Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics
Abstract
1. Introduction
2. Bone Disease: Pathogenesis and Current Therapies
2.1. Bone Metastases
2.2. Osteosarcoma
2.3. Osteoporosis
2.4. Osteomyelitis
2.5. Bone Regeneration
3. Drug Delivery Systems for Bone Diseases
- Liposomes: spherical vesicles with an aqueous core surrounded by a single or multiple bilayer membrane, proven to be suitable tools also in several bone diseases [40].
- NPs: solid, colloidal particles of different materials which can be functionalized or coated to improve stability and receptor binding [43].
- Nanodiamonds: carbon-based nanomaterials which exhibit a large surface area and could be functionalized to conjugate various compounds or drugs, suitable as photoluminescent probes [54].
- Nanocrystals: usually stabilized by surfactants and/or polymers to prolong the circulating time in the blood and facilitating tumor accumulation [55].
- Polymeric gels: solid–liquid systems with polymeric matrix broadly crosslinked forming a 3D network of particular interests for biomedical applications [58].
- Matrices: consisting in homogenous dispersions of solid drug in a polymer mixture [59].
- Nanocapsules: systems with a liquid/solid core with a protective coating which delays the release of the entrapped active compounds [60].
- Scaffolds: biocompatible templates which are similar to a platform and promote the attachment and the growth of cells, thus used for tissue regeneration and surgical repairs [63].
- Nanoplatforms: biocompatible and biodegradable engineered systems based on NPs, liposomes, micelles, and dendrimers, capable to load both hydrophilic and lipophilic drugs and molecules, making them attractive systems mainly in cancer therapy [64].
3.1. Bone Metastases
3.2. Osteosarcoma
3.3. Osteoporosis
3.4. Osteomyelitis
3.5. Bone Regeneration
4. Summary of the Commonest Drug Delivery Systems for Bone Diseases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACP | Amorphous calcium phosphate |
| ALN | Alendronate |
| ASP | Aspartic acid |
| Au@MSNPs | Gold nanorods enclosed inside mesoporous silica nanoparticles |
| BG | Bioactive glass |
| BMP | Bone morphogenetic protein |
| Bip | 3,5-di(ethylamino-2,2-bisphosphono) benzoic acid |
| BPs | Bisphosphonates |
| BSA | Bovine serum albumin |
| CA | Citric acid |
| CaP | Calcium phosphate |
| CaPCs | Calcium phosphate cements |
| CaPSC | Calcium phosphate silicate cements |
| CDHA | Calcium deficient hydroxyapatite |
| CPNs | Coordination polymer nanoparticles |
| CPPA | Calcium phosphate-phosphorylated adenosine |
| CSC | Calcium silicate cements |
| DOX | Doxorubicin |
| DSP | Diamminedichlorosuccinate-platinum |
| DTPA | Diethylenetriaminepentaacetic acid |
| DTX | Docetaxel |
| EMA | European Medicines Agency |
| EVs | Extracellular vesicles |
| FDA | Food and Drug Administration |
| FGF | Fibroblast growth factor |
| GI | Gastrointestinal |
| GLU | Glutamic acid |
| GS | Gentamicin sulfate |
| HA | Hydroxyapatite |
| HAC | Hydroxyapatite cement |
| HAP | Hydroxyapatite powder |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| I.V. | Intravenous |
| MA | Monoethyl adipate |
| MMPs | Matrix metalloproteinases |
| MRI | Magnetic resonance |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSCs | Mesenchymal stromal cells |
| MSNPs | Mesoporous silica nanoparticles |
| MTP-PE | Muramyl tripeptide phosphatidyl ethanolamine |
| MTX | Methotrexate |
| N-BPs | Nitrogen-containing bisphosphonates |
| NIR | Near infrared |
| NPs | Nanoparticles |
| OCP | Octacalcium phosphate |
| OS | Osteosarcoma |
| P/L | Power to liquid |
| PAMAM | Polyamidoamine |
| PDGF | Platelet-derived growth factor |
| PEG | Polyethylene glycol |
| PEPn Na | Poly(ethylene sodium phosphate) |
| PL | Plumbagin |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic) acid |
| PMMA | Poly(methyl methacrylate) |
| PNs | Polymeric nanoparticles |
| PTH | Parathyroid hormone |
| PTT | Photothermal therapy |
| PTX | Paclitaxel |
| RA | Risedronate |
| RANKL | Nuclear factor kappa-B ligand |
| ROS | Reactive oxygen species |
| SERMs | Selective estrogen receptor modulators |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| TCP | Tricalcium phosphate |
| TEs | Trace elements |
| TGF-β | Transforming growth factor-β |
| TNF | Tumor necrosis factor |
| TOB | Tobramycin |
| TSECs | Tissue-selective estrogen complexes |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| W/O | Water in oil |
| ZOL | Zoledronate |
References
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of nanomaterials for bone-targeted drug delivery. Drug Discovery Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.; Baradari, H.; Champion, E.; Damia, C.; Viana-Trecant, M. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: A review of the parameters affecting the loading and release of the therapeutic substance. J. Control. Release 2017, 252, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Uskoković, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. Part B 2011, 96B, 152–191. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E. Drug delivery systems in hard tissue engineering. SF J. Biotechnol. Biomed. Eng. 2018, 1, 1001–1006. [Google Scholar]
- Ke Ren, A.D. Drug delivery strategies for treating osteoporosis. Orthop. Muscular Syst. 2014, S2, 8–11. [Google Scholar] [CrossRef]
- Carbone, E.J.; Rajpura, K.; Allen, B.N.; Cheng, E.; Ulery, B.D.; Lo, K.W.H. Osteotropic nanoscale drug delivery systems based on small molecule bone-targeting moieties. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 37–47. [Google Scholar] [CrossRef]
