Hydrothermal Synthesis and Gas Sensing of Monoclinic MoO3 Nanosheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Hydrothermal Reactions
2.3. Characterization
2.4. Gas Sensing Tests
3. Results and Discussion
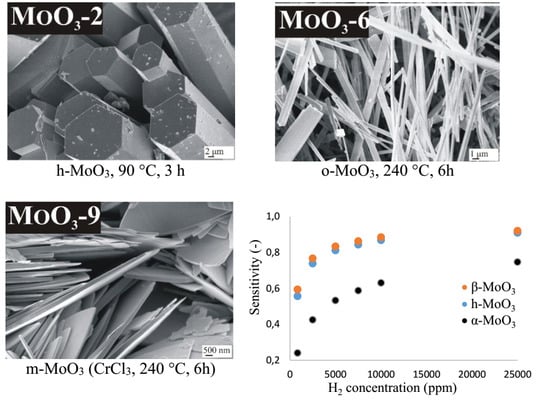
3.1. Role of Reaction Temperature and Duration on the Formation of MoO3
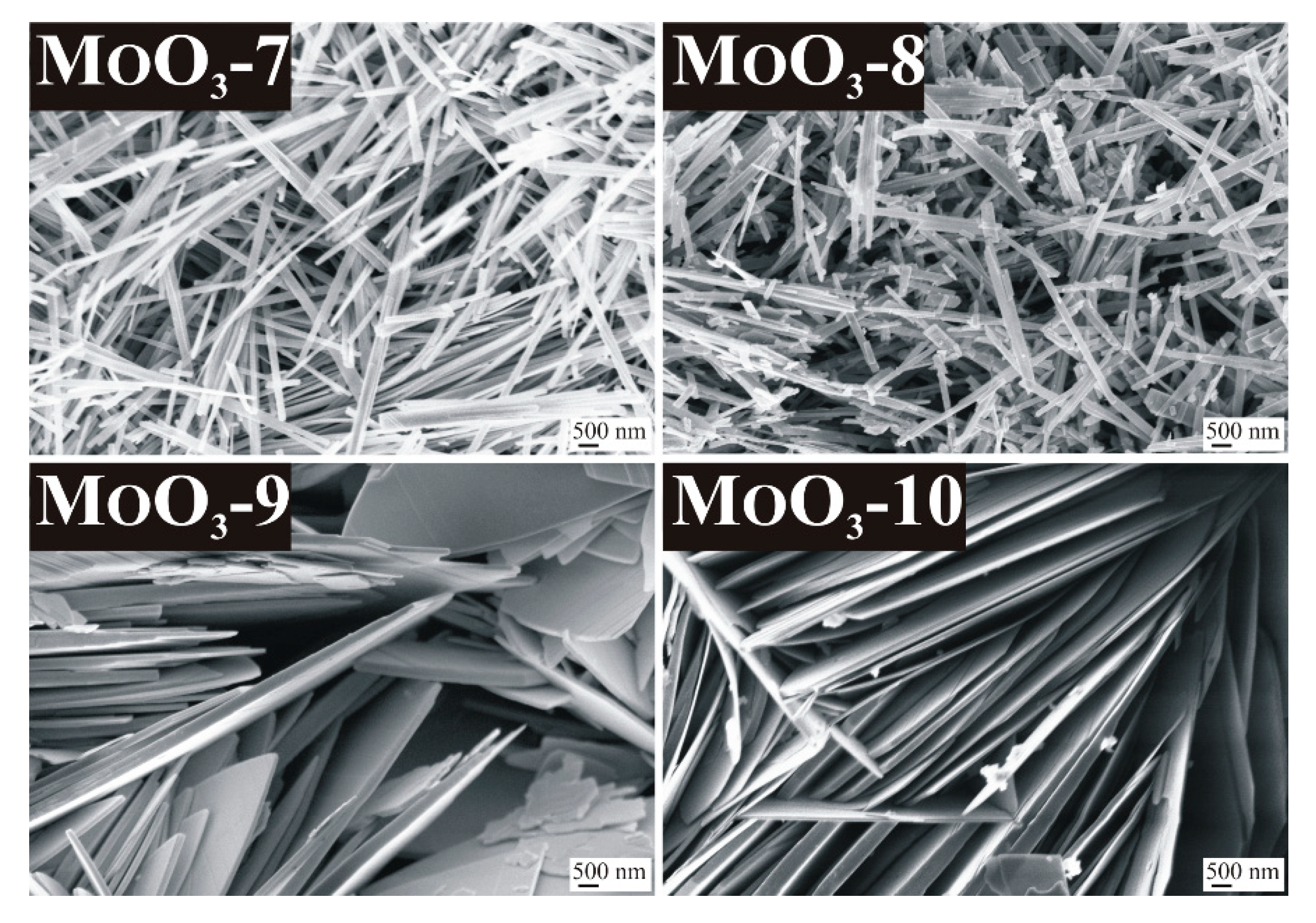
3.2. Role of Additives on the Formation of MoO3
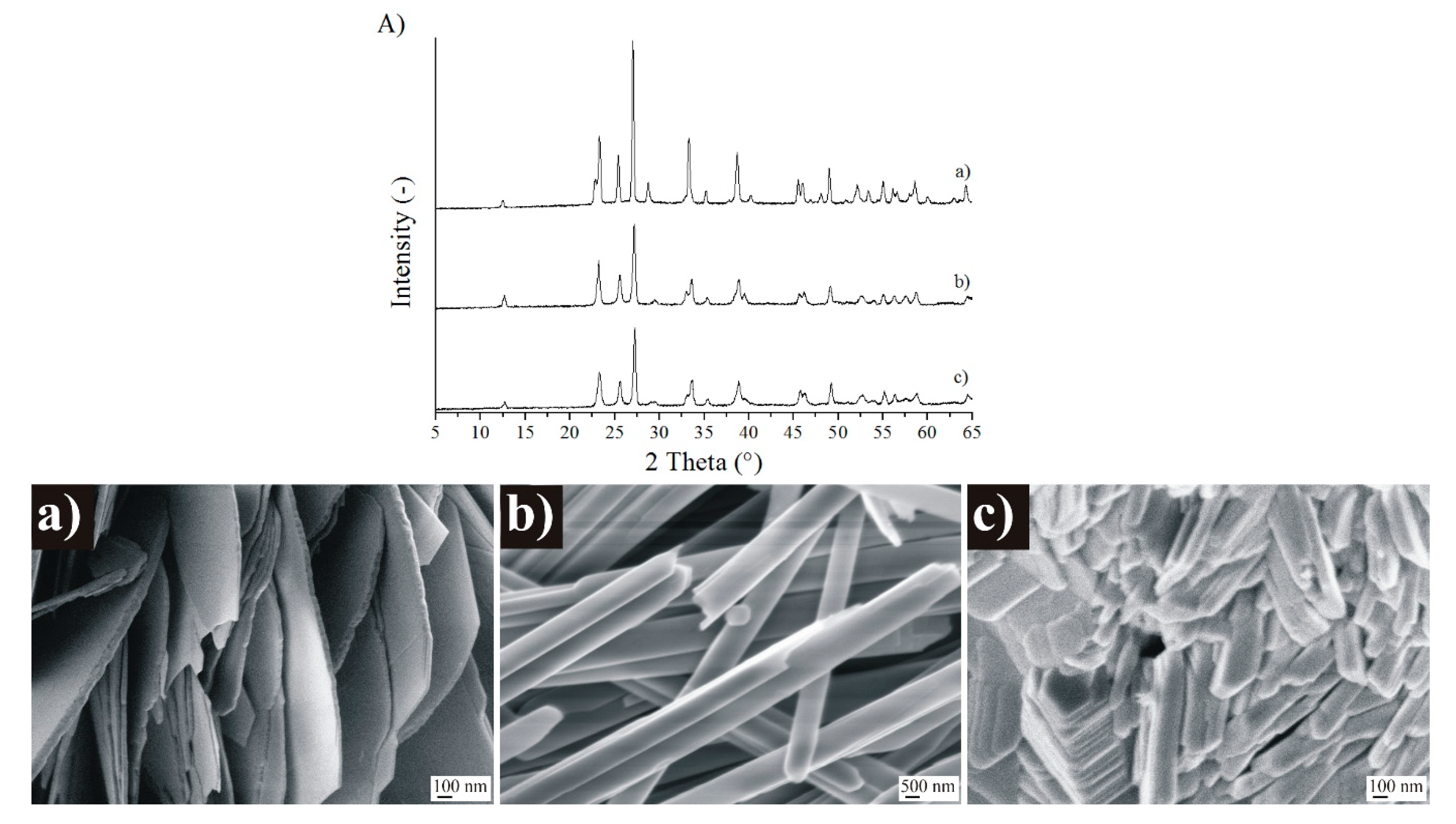
3.3. Further Investigation of the Different MoO3 Phases
3.4. Gas Sensing
3.4.1. Checking Crystalline Phases and Morphology at 500 °C
3.4.2. Gas Sensing Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krobkrong, N.; Itthibenchapong, V.; Khongpracha, P.; Faungnawakij, K. Deoxygenation of oleic acid under an inert atmosphere using molybdenum oxide-based catalysts. Energy Convers. Manag. 2018, 167, 1–8. [Google Scholar] [CrossRef]
- Alam, U.; Kumar, S.; Bahnemann, D.; Koch, J.; Tegenkamp, C.; Muneer, M. Harvesting visible light with MoO3 nanorods modified by Fe(iii) nanoclusters for effective photocatalytic degradation of organic pollutants. Phys. Chem. Chem. Phys. 2018, 20, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- Yogananda, H.S.; Nagabhushana, H.; Darshan, G.P.; Basavaraj, R.B. MoO3 nanostructures from EGCG assisted sonochemical route: Evaluation of its application towards forensic and photocatalysis. J. Alloys Compd. 2018, 745, 874–891. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wu, X.; He, G.; Xu, R.; Lu, X.; Wang, F.R.; Parkin, I.P. Phase and morphological control of MoO3−x nanostructure for efficient cancer theragnosis therapy. Nanoscale 2017, 9, 11012–11016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Zhao, J.; Zhao, Y.; Huang, Z.; Li, Y.; Wu, G. Synthesis of α-MoO3 nanobelts with preferred orientation and good photochromic performance. CrystEngComm 2016, 18, 6502–6512. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Cheed-im, U.; Thongtem, T.; Thongtem, S. Influence of Gd dopant on photocatalytic properties of MoO3 nanobelts. Mater. Lett. 2016, 173, 158–161. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Sanjini, N.S.; Velmathi, S.; Bose, A.C. Preparation of h-MoO3 and a-MoO3 nanocrystals: Comparative study on photocatalytic degradation of methylene blue under visible light irradiation. Phys. Chem. Chem. Phys. 2013, 15, 14761–14769. [Google Scholar] [CrossRef]
