-efficiency surface-enhanced raman scattering detection. Nanomaterials 2019, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.W.; Lin, P.H. Core/shell Ag@silicate nanoplatelets and poly(vinyl alcohol) spherical nanohybrids fabricated by coaxial electrospraying as highly sensitive SERS substrates. RSC Adv. 2016, 6, 67204–67211. [Google Scholar] [CrossRef]
- Karabay, S.; Ertürk, A.T.; Zeren, M.; Yamanoğlu, R.; Karakulak, E. Failure analysis of wire-breaks in aluminum conductor production and investigation of early failure reasons for transmission lines. Eng. Fail. Anal. 2018, 83, 47–56. [Google Scholar] [CrossRef]
- Li, J.W.; Tsen, W.C.; Tsou, C.H.; Suen, M.C.; Chiu, C.W. Synthetic environmentally friendly castor oil based-polyurethane with carbon black as a microphase separation promoter. Polymers 2019, 11, 1333. [Google Scholar] [CrossRef] [Green Version]
- Schütt, F.; Signetti, S.; Krüger, H.; Röder, S.; Smazna, D.; Kaps, S.; Gorb, S.N.; Mishra, Y.K.; Pugno, N.M.; Adelung, R. Hierarchical self-entangled carbon nanotube tube networks. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Bretheau, L.; Joel, I.; Wang, J.; Pisoni, R.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Tunnelling spectroscopy of Andreev states in graphene. Nature Phys. 2017, 13, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.K. Copper micro-labyrinth with graphene skin: New transparent flexible electrodes with ultimate low sheet resistivity and superior stability. Nanomaterials 2016, 6, 161. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Chen, W.; Bai, S.; Liu, Y.; Wang, Q. Fabrication of an ultralight flame-induced high conductivity hybrid sponge based on poly (vinyl alcohol)/silver nitrate composite. Mater. Des. 2018, 139, 96–103. [Google Scholar] [CrossRef]
- Fang, G.; Bi, X. Silver-catalysed reactions of alkynes: Recent advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Yang, T.; Liu, T.; Zou, Y.; Jiang, J. Graphene oxide wrapped individual silver nanocomposites with improved stability for surface-enhanced Raman scattering. RSC Adv. 2015, 5, 55801–55807. [Google Scholar] [CrossRef]
- Song, J.; Huang, Y.; Fan, Y.; Zhao, Z.; Yu, W.; Rasco, B.A.; Lai, K. Detection of prohibited fish drugs using silver nanowires as substrate for surface-enhanced Raman scattering. Nanomaterials 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Odoom-Wubah, T.; Huang, J. Biosynthesis of Ag-Pd bimetallic alloy nanoparticles through hydrolysis of cellulose triggered by silver sulfate. RSC Adv. 2018, 8, 30340–30345. [Google Scholar] [CrossRef] [Green Version]
- Atwa, Y.; Maheshwari, N.; Goldthorpe, I.A. Silver nanowire coated threads for electrically conductive textiles. J. Mater. Chem. C 2015, 3, 3908–3912. [Google Scholar] [CrossRef] [Green Version]
- Borkowski, A.; Cłapa, T.; Szala, M.; Gąsiński, A.; Selwet, M. Synthesis of SiC/Ag/cellulose nanocomposite and its antibacterial activity by reactive oxygen species generation. Nanomaterials 2016, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Ma, Y.; Liu, Y.; Wang, Y.; Zhang, Q. Silver nanoparticles decorated by amino groups on the periphery of litchi-like P(MMA-AA-DVB)@Fe3O4 microspheres for the catalytic reduction of methyl orange. Catal. Letters 2019, 149, 2873–2886. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; Castro, D.P.C.; Ortiz-Trujillo, I.C.; Palacio, L.E.B.; Upegui, B.J.G.; Mora, N.J.E.; Cornelio, J.A.C. Compounds of carbon nanotubes decorated with silver nanoparticles via in-situ by chemical vapor deposition (CVD). J. Mater. Res. Technol. 2019, 8, 5893–5898. [Google Scholar] [CrossRef]
- Chiu, C.W.; Lee, Y.C.; Ou, G.B.; Cheng, C.C. Controllable 3D hot-junctions of silver nanoparticles stabilized by amphiphilic tri-block copolymer/graphene oxide hybrid surfactants for use as surface-enhanced Raman scattering substrates. Ind. Eng. Chem. Res. 2017, 56, 2935–2942. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kaith, B.S.; Shanker, U.; Gupta, B. γ-radiation induced synthesis of antibacterial silver nanocomposite scaffolds derived from natural gum Boswellia serrata. J. Drug Deliv. Sci. Technol. 2020, 56, 101550. [Google Scholar] [CrossRef]
- Ma, P.C.; Tang, B.Z.; Kim, J.K. Effect of CNT decoration with silver nanoparticles on electrical conductivity of CNT-polymer composites. Carbon 2008, 46, 1497–1505. [Google Scholar] [CrossRef]