- Yang, L.; Webster, T.J. Nanotechnology controlled drug delivery for treating bone diseases. Expert Opin. Drug Deliv. 2009, 6, 851–864. [Google Scholar] [CrossRef]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem. Commun. 2014, 50, 14071–14081. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V. Facilitating the translation of nanomedicines to a clinical product: Challenges and opportunities. Drug Discov. Today 2018, 23, 974–991. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Ando, K.; Mori, K.; Corradini, N.; Redini, F.; Heymann, D. Mifamurtide for the treatment of nonmetastatic osteosarcoma. Expert Opin. Pharm. 2011, 12, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gogia, J.S.; Meehan, J.P.; Di Cesare, P.E.; Jamali, A.A. Local antibiotic therapy in osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S. Prospective of calcium phosphate cements for bone regeneration in relation to physicochemical, mechanical and biological properties. Sm J. Orthop. 2018, 4, 1–12. [Google Scholar]
- Gu, W.; Wu, C.; Chen, J.; Xiao, Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 43–49. [Google Scholar] [CrossRef]
- Brown, J.P.; Morin, S.; Leslie, W.; Papaioannou, A.; Cheung, A.M.; Davison, K.S.; Goltzman, D.; Hanley, D.A.; Hodsman, A.; Josse, R.; et al. Bisphosphonates for treatment of osteoporosis. Can. Fam. Physician 2014, 60, 324–333. [Google Scholar]
- Vinay, R.; KusumDevi, V. Potential of targeted drug delivery system for the treatment of bone metastasis. Drug Deliv. 2016, 23, 21–29. [Google Scholar] [CrossRef]
- Saikali, Z.; Singh, G. Doxycycline and other tetracyclines in the treatment of bone metastasis. Anticancer Drugs 2003, 14, 773–778. [Google Scholar] [CrossRef]
- Escudier, B.; Powles, T.; Motzer, R.J.; Olencki, T.; Frontera, O.A.; Oudard, S.; Rolland, F.; Tomczak, P.; Castellano, D.; Appleman, L.J.; et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J. Clin. Oncol. 2018, 36, 765–772. [Google Scholar] [CrossRef]
- Maisano, R.; Pergolizzi, S.; Cascinu, S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit. Rev. Oncol. Hematol. 2001, 40, 239–250. [Google Scholar] [CrossRef]
- Thanos, L.; Mylona, S.; Galani, P.; Tzavoulis, D.; Kalioras, V.; Tanteles, S.; Pomoni, M. Radiofrequency ablation of osseous metastases for the palliation of pain. Skelet. Radiol. 2008, 37, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Callstrom, M.R.; Dupuy, D.E.; Solomon, S.B.; Beres, R.A.; Littrup, P.J.; Davis, K.W.; Paz-Fumagalli, R.; Hoffman, C.; Atwell, T.D.; Charboneau, J.W.; et al. Percutaneous image-guided cryoablation of painful metastases involving bone: Multicenter trial. Cancer 2013, 119, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.; Chung, C.K. The role of stereotactic radiosurgery in Metastasis to the Spine. J. Korean Neurosurg. Soc. 2012, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hemant, S. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2010, 2, 1–19. [Google Scholar]
- Wittig, J.C.; Bickels, J.; Priebat, D.; Jelinek, J.; Kellar-Graney, K.; Shmookler, B.; Malawer, M.M. Osteosarcoma: A multidisciplinary approach to diagnosis and treatment. Am. Fam. Physician 2002, 65, 1123–1132. [Google Scholar]
- Messerschmitt, P.J.; Garcia, R.M.; Abdul-Karim, F.W.; Greefield, E.M.; Getty, P.J. Osteosarcoma. J. Am. Acad. Othopaedic Surg. 2009, 17, 515–527. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K. Prevention and rehabilitation of osteoporosis. Wien. Med. Wochenschr. 2016, 166, 22–27. [Google Scholar] [CrossRef]
- Pazianas, M.; Abrahamsen, B. Osteoporosis treatment: Bisphosphonates reign to continue for a few more years, at least? Ann. N. Y. Acad. Sci. 2016, 1376, 5–13. [Google Scholar] [CrossRef]
- Nemeth, E.F.; Shoback, D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pr. Res. Clin. Endocrinol. Metab. 2013, 27, 373–384. [Google Scholar] [CrossRef]
- Uskoković, V. Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis. Crit Rev. Drug Carrier Syst. 2015, 32, 1–59. [Google Scholar] [CrossRef]
- Uskoković, V.; Desai, T.A. Nanoparticulate drug delivery platforms for advancing bone infection therapies. Expert Opin. Drug Deliv. 2014, 11, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.A.; Uskoković, V. Calcium phosphate nanoparticles: A future therapeutic platform for the treatment of osteomyelitis? Ther. Deliv. 2013, 4, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, J.; Yang, J.; Li, B. Nanomaterials promise better bone repair. Mater. Today 2016, 19, 451–463. [Google Scholar] [CrossRef]
- Aronson, J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J. Bone Jt. Surg. 1997, 79-A, 1243–1258. [Google Scholar]
- Spiegelberg, B.; Parratt, T.; Dheerendra, S.K.; Khan, W.S.; Jennings, R.; Marsh, D.R. Ilizarov principles of deformity correction. Ann. R. Coll. Surg. Engl. 2010, 92, 101–105. [Google Scholar] [CrossRef]
- Tamimi, F.; Kumarasami, B.; Doillon, C.; Gbureck, U.; Le Nihouannen, D.; Cabarcos, E.L.; Barralet, J.E. Brushite-collagen composites for bone regeneration. Acta Biomater. 2008, 4, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Bellavia, D.; Raimondi, L.; Costa, V.; De Luca, A.; Carina, V.; Maglio, M.; Fini, M.; Alessandro, R.; Giavaresi, G. Engineered exosomes: A new promise for the management of musculoskeletal diseases. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1893–1901. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Maravajhala, V.; Dasari, N.; Sepuri, A.; Joginapalli, S. Design and evaluation of niacin microspheres. Indian J. Pharm. Sci. 2009, 71, 663–669. [Google Scholar] [CrossRef]