- Illyaskutty, N.; Kohler, H.; Trautmann, T.; Schwotzer, M.; Mahadevan Pillai, V.P. Hydrogen and ethanol sensing properties of molybdenum oxide nanorods based thin films: Effect of electrode metallization and humid ambience. Sens. Actuators B Chem. 2013, 187, 611–621. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Lu, Y.; Podval’naya, N.V.; Chen, W.; Zakharova, G.S. Hydrothermal synthesis of h-MoO3 microrods and their gas sensing properties to ethanol. Appl. Surf. Sci. 2015, 359, 114–119. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, L.; Zhang, Y.; Cao, X. Hydrothermal synthesis of hexagonal MoO3 and its reversible electrochemical behavior as a cathode for Li-ion batteries. Electron. Mater. Lett. 2013, 9, 693–696. [Google Scholar] [CrossRef]
- Han, B.; Lee, K.; Lee, Y.; Kim, S.; Park, H.; Hwang, B.; Kwak, D.; Park, K. MoO3 nanostructured electrodes prepared via hydrothermal process for lithium ion batteries. Int. J. Electrochem. Sci. 2015, 10, 4232–4240. [Google Scholar]
- Dhanasankar, M.; Purushothaman, K.K.; Muralidharan, G. Optical, structural and electrochromic studies of molybdenum oxide thin films with nanorod structure. Solid State Sci. 2010, 12, 246–251. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Rajeswari Yogamalar, N.; Bose, A.C. Hydrothermally synthesized h-MoO3 and α-MoO3 nanocrystals: New findings on crystal-structure-dependent charge transport. Cryst. Growth Des. 2016, 16, 1984–1995. [Google Scholar] [CrossRef]
- Kumar, V.V.; Gayathri, K.; Anthony, S.P. Synthesis of α-MoO3 nanoplates using organic aliphatic acids and investigation of sunlight enhanced photodegradation of organic dyes. Mater. Res. Bull. 2016, 76, 147–154. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenum oxides—From fundamentals to functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Madarász, J.; Pokol, G.; Király, P.; Tárkányi, G.; Saukko, S.; Mizsei, J.; Tóth, A.L.; Szabó, A.; Varga-Josepovits, K. Stability and controlled composition of hexagonal WO3. Chem. Mater. 2008, 20, 4116–4125. [Google Scholar] [CrossRef]