- Chiu, C.W.; Ou, G.B.; Tsai, Y.H.; Lin, J.J. Immobilization of silver nanoparticles on exfoliated mica nanosheets to form highly conductive nanohybrid films. Nanotechnology 2015, 26, 465702. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Arduini, F.; Carbone, M.; Sansone, L.; Cacciotti, I.; Moscone, D.; Palleschi, G. Screen-printed electrodes modified with carbon nanomaterials: A comparison among carbon black, carbon nanotubes and graphene. Electroanalysis 2015, 27, 2230–2238. [Google Scholar] [CrossRef]
- Cheong, Y.K.; Arce, M.P.; Benito, A.; Chen, D.; Luengo Crisóstomo, N.; Kerai, L.V.; Rodríguez, G.; Valverde, J.L.; Vadalia, M.; Cerpa-Naranjo, A.; et al. Synergistic antifungal study of PEGylated graphene oxides and copper nanoparticles against candida albicans. Nanomaterials 2020, 10, 819. [Google Scholar] [CrossRef]
- Coppola, B.; Di Maio, L.; Incarnato, L.; Tulliani, J.M. Preparation and characterization of polypropylene/carbon nanotubes (PP/CNTs) nanocomposites as potential strain gauges for structural health monitoring. Nanomaterials 2020, 10, 814. [Google Scholar] [CrossRef]
- Kandare, E.; Khatibi, A.A.; Yoo, S.; Wang, R.; Ma, J.; Olivier, P.; Gleizes, N.; Wang, C.H. Improving the through-thickness thermal and electrical conductivity of carbon fibre/epoxy laminates by exploiting synergy between graphene and silver nano-inclusions. Compos. Part A 2015, 69, 72–82. [Google Scholar] [CrossRef]
- Chiu, C.W.; Ou, G.B. Facile preparation of highly electrically conductive films of silver nanoparticles finely dispersed in polyisobutylene-b-poly (oxyethylene)-b-polyisobutylene triblock copolymers and graphene oxide hybrid surfactants. RSC Adv. 2015, 5, 102462–102468. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.W.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.W.; Hong, P.D.; Lin, J.J. Clay-mediated synthesis of silver nanoparticles exhibiting low-temperature melting. Langmuir 2011, 27, 11690–11696. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; He, P.; Yang, J. Screen printed silver nanowire and graphene oxide hybrid transparent electrodes for long-term electrocardiography monitoring. J. Phys. D 2019, 52, 455401. [Google Scholar] [CrossRef]
- Baek, J.Y.; An, J.H.; Choi, J.M.; Park, K.S.; Lee, S.H. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Actuator A 2008, 143, 423–429. [Google Scholar] [CrossRef]






| Sample | Weight Ratios | Average Ag Particle Size (nm) a | Sheet Resistance (Ω/sq) b |
|---|---|---|---|
| CB | -- | -- | 2.3 × 101 |
| CNT | -- | -- | 9.2 × 102 |
| GO | -- | -- | 8.6 × 106 |
| AgNPs/PIB-POE-PIB | 1:1 | 29.9 | 4.5 × 104 |
| AgNPs/PIB-POE-PIB/CB | 5:5:1 | 26.2 | 2.8 × 10−1 |
| 10:10:1 | 25.4 | 2.0 × 10−2 (4.0 × 10−1) c | |
| 20:20:1 | 28.4 | 4.4 × 10−1 | |
| AgNPs/PIB-POE-PIB/CNT | 5:5:1 | 29.8 | 5.2 × 100 |
| 10:10:1 | 28.1 | 6.5 × 100 | |
| 20:20:1 | 32.8 | 9.4 × 10−2 (1.8 × 10−1) c | |
| AgNPs/PIB-POE-PIB/GO | 5:5:1 | -- | 3.4 × 10−1 |
| 10:10:1 | -- | 1.5 × 10−1 | |
| 20:20:1 | 16.7 | 1.2 × 10−2 (2.4 × 10−2) c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-W.; Li, J.-W.; Huang, C.-Y.; Yang, S.-S.; Soong, Y.-C.; Lin, C.-L.; Lee, J.C.-M.; Lee Sanchez, W.A.; Cheng, C.-C.; Suen, M.-C. Controlling the Structures, Flexibility, Conductivity Stability of Three-Dimensional Conductive Networks of Silver Nanoparticles/Carbon-Based Nanomaterials with Nanodispersion and their Application in Wearable Electronic Sensors. Nanomaterials 2020, 10, 1009. https://doi.org/10.3390/nano10051009
Chiu C-W, Li J-W, Huang C-Y, Yang S-S, Soong Y-C, Lin C-L, Lee JC-M, Lee Sanchez WA, Cheng C-C, Suen M-C. Controlling the Structures, Flexibility, Conductivity Stability of Three-Dimensional Conductive Networks of Silver Nanoparticles/Carbon-Based Nanomaterials with Nanodispersion and their Application in Wearable Electronic Sensors. Nanomaterials. 2020; 10(5):1009. https://doi.org/10.3390/nano10051009
Chicago/Turabian StyleChiu, Chih-Wei, Jia-Wun Li, Chen-Yang Huang, Shun-Siang Yang, Yu-Chian Soong, Chih-Lung Lin, Jimmy Chi-Min Lee, William Anderson Lee Sanchez, Chih-Chia Cheng, and Maw-Cherng Suen. 2020. "Controlling the Structures, Flexibility, Conductivity Stability of Three-Dimensional Conductive Networks of Silver Nanoparticles/Carbon-Based Nanomaterials with Nanodispersion and their Application in Wearable Electronic Sensors" Nanomaterials 10, no. 5: 1009. https://doi.org/10.3390/nano10051009
APA StyleChiu, C.-W., Li, J.-W., Huang, C.-Y., Yang, S.-S., Soong, Y.-C., Lin, C.-L., Lee, J. C.-M., Lee Sanchez, W. A., Cheng, C.-C., & Suen, M.-C. (2020). Controlling the Structures, Flexibility, Conductivity Stability of Three-Dimensional Conductive Networks of Silver Nanoparticles/Carbon-Based Nanomaterials with Nanodispersion and their Application in Wearable Electronic Sensors. Nanomaterials, 10(5), 1009. https://doi.org/10.3390/nano10051009