- Noguez Méndez, N.A.; Quirino Barreda, C.T.; Vega, A.F.; Miranda Calderon, J.E.; Urioste, C.G.; Palomec, X.C.; Martínez, A.R.; Díaz, M.P. Design and development of pharmaceutical microprocesses in the production of nanomedicine. In Nanostructures for Oral Medicine, 1st ed.; Andronescu, E., Grumezescu, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 669–697. [Google Scholar]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, A. Beads as drug carriers. In Polymer Macro- and Micro-Gel Beads: Fundamentals and Applications, 1st ed.; Springer: New York, NY, USA, 2010; pp. 191–230. [Google Scholar]
- Bhattarai, R.S.; Dhandapani, N.V.; Shrestha, A. Drug delivery using alginate and chitosan beads: An Overview. Chron. Young Sci. 2011, 2, 192–196. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar]
- Zhu, X.; Anquillare, E.L.B.; Farokhzad, O.C.; Shi, J. Polymer- and protein-based nanotechnologies for cancer theranostics. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Elsevier: New York, NY, USA, 2014; pp. 419–436. [Google Scholar]
- Irby, D.; Du, C.; Li, F. Lipid–Drug Conjugate for Enhancing Drug Delivery. Mol. Pharmcol. 2017, 14, 1325–1338. [Google Scholar] [CrossRef]
- Sa, Y.; Gao, Y.; Wang, M.; Wang, T.; Feng, X.; Wang, Z.; Wang, Y.; Jiang, T. Bioactive calcium phosphate cement with excellent injectability, mineralization capacity and drug-delivery properties for dental biomimetic reconstruction and minimum intervention therapy. RSC Adv. 2016, 6, 27349–27359. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Barsoum, M.W. Fundamentals of Ceramics; Cantor, B., Goringe, M.J., Eds.; Taylor & Francis Group: New York, NY, USA, 2003. [Google Scholar]
- Zang, S.; Chang, S.; Shahzad, M.B.; Sun, X.; Jiang, X.; Yang, H. Ceramics-based Drug Delivery System: A Review and Outlook. Rev. Adv. Mater. Sci. 2019, 58, 82–97. [Google Scholar] [CrossRef]
- Ansari, S.A.; Satar, R.; Jafri, M.A.; Rasool, M.; Ahmad, W.; Zaidi, S.K. Role of nanodiamonds in drug delivery and stem cell therapy. Iran J. Biotechnol. 2016, 14, 70–81. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Wu, W. Injected nanocrystals for targeted drug delivery. Acta Pharm. Sin. B 2016, 6, 106–113. [Google Scholar] [CrossRef]
- García, M.C.; Uberman, P.M. Nanohybrid Filler-Based Drug-Delivery System. In Nanocarriers for Drug Delivery, 1st ed.; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Kumar Mishra, R., Thomas, S., Eds.; Elsevier: Cambridge, MA, USA, 2019; pp. 43–79. [Google Scholar]
- Prakash, S.; Malhotra, M.; Shao, W.; Tomaro-Duchesneau, C.; Abbasi, S. Polymeric nanohybrids and functionalized carbon nanotubes as drug delivery carriers for cancer therapy. Adv. Drug Deliv. Rev. 2011, 63, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Jagur-Grodzinski, J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol. 2010, 21, 27–47. [Google Scholar] [CrossRef]
- Pundir, S.; Badola, A.; Sharma, D. Sustained release matrix technology and recent advance in matrix drug delivery system: A review. Int. J. Drug Res. Technol. 2013, 3, 12–20. [Google Scholar]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The weapons for novel drug delivery systems. BioImpacts 2012, 2, 71–81. [Google Scholar] [PubMed]
- Wang, M. Composite coatings for implants and tissue engineering scaffolds. In Biomedical Composites, 1st ed.; Ambrosio, L., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 127–177. [Google Scholar]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar] [CrossRef]
- Fang, F.; Aabith, S.; Homer-Vanniasinkam, S.; Tiwari, M.K. High-resolution 3D printing for healthcare underpinned by small-scale fluidics. In 3D Printing in Medicine; Kalaskar, D.M., Ed.; Woodhead Publishing: London, UK, 2017; pp. 167–206. [Google Scholar]
- Mukerabigwi, J.F.; Ge, Z.; Kataoka, K. Therapeutic nanoreactors as in vivo nanoplatforms for cancer therapy. Chem. A Eur. J. 2018, 24, 15706–15724. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Huang, Z.; Jiang, Y.; Sun, X.; Shen, Y.; Chu, W.; Zhao, C. Bisphosphonate-functionalized coordination polymer nanoparticles for the treatment of bone metastatic breast cancer. J. Control. Release 2017, 264, 76–88. [Google Scholar] [CrossRef]
- Hirano, Y.; Iwasaki, Y. Bone-specific poly(ethylene sodium phosphate)-bearing biodegradable nanoparticles. Colloids Surf. B Biointerfaces 2017, 153, 104–110. [Google Scholar] [CrossRef]
- Chu, W.; Huang, Y.; Yang, C.; Liao, Y.; Zhang, X.; Yan, M.; Cui, S.; Zhao, C. Calcium phosphate nanoparticles functionalized with alendronate-conjugated polyethylene glycol (PEG) for the treatment of bone metastasis. Int. J. Pharm. 2017, 516, 352–363. [Google Scholar] [CrossRef]
- Ramanlal Chaudhari, K.; Kumar, A.; Megraj Khandelwal, V.K.; Ukawala, M.; Manjappa, A.S.; Mishra, A.K.; Monkkonen, J.; Ramachandra Murthy, R.S. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J. Control. Release 2012, 158, 470–478. [Google Scholar] [CrossRef]
- Aziz, M.H.; Dreckschmidt, N.E.; Verma, A.K. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Cui, Z.; Yang, S.; Ji, D.; Wang, Y.; Yang, Y.; Han, X.; Fan, Q.; Qin, A.; Wang, T.; et al. Targeting osteocytes to attenuate early breast cancer bone metastasis by theranostic upconversion nanoparticles with responsive plumbagin release. ACS Nano 2017, 11, 7259–7273. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Lin, W.; Li, X.; Wang, H.; Xiao, X.; Guo, Z. Synergistic dual-modified liposome improves targeting and therapeutic efficacy of bone metastasis from breast cancer. Drug Deliv. 2017, 24, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Takeda, Y.; Honda, Y.; Sakurai, K.; Suzuki, O. Synthesis of calcium phosphate-binding liposome for drug delivery. Bioorg. Med. Chem. Lett. 2009, 19, 4148–4150. [Google Scholar] [CrossRef] [PubMed]