- Badr, A.M.; El-Anssary, E.H.; Elshaikh, H.A.; Afify, H.H. Hydrothermal synthesis and influence of later heat treatment on the structural evolution, optical and electrical properties of nanostructured α-MoO3 single crystals. J. Phys. D Appl. Phys. 2017, 50, 505111. [Google Scholar] [CrossRef]
- Bouzidi, A.; Benramdane, N.; Tabet-derraz, H.; Mathieu, C.; Khelifa, B.; Desfeux, R. Effect of substrate temperature on the structural and optical properties of MoO3 thin films prepared by spray pyrolysis technique. Mater. Sci. Eng. B 2003, 97, 5–8. [Google Scholar] [CrossRef]
- Bai, S.; Chen, S.; Chen, L.; Zhang, K.; Luo, R.; Li, D.; Liu, C.C. Ultrasonic synthesis of MoO3 nanorods and their gas sensing properties. Sens. Actuators B Chem. 2012, 174, 51–58. [Google Scholar] [CrossRef]
- Yan, H.; Song, P.; Zhang, S.; Zhang, J.; Yang, Z.; Wang, Q. Au nanoparticles modified MoO3 nanosheets with their enhanced properties for gas sensing. Sens. Actuators B Chem. 2016, 236, 201–207. [Google Scholar] [CrossRef]
- Chang, W.C.; Qi, X.; Kuo, J.C.; Lee, S.C.; Ng, S.K.; Chen, D. Post-deposition annealing control of phase and texture for the sputtered MoO3 films. CrystEngComm 2011, 13, 5125–5132. [Google Scholar] [CrossRef]
- Haro-Poniatowski, E.; Jouanne, M.; Morhange, J.F.; Julien, C.; Diamant, R.; Fernández-Guasti, M.; Fuentes, G.A.; Alonso, J.C. Micro-Raman characterization of WO3 and MoO3 thin films obtained by pulsed laser irradiation. Appl. Surf. Sci. 1998, 127–129, 674–678. [Google Scholar] [CrossRef]
- Maiti, P.; Guha, P.; Singh, R.; Dash, J.K.; Satyam, P.V. Optical band gap, local work function and field emission properties of MBE grown B-MoO3 nanoribbons. Appl. Surf. Sci. 2019, 476, 691–700. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.-H.; Sun, Y.-J.; Zhou, X.-W.; Chou, K.-C. Preparation of ultrafine β-MoO3 from industrial grade MoO3 powder by the method of sublimation. J. Phys. Chem. C 2016, 120, 19821–19829. [Google Scholar] [CrossRef]