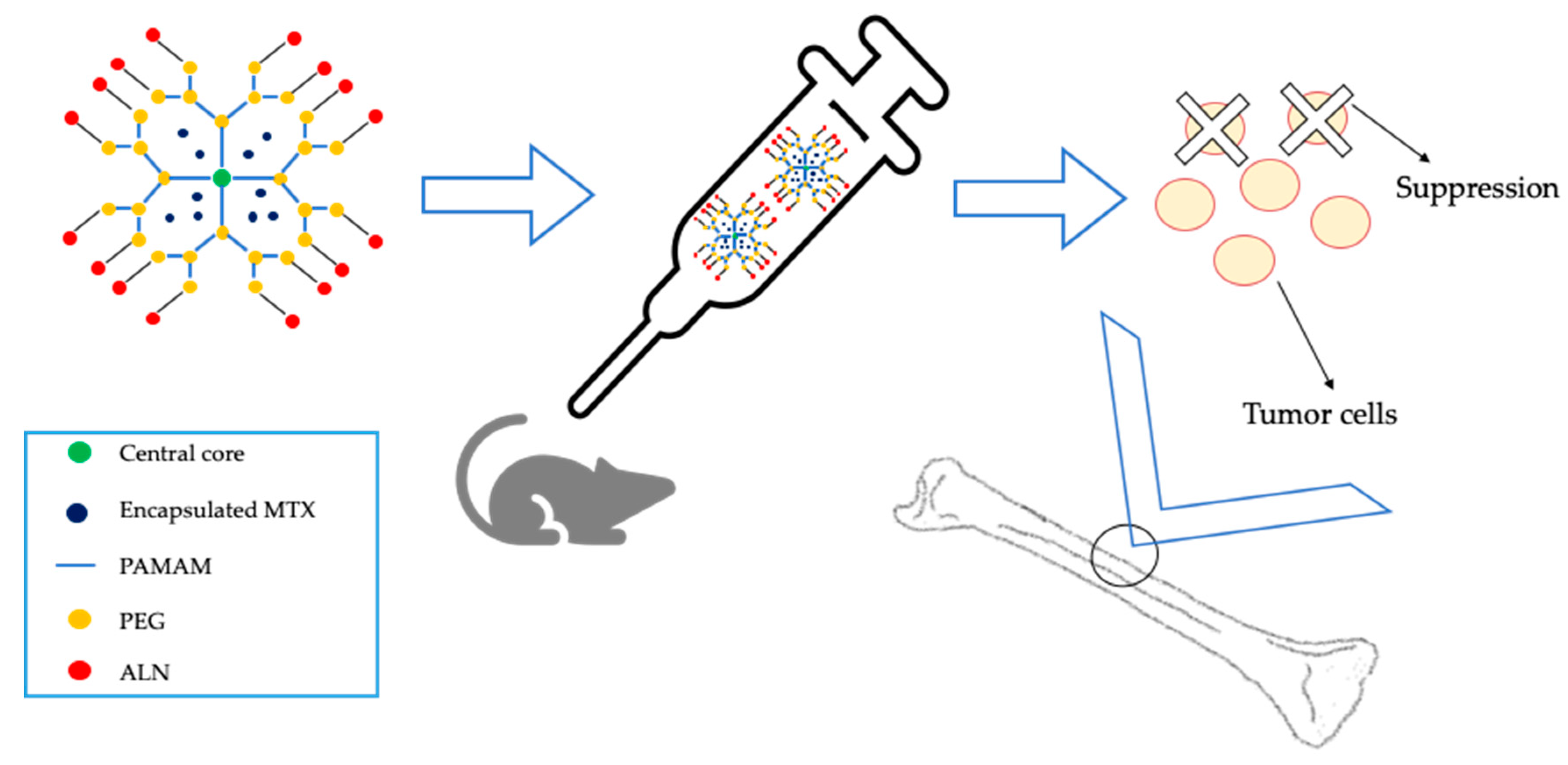
- Yamashita, S.; Katsumi, H.; Sakane, T.; Yamamoto, A. Bone-targeting dendrimer for the delivery of methotrexate and treatment of bone metastasis. J. Drug Target. 2018, 26, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Clementi, C.; Miller, K.; Mero, A.; Satchi-Fainaro, R.; Pasut, G. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. Mol. Pharm. 2011, 8, 1063–1072. [Google Scholar] [CrossRef]
- Arnett, T. Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 2003, 62, 511–520. [Google Scholar] [CrossRef]
- Ye, W.-L.; Zhao, Y.-P.; Na, R.; Li, F.; Mei, Q.-B.; Zhao, M.-G.; Zhou, S.-Y. Actively Targeted Delivery of Doxorubicin to Bone Metastases by a pH-Sensitive Conjugation. J. Pharm. Sci. 2015, 104, 2293–2303. [Google Scholar] [CrossRef]
- Nishikawa, M.; Hashida, M.; Takakura, Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv. Drug Deliv. Rev. 2009, 61, 319–326. [Google Scholar] [CrossRef]
- Zheng, Y.; Nishikawa, M.; Ikemura, M.; Yamashita, F.; Hashida, M. Development of bone-targeted catalase derivatives for inhibition of bone metastasis of tumor cells in mice. J. Pharm. Sci. 2012, 101, 552–557. [Google Scholar] [CrossRef] [PubMed]
- El-Mabhouh, A.; Angelov, C.; McEwan, A.; Jia, G.; Mercer, J. Preclinical Investigations of Drug and Radionuclide Conjugates of Bisphosphonates for the Treatment of Metastatic Bone Cancer. Cancer Biother. Radiopharm. 2004, 19, 627–640. [Google Scholar] [PubMed]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A smart hyperthermia nanofiber with switchable drug release for inducing cancer apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Sun, W.; Ge, K.; Jin, Y.; Han, Y.; Zhang, H.; Zhou, G.; Yang, X.; Liu, D.; Liu, H.; Liang, X.-J.; et al. Bone-targeted nanoplatform combining zoledronate and photothermal therapy to treat breast cancer bone metastasis. ACS Nano 2019, 13, 7556–7567. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Heredia, M.A.; Bernard Kamphuis, G.J.; Thüne, P.C.; Cumhur Öner, F.; Jansen, J.A.; Frank Walboomers, X. An injectable calcium phosphate cement for the local delivery of paclitaxel to bone. Biomaterials 2011, 32, 5411–5416. [Google Scholar] [CrossRef]
- Min, L.; Xiao-Ping, Z.; Zhi-Yuan, C.; Jia-Ming, L.; Qing, L.; Zhi-Li, L. Extracellular vesicles-mediated signaling in the osteosarcoma microenvironment: Roles and potential therapeutic targets. J. Bone Oncol. 2018, 12, 101–104. [Google Scholar]
- Abello, J.; Nguyen, T.D.T.; Marasini, R.; Aryal, S.; Weiss, M.L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 2019, 9, 2325–2345. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef]
- Lee, M.S.; Dees, E.C.; Wang, A.Z. Nanoparticle-delivered chemotherapy: Old drugs in new packages. Oncol. J. 2017, 198–208. [Google Scholar]
- Wu, V.M.; Mickens, J.; Uskoković, V. Bisphosphonate-functionalized hydroxyapatite nanoparticles for the delivery of the bromodomain inhibitor JQ1 in the treatment of osteosarcoma. ACS Appl. Mater. Interfaces 2017, 9, 25887–25904. [Google Scholar] [CrossRef]
- Barroug, A.; Kuhn, L.T.; Gerstenfeld, L.C.; Glimcher, M.J. Interactions of cisplatin with calcium phosphate nanoparticles: In vitro controlled adsorption and release. J. Orthop. Res. 2004, 22, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Nazmi, K.; De Boer, J.P.; Zandieh-Doulabi, B.; Forouzanfar, T.; Helder, M.N. EphA2 Targeted doxorubicin-nanoliposomes for osteosarcoma treatment. Pharm. Res. 2017, 34, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Zandieh-Doulabi, B.; Forouzanfar, T.; Helder, M.N. Codelivery of doxorubicin and JIP1 siRNA with novel EphA2-targeted pegylated cationic nanoliposomes to overcome osteosarcoma multidrug resistance. Int. J. Nanomed. 2018, 13, 3853–3866. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Wei, L.; Duolikun, D.; Hou, X.-D.; Chen, F.; Liu, J.-J.; Zheng, L.-P. Preparation of porous calcium phosphate microspheres with phosphate-containing molecules at room temperature for drug delivery and osteogenic differentiation. RSC Adv. 2018, 8, 25480–25488. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Sun, T.-W.; Chen, F.; Zuo, D.-Q.; Wang, H.-S.; Hua, Y.-Q.; Cai, Z.-D.; Tan, J. Calcium phosphate-phosphorylated adenosine hybrid microspheres for anti-osteosarcoma drug delivery and osteogenic differentiation. Biomaterials 2017, 121, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tanzawa, Y.; Tsuchiya, H.; Shirai, T.; Nishida, H.; Hayashi, K.; Takeuchi, A.; Kawahara, M.; Tomita, K. Potentiation of the antitumor effect of calcium phosphate cement containing anticancer drug and caffeine on rat osteosarcoma. J. Orthop. Sci. 2011, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Okada, K.; Takahashi, S.; Suzuki, N.; Shimada, Y.; Itoi, E. Doxorubicin-loaded calcium phosphate cement in the management of bone and soft tissue tumors. In Vivo (Brooklyn) 2006, 20, 55–60. [Google Scholar]
- Hess, U.; Shahabi, S.; Treccani, L.; Streckbein, P.; Heiss, C.; Rezwan, K. Co-delivery of cisplatin and doxorubicin from calcium phosphate beads/matrix scaffolds for osteosarcoma therapy. Mater. Sci. Eng. C 2017, 77, 427–435. [Google Scholar] [CrossRef]
- Gonçalves, M.; Figueira, P.; Maciel, D.; Rodrigues, J.; Qu, X.; Liu, C.; Tomás, H.; Li, Y. pH-sensitive Laponite®/doxorubicin/alginate nanohybrids with improved anticancer efficacy. Acta Biomater. 2014, 10, 300–307. [Google Scholar] [CrossRef]
- Mepact: EPAR. Available online: https://www.ema.europa.eu/en/documents/product-information/mepact-epar-product-information_en.pdf (accessed on 24 April 2020).
- Arkfeld, D.G.; Rubenstein, E. Quest for the Holy Grail to cure arthritis and osteoporosis: Emphasis on bone drug delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 939–944. [Google Scholar] [CrossRef]
- Gong, T.; Chen, Y.; Zhang, Y.; Zhang, Y.; Liu, X.; Troczynski, T.; Häfeli, U.O. Osteogenic and anti-osteoporotic effects of risedronate-added calcium phosphate silicate cement. Biomed. Mater. 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Wang, Z.; Zhang, Y.; Sun, C.; Yang, Q.; Troczynski, T.; Häfeli, U.O. Preparation, characterization, release kinetics, and in vitro cytotoxicity of calcium silicate cement as a risedronate delivery system. J. Biomed. Mater. Res. Part A 2014, 102 A, 2295–2304. [Google Scholar] [CrossRef]
- Jindong, Z.; Hai, T.; Jiayang, W.; Gang, L. Local treatment of osteoporosis with alendronate-loaded calcium phosphate cement. Chin. Med. J. (Engl.) 2014, 127, 3906–3914. [Google Scholar]
- Jindong, Z.; Hai, T.; Junchao, G.; Bo, W.; Li, B.; Qiang, W.B. Evaluation of a novel osteoporotic drug delivery system in vitro: Alendronate-loaded calcium phosphate cement. Orthopedics 2010, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, X.; Ge, B. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: A pilot study. Clin. Orthop. Relat. Res. 2010, 468, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Ishii, Y. Mechanical properties of the femur after injection of calcium phosphate cement containing arbekacin sulfate and polylactic acid in a rat model of experimental osteoporosis. J. Orthop. Sci. 2002, 7, 370–378. [Google Scholar] [CrossRef]
- Liu, Y.; Schouten, C.; Boerman, O.; Wu, G.; Jansen, J.A.; Hunziker, E.B. The kinetics and mechanism of bone morphogenetic protein 2 release from calcium phosphate-based implant-coatings. J. Biomed. Mater. Res. Part A 2018, 106A, 2363–2371. [Google Scholar] [CrossRef]
- Pyo, S.W.; Kim, Y.M.; Kim, C.S.; Lee, I.S.; Park, J.U. Bone Formation on Biomimetic Calcium Phosphate–Coated and Zoledronate-Immobilized Titanium Implants in Osteoporotic Rat Tibiae. Int. J. Oral Maxillofac. Implant. 2014, 29, 478–484. [Google Scholar] [CrossRef]
- Peter, B.; Pioletti, D.P.; Laïb, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.-Y.; Bouler, J.-M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Fini, M.; Bigi, A. Antiresorptive and anti-angiogenetic octacalcium phosphate functionalized with bisphosphonates: An in vitro tri-culture study. Acta Biomater. 2017, 54, 419–428. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Yang, X.; Li, Z.; Sun, X.; Lin, M.; Yang, G.; Gou, Z. Micronutrients-incorporated calcium phosphate particles with protective effect on osteoporotic bone tissue. J. Nutr. Heal. Aging 2013, 17, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Pareta, R.A.; Taylor, E.; Webster, T.J. Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnology 2008, 19, 265101. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Ito, T.; Otsuka, M.; Ben-Nissan, B.; Milthorpe, B. The effectiveness of the controlled release of simvastatin from β-TCP macrosphere in the treatment of OVX mice. J. Tissue Eng. Regen. Med. 2016, 10, E195–E203. [Google Scholar] [CrossRef] [PubMed]
- Liporace, F.A.; Breitbart, E.A.; Yoon, R.S.; Doyle, E.; Paglia, D.N.; Lin, S. The effect of locally delivered recombinant human bone morphogenetic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J. Orthop. Traumatol. 2015, 16, 151–159. [Google Scholar] [CrossRef]
- Ryu, T.-K.; Kang, R.-H.; Jeong, K.-Y.; Jun, D.-R.; Koh, J.-M.; Kim, D.; Bae, S.K.; Choi, S.-W. Bone-targeted delivery of nanodiamond-based drug carriers conjugated with alendronate for potential osteoporosis treatment. J. Control. Release 2016, 232, 152–160. [Google Scholar] [CrossRef]
- Ito, T.; Takemasa, M.; Makino, K.; Otsuka, M. Preparation of calcium phosphate nanocapsules including simvastatin/deoxycholic acid assembly, and their therapeutic effect in osteoporosis model mice. J. Pharm. Pharmcol. 2013, 65, 494–502. [Google Scholar] [CrossRef]
- Ito, T.; Saito, M.; Uchino, T.; Senna, M.; Iafisco, M.; Prat, M.; Rimondini, L.; Otsuka, M. Preparation of injectable auto-forming alginate gel containing simvastatin with amorphous calcium phosphate as a controlled release medium and their therapeutic effect in osteoporosis model rat. J. Mater. Sci. Mater. Med. 2012, 23, 1291–1297. [Google Scholar] [CrossRef]
- Kim, C.W.; Yun, Y.-P.; Lee, H.J.; Hwang, Y.-S.; Kwon, I.K.; Lee, S.C. In situ fabrication of alendronate-loaded calcium phosphate microspheres: Controlled release for inhibition of osteoclastogenesis. J. Control. Release 2010, 147, 45–53. [Google Scholar] [CrossRef]
- Verron, E.; Gauthier, O.; Janvier, P.; Pilet, P.; Lesoeur, J.; Bujoli, B.; Guicheux, J.; Bouler, J.-M. In vivo bone augmentation in an osteoporotic environment using bisphosphonate-loaded calcium deficient apatite. Biomaterials 2010, 31, 7776–7784. [Google Scholar] [CrossRef]
- Rogers-Foy, J.M.; Powers, D.L.; Brosnan, D.A.; Barefoot, S.F.; Friedman, R.J.; LaBerge, M. Hydroxyapatite composites designed for antibiotic drug delivery and bone reconstruction: A caprine model. J. Investig. Surg. 1999, 12, 263–275. [Google Scholar] [CrossRef]
- Lulu, G.A.; Karunanidhi, A.; Mohamad Yusof, L.; Abba, Y.; Fauzi, F.M.; Othman, F. In vivo efficacy of tobramycin-loaded synthetic calcium phosphate beads in a rabbit model of staphylococcal osteomyelitis. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- Ghosh, S.; Wu, V.; Pernal, S.; Uskoković, V. Self-setting calcium phosphate cements with tunable antibiotic release rates for advanced antimicrobial applications. ACS Appl. Mater. Interfaces 2016, 8, 7691–7708. [Google Scholar] [CrossRef] [PubMed]
- Schnieders, J.; Gbureck, U.; Vorndran, E.; Schossig, M.; Kissel, T. The effect of porosity on drug release kinetics from vancomycin microsphere/calcium phosphate cement composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99 B, 391–398. [Google Scholar] [CrossRef]
- Lazarettos, J.; Efstathopoulos, N.; Papagelopoulos, P.J.; Savvidou, O.D.; Kanellakopoulou, K.; Giamarellou, H.; Giamarellos-Bourboulis, E.J.; Nikolaou, V.; Kapranou, A.; Papalois, A.; et al. A bioresorbable calcium phosphate delivery systems with teicoplanin for treating MRSA osteomyelitis. Clin. Orthop. Relat. Res. 2004, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, H.P.; Faber, C.; Bronckers, A.L.J.J.; Nieuw Amerongen, A.V.; Wuismann, P.I.J.M. Osteomyelitis prevention in rabbits using antimicrobial peptide hLF1-11- or gentamicin-containing calcium phosphate cement. J. Antimicrob. Chemother. 2004, 54, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Joosten, U.; Joist, A.; Frebel, T.; Brandt, B.; Diederichs, S.; Von Eiff, C. Evaluation of an in situ setting injectable calcium phosphate as a new carrier material for gentamicin in the treatment of chronic osteomyelitis: Studies in vitro and in vivo. Biomaterials 2004, 25, 4287–4295. [Google Scholar] [CrossRef]
- Bastari, K.; Arshath, M.; Ng, Z.H.M.; Chia, J.H.; Yow, Z.X.D.; Sana, B.; Tan, M.F.C.; Lim, S.; Loo, S.C.J. A controlled release of antibiotics from calcium phosphate-coated poly(lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm. J. Mater. Sci. Mater. Med. 2014, 25, 747–757. [Google Scholar] [CrossRef]
- Uskoković, V.; Desai, T.A. In vitro analysis of nanoparticulate hydroxyapatite/chitosan composites as potential drug delivery platforms for the sustained release of antibiotics in the treatment of osteomyelitis. J. Pharm. Sci. 2014, 103, 567–579. [Google Scholar] [CrossRef]
- Uskoković, V.; Desai, T.A. Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. Part 1: Preparation and drug release. J. Biomed. Mater. Res. Part A 2013, 101, 1416–1426. [Google Scholar]
- Uskoković, V.; Desai, T.A. phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. Part 2: Antibacterial and osteoblastic response. J. Biomed. Mater. Res. Part A 2013, 101, 1427–1436. [Google Scholar]
- Sunita Prem, V.; Sampath Kumar, T.S. Tailoring calcium-deficient hydroxyapatite nanocarriers for enhanced release of antibiotics. J. Biomed. Nanotechnol. 2008, 4, 203–209. [Google Scholar]
- Sampath Kumar, T.S.; Madhumathi, K.; Rubaiya, Y.; Doble, M. Dual mode antibacterial activity of ion substituted calcium phosphate nanocarriers for bone infections. Front. Bioeng. Biotechnol. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Uskoković, V.; Hoover, C.; Vukomanović, M.; Uskoković, D.P.; Desai, T.A. Osteogenic and antimicrobial nanoparticulate calcium phosphate and poly-(d, l-lactide-co-glycolide) powders for the treatment of osteomyelitis. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Thanyaphoo, S.; Kaewsrichan, J. Synthesis and evaluation of novel glass ceramics as drug delivery systems in osteomyelitis. J. Pharm. Sci. 2012, 101, 2870–2882. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Li, P.; Teng, H.; Fan, D.; Du, W.; Guo, Z. Progress and applications of polyphosphate in bone and cartilage regeneration. Biomed. Res. Int. 2019, 2019, 5141204. [Google Scholar] [CrossRef]
- Zeng, Y.; Hoque, J.; Varghese, S. Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater. 2019, 93, 152–168. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Roether, J.A.; Dubey, P.; Roy, I.; Boccaccini, A.R. Composite polymer-bioceramic scaffolds with drug delivery capability for bone tissue engineering. Expert Opin. Drug Deliv. 2013, 10, 1353–1365. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Selvaratnam, B.; Koodali, R.T.; Sun, H. Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering. J. Control. Release 2018, 279, 69–78. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Wang, P.; Wang, L.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Ren, K.; Zhao, L.; Xu, H.H.K. Engineering bone regeneration with novel cell-laden hydrogel microfiber-injectable calcium phosphate scaffold. Mater. Sci. Eng. C 2017, 75, 895–905. [Google Scholar] [CrossRef]
- Frasca, S.; Norol, F.; Le Visage, C.; Collombet, J.-M.; Letourneur, D.; Holy, X.; Sari Ali, E. Calcium-phosphate ceramics and polysaccharide-based hydrogel scaffolds combined with mesenchymal stem cell differently support bone repair in rats. J. Mater. Sci. Mater. Med. 2017, 28, 1–13. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Li, C.; Weir, M.D.; Wang, P.; Reynolds, M.A.; Zhao, L.; Xu, H.H.K. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, K.; Azadpour, P.; Moztarzadeh, F.; Urbanska, A.M.; Mozafari, M. Tissue-engineered chitosan/bioactive glass bone scaffolds integrated with PLGA nanoparticles: A therapeutic design for on-demand drug delivery. Mater. Lett. 2015, 138, 16–20. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Xiao, G.-Y.; Wang, X.; Dong, Z.-G.; Ma, Z.-Y.; Li, L.; Li, Y.-H.; Pan, X.; Nie, L. Biocompatibility and osteogenesis of calcium phosphate composite scaffolds containing simvastatin-loaded PLGA microspheres for bone tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103A, 3250–3258. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ye, J. In vitro degradation, biocompatibility, and in vivo osteogenesis of poly(lactic-co-glycolic acid)/calcium phosphate cement scaffold with unidirectional lamellar pore structure. J. Biomed. Mater. Res. Part A 2012, 100A, 3239–3250. [Google Scholar] [CrossRef]
- Park, K.-W.; Yun, Y.-P.; Kim, S.E.; Song, H.-R. The effect of alendronate loaded biphasic calcium phosphate scaffolds on bone regeneration in a rat tibial defect model. Int. J. Mol. Sci. 2015, 16, 26738–26753. [Google Scholar] [CrossRef]
- Kim, S.E.; Yun, Y.-P.; Lee, D.-W.; Kang, E.Y.; Jeong, W.J.; Lee, B.; Jeong, M.S.; Kim, H.J.; Park, K.; Song, H.-R. Alendronate-eluting biphasic calcium phosphate (BCP) scaffolds stimulate osteogenic differentiation. Biomed. Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite hydrogels for bone regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef]
- Tangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked extracellular matrix of calcified soft tissue based on chitosan/gelatin/compounded calcium phosphate hydrogel to design ex vivo model for heterotopic ossification. Mater. Des. 2017, 134, 486–493. [Google Scholar] [CrossRef]
- Yu, P.; Bao, R.-Y.; Shi, X.-J.; Yang, W.; Yang, M.-B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017, 155, 507–515. [Google Scholar] [CrossRef]
- Weinand, C.; Pomerantseva, I.; Neville, C.M.; Gupta, R.; Weinberg, E.; Madisch, I.; Shapiro, F.; Abukawa, H.; Troulis, M.J.; Vacanti, J.P. Hydrogel-β-TCP scaffolds and stem cells for tissue engineering bone. Bone 2006, 38, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R.; Pohlit, H.; Schüler, T.; Panthöfer, M.; Unger, R.E.; Frey, H.; Tremel, W. Transformation of vaterite nanoparticles to hydroxycarbonate apatite in a hydrogel scaffold: Relevance to bone formation. J. Mater. Chem. B 2015, 3, 7079–7089. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Krawczyk, G.; Pamula, E.; Declercq, H.A.; Schaubroeck, D.; Bucko, M.M.; Balcaen, L.; Van Der Voort, P.; Bliznuk, V.; van der Vreken, N.M.F.; et al. Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means. J. Tissue Eng. Regen. Med. 2016, 10, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, T.; Ogasawara, H.; Tanaka, M.; Higuchi, M.; Kinoshita, T. Calcium phosphate biomineralization in peptide hydrogels for injectable bone-filling materials. Soft Matter 2012, 8, 11531–11536. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Jansen, J.A.; Mikos, A.G. Functionalization of oligo(poly(ethylene glycol)fumarate) hydrogels with finely dispersed calcium phosphate nanocrystals for bone-substituting purposes. J. Biomater. Sci. Polym. Ed. 2007, 18, 1547–1564. [Google Scholar] [CrossRef]
- Qu, X.; He, F.; Tan, H.; Yu, Y.; Axrap, A.; Wang, M.; Dai, K.; Zhang, Z.; Yang, F.; Wang, S.; et al. Self-assembly of dual drug-delivery coating for synergistic bone regeneration. J. Mater. Chem. B 2016, 4, 4901–4912. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Farag, M.M.; El-Rashedi, A.M.I. Bioactive glass nanoparticles designed for multiple deliveries of lithium ions and drugs: Curative and restorative bone treatment. Eur. J. Pharm. Sci. 2016, 91, 243–250. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, G.-S.; Shin, U.S.; Kim, H.-W. Self-hardening microspheres of calcium phosphate cement with collagen for drug delivery and tissue engineering in bone repair. J. Am. Ceram. Soc. 2011, 94, 351–354. [Google Scholar] [CrossRef]
- Ribeiro, C.C.; Barrias, C.C.; Barbosa, M.A. Calcium phosphate-alginate microspheres as enzyme delivery matrices. Biomaterials 2004, 25, 4363–4373. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 1–19. [Google Scholar] [CrossRef]
- Masaeli, R.; Jafarzadeh Kashi, T.S.; Dinarvand, R.; Rakhshan, V.; Shahoon, H.; Hooshmand, B.; Mashhadi Abbas, F.; Raz, M.; Rajabnejad, A.; Eslami, H.; et al. Efficacy of the biomaterials 3 wt%-nanostrontium-hydroxyapatite-enhanced calcium phosphate cement (nanoSr-CPC) and nanoSr-CPC-incorporated simvastatin-loaded poly(lactic-co-glycolic-acid) microspheres in osteogenesis improvement: An explorative multi-phas. Mater. Sci. Eng. C 2016, 69, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Giardino, R.; Bigi, A. Nanocomposites of hydroxyapatite with aspartic acid and glutamic acid and their interaction with osteoblast-like cells. Biomaterials 2006, 27, 4428–4433. [Google Scholar] [CrossRef] [PubMed]
- Dittler, M.L.; Unalan, I.; Grünewald, A.; Beltrán, A.M.; Grillo, C.A.; Destch, R.; Gonzalez, M.C.; Boccaccini, A.R. Bioactive glass (45S5)-based 3D scaffolds coated with magnesium and zinc-loaded hydroxyapatite nanoparticles for tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 182, 110346. [Google Scholar] [CrossRef] [PubMed]





| Types | Pathophysiology | Primary Cancer Sites |
|---|---|---|
| Osteolytic | Destruction of normal bone by ostoclasts | Multiple myeloma, melanoma, non-small cell lung cancer, non-Hodgkin lymphoma, thyroid cancer or Langerhans-cell histiocytosis, breast cancer, renal cell carcinoma |
| Osteoblastic | Deposition of new bone by osteoblasts | Prostate cancer, small cell lung cancer, Hodgkin lymphoma or medulloblastoma |
| Mixed | Osteolytic and osteoblastic lesions or both types present in the same lesion | Breast cancer, gastrointestinal (GI) cancer and squamous cancer |
| Agent | Mechanism of Action | Side Effects |
|---|---|---|
| Bisphosponates (BPs) | Inhibition of bone demineralization by the interruption of enhanced osteolysis and tumor growth [16] | Fever, arthralgia, myalgia, anemia, nausea and peripheral edema. Osteonecrosis of the jaw (rare, difficult to establish casative evidence) [17] |
| Tetracyclines | Inhibition of matrix metalloproteinases (MMPs) involved in bone metastasis [17] | Dose-limiting toxicity e.g., fatigue and nausea, development of resistance [18] Generally well tolerated in adults |
| Denosumab | Inhibition of RANKL which prevents the development of osteoclasts | Similar to those exhibited by BPs, but reversible after treatment interruption [15] |
| Cabozantinib | Inhibition of vascular endothelial growth factor receptor-2 (VEGFR2), MET, KIT and mutationally activated RET [15] | Fatigue, diarrhea and palmar-plantar erythrodysesthesia syndrome [19] |
| Radionuclide therapy | Systemic administration of radioisotopes, but the mechanism of pain relief is uncertain [20] | Myelosuppression and pain flare [15] |
| Ablation | Use of chemical agents (ethanol, acetic acid) or local deposition of some form of energy (e.g., for radiofrequency and cryoablation) to destroy tumor cells [21] | Neurologic injuries, neuropathic pain and infection in the treatment area (especially for radiofrequency and cryoablation) [22] |
| Types | Subtypes | Frequency |
|---|---|---|
| Intramedullary | Conventional (osteoblastic/chondroblastic/fibroblastic) | 80% |
| Telangiectatic | <4% | |
| Low-grade | 1–2% | |
| Small cell | 1.5% | |
| Cortex-associated | Parosteal | 1–6% |
| Periosteal | 1–2% | |
| High-grade surface | <1% |
| Agent | Mechanism of Action | Side Effects |
|---|---|---|
| DOX | Intercalation at points of local uncoiling of the DNA double helix and inhibition of DNA/RNA synthesis | Cardiomyopathy, emesis, alopecia, mucositis, myelosuppression |
| Cisplatin | Formation of DNA cross-links with inhibition of the synthesis of the tumor DNA and denaturation of DNA double helix | Acute/chronic renal failure, ototoxicity, emesis, myelosuppression, alopecia, hypomagnesemia |
| Ifosfamide, with mesna | Cross-linking of DNA strands with inhibition of DNA/protein synthesis | Hemorrhagic cystitis, renal failure, myelosuppression, alopecia, emesis, encephalopathy |
| High-dose methotrexate (MTX), with leucovorin calcium rescue | Inhibition of purine/thymidylic acid synthesis by binding dihydrofolate reductase | Renal failure, mucositis, myelosuppression, nervous system effects (rare) |
| Drug | Advantages | Disadvantages |
|---|---|---|
| N-BPs | Bone selectivity, inexpensive, longtime use, apoptosis of osteoclasts inductors (but don’t eliminate them) | Effects on renal functions, atypical femoral fractures (osteonecrosis of the jaw), GI adverse effect, acute phase reactions |
| Denosumab | No effect on renal function, low frequency of administration, no bone accumulation, reversible effects | No bone selectivity, atypical femoral fractures, increased risk of infections, parenteral administration, reduction in bone formation, rebound bone resorption |
| Class | Drug | Characteristics |
|---|---|---|
| Cathepsin K inhibitors | Odanacatib | Ability to decrease the rate of bone resorption, preserving other osteoclasts functions Orally active, weekly administered Phase III fracture clinical trial |
| SERMs | Raloxifene | Ability to decrease the risk of vertebral fractures by 30% First FDA approved SERM |
| Bazedoxifene in association with TSECs | Treatment of moderate/severe hot flashes associated with menopause Approved by FDA in 2013 and in the European Union in 2009 (to be used alone) | |
| Lasofoxifene | Double event rate for venous thromboembolic events |
| Drug | Origin | Characteristics | Disadvantages |
|---|---|---|---|
| Romosozumab | Humanized mouse monoclonal antibody | Increment of lumbar spine and hip bone mass density during the first year of treatment Phase II studies | Benign safety profile need to be confirmed Probable development of antibodies against them Probable risk of vascular calcification Parenteral administration |
| Blosozumab | Humanized mouse monoclonal antibody | Under investigation |
| Material | Type of material | Advantages | Disadvantages |
|---|---|---|---|
| Bone | Autologous bone | Biocompatible with high bone fusion rate | Limited sources |
| Allogenic bone | Relatively high bone fusion rate | Immune rejection | |
| Heterologous bone | Wide variety of sources | Severe immune rejection and poor bone formation | |
| Bone cement | Non-bioactive bone cement | Easy fit, good hardening properties | Poor biocompatibility, non-osteoinductive, non-osteoconductive |
| Bioactive bone cement | High strength, stability and bone induction activity | Insufficient mechanical properties, expensive | |
| Metal | Stainless steel | Easy processing, inexpensive | High stiffness, poor biocompatibility |
| Titanium alloy | Biocompatible, corrosion resistance | Poor wear resistance | |
| Cobalt chromium alloy | Biocompatible, high corrosion resistance | Low ductility | |
| Ceramic | Aluminium oxide | Inertness, high corrosion resistance | Possible local stress |
| Apatite-wollas-tonite glass ceramic | Good biological activity | Brittleness, poor flexibility | |
| Polymer | Poly(lactic-co-glycolic) acid (PLGA) | Biocompatible, degradable | Possible disruption |
| PMMA | Corrosion resistance, easy fit | Poor biocompatible | |
| Chitosan | High degradable and biocompatible, porous structure, good mechanical properties | Non-osteoinductive, inadequate bone formation ability, low solubility | |
| Alginate | Easy to manipulate, non-toxic, biodegradable, less expensive | Low mechanical stability |
| System | Applications | Advantages/Disadvantages | References |
|---|---|---|---|
| Beads | OS | Carrier of low molecular weight drugs Best results with alginate/chitosan association Time-dependent, but burst and inconsistent release profile Non-biodegradable, need to be surgical removed | [44,45,120] |
| Cements | Bone regeneration Bone metastases Osteoporosis Osteomyelitis OS | Ability to harden in vivo and to form bond with bone, low temperature setting reaction, excellent bioactivity, osteoconductivity Low strength, lack of full injectability, slow resorption rate, limited amount of incorporated drug, heterogeneity of drug repartition | [50,51,83,94,95,100,101,102,103,104,105,121,122,123,124,125,161,162] |
| Ceramics | Osteomyelitis | Simple preparation, modifiable size and structure, desirable stability under physiological conditions, low toxicity, good biocompatibility High cost, low encapsulation, uncontrollable dose | [2,52,53,133,137] |
| Conjugates | Bone metastases Osteoporosis | Good aqueous solubility, stability and controlled delivery Better oral bioavailability, tumor targeting, reduced toxicity for lipid-drug conjugates | [48,49,77,79,80] |
| Dendrimers | Bone metastases | Drug/oligonucleotides easily encapsulated Monodisperse size, good water solubility Modifiable surface Markable cytotoxicity for cationic surface end groups | [47,73,75] |
| EVs | OS | Cargo of cellular content, drugs and biomolecules | [38,39,84,85,86] |
| Gel | Osteoporosis | Controlled and continuous drug release High therapeutic effects, capable of auto-forming in situ | [58,116] |
| Hydrogels | Bone regeneration | Sol-gel transitions due to body temperature or pH variations Possibility to be tailored for a better geometry, degradation rate and release profile Development of a hydrophilic environment suitable for new bone growth, fast setting time Suitable for dental and orthopedic applications | [142,148,149,150,151,152,153,154,155,156] |
| Implants | Osteoporosis | Biocompatibility, prolonged drug release, tailored biodegradation kinetics Suitable for cardiovascular, neuroprosthetic and orthopedic purpose Required surgical procedures with some side effects like bleeding, infections or nerve injuries (for dental implants) High production costs and the possible presence of degradation fragments which impair new grown bone | [62,106,107,108,119] |
| Liposomes | Bone metastases OS | Good biocompatibility and loading properties Possible prolonged/targeted drug delivery | [40,71,72,90,91] |
| Matrices | Osteoporosis | Improved patient compliance, treatment efficiency, reduced costs, possibility to reduce drug administrated Probability of dose dumping, poor in vitro/in vivo correlations | [59,118] |
| Nanocapsules | Osteoporosis | Protective coating which delays compounds release, high reproducibility, broad range of application | [60,115] |
| Nanocrystals | Bone regeneration | Suitable for intravenously injection, slowly blood dissolution, improved biodistribution Opsonization could prolong circulating time, promote tumor accumulation, improve targeting efficiency | [55,163] |
| Nanodiamonds | Osteoporosis | Large surface area, easiness surface functionalization, suitable as photoluminescent probes Negligible cytotoxicity, high biocompatibility | [54,114] |
| Nanohybrids | OS | Capable to incapsulate drug with low water solubility, possible tool for non-invasive tumor targeting Good biocompatibility and biodegradability | [56,57,97] |
| Nanoplatforms | Bone metastases Osteomyelitis | Biocompatible and biodegradable Tunable size, changeable membrane permeability, possible bound with targeting ligand, capable to load hydrophilic/lipophilic drugs Responsive to pH, ionic strength, temperature | [30,31,64,81,82,143] |
| NPs | Bone regeneration Bone metastases Osteoporosis Osteomyelitis OS | High soluble, bioavailable Possible surface coating or functionalization to improve stability and bone targeting | [32,43,46,65,66,67,68,69,70,87,88,89,110,111,126,127,128,129,130,131,132,157,158] |
| Scaffolds | Bone regeneration Osteomyelitis | Biocompatible templates, promote attachment and growth of bone cells, induce osteogenesis Possible drug carriers | [61,63,96,138,139,140,141,144,145,146,147,164] |
| Spheres and microspheres | Bone regeneration Osteoporosis Osteomyelitis OS | Reduced side effects and improved efficiency of cytotoxic anticancer drugs Alternative routes: ophthalmic, topical, nasal, intramuscular Good choice for low/high molecular weight drugs, DNA fragments, peptides, proteins | [41,42,92,93,112,117,159,160] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials 2020, 10, 875. https://doi.org/10.3390/nano10050875
Chindamo G, Sapino S, Peira E, Chirio D, Gonzalez MC, Gallarate M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials. 2020; 10(5):875. https://doi.org/10.3390/nano10050875
Chicago/Turabian StyleChindamo, Giulia, Simona Sapino, Elena Peira, Daniela Chirio, Mónica Cristina Gonzalez, and Marina Gallarate. 2020. "Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics" Nanomaterials 10, no. 5: 875. https://doi.org/10.3390/nano10050875
APA StyleChindamo, G., Sapino, S., Peira, E., Chirio, D., Gonzalez, M. C., & Gallarate, M. (2020). Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials, 10(5), 875. https://doi.org/10.3390/nano10050875