- Mizushima, T.; Moriya, Y.; Phuc, N.H.H.; Ohkita, H.; Kakuta, N. Soft chemical transformation of α-MoO3 to β-MoO3 as a catalyst for vapor-phase oxidation of methanol. Catal. Commun. 2011, 13, 10–13. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Nguyen, P.H.D.; Vo, T.T.; Nguyen, H.H.P.; Luu, C.L. Facile method for synthesis of nanosized β–MoO3 and their catalytic behavior for selective oxidation of methanol to formaldehyde. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045010. [Google Scholar] [CrossRef]
- Alsaif, M.M.Y.A.; Balendhran, S.; Field, M.R.; Latham, K.; Wlodarski, W.; Ou, J.Z.; Kalantar-Zadeh, K. Two dimensional α-MoO3 nanoflakes obtained using solvent-assisted grinding and sonication method: Application for H2 gas sensing. Sens. Actuators B Chem. 2014, 192, 196–204. [Google Scholar] [CrossRef]
- Arachchige, H.M.M.M.; Comini, E.; Zappa, D.; Sberveglieri, G. Gas Sensing Properties of MoO3. Proceedings 2017, 1, 449. [Google Scholar] [CrossRef] [Green Version]
- Mane, A.A.; Moholkar, A.V. Orthorhombic MoO3 nanobelts based NO2 gas sensor. Appl. Surf. Sci. 2017, 405, 427–440. [Google Scholar] [CrossRef]
- Martínez, H.M.; Torres, J.; Rodríguez-García, M.E.; López Carreño, L.D. Gas sensing properties of nanostructured MoO3 thin films prepared by spray pyrolysis. Phys. B Condens. Matter 2012, 407, 3199–3202. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Bakos, L.P.; Nóra, J.; Moura da Silva Bezerra da Costa, U.C.; László, K.; Lábár, J.; Igricz, T.; Varga-Josepovits, K.; Pasierb, P.; Färm, E.; et al. Photocatalytic and gas sensitive multiwalled carbon prepared by atomic layer deposition. Nanomaterials 2020, 10, 252. [Google Scholar]
- Liu, Y.; Feng, P.; Wang, Z.; Jiao, X.; Akhtar, F. Novel fabrication and enhanced photocatalytic MB degradation of hierarchical porous monoliths of MoO3 nanoplates. Sci. Rep. 2017, 7, 1845. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Bose, A.C. Hydrothermal synthesis of hexagonal and orthorhombic MoO3 nanoparticles. J. Alloys Compd. 2011, 509, 8105–8110. [Google Scholar] [CrossRef]
- Sinaim, H.; Jin, D.; Sung, J.; Phuruangrat, A.; Thongtem, S. Free-polymer controlling morphology of L-MoO3 nanobelts by a facile hydrothermal synthesis, their electrochemistry for hydrogen evolution reactions and optical properties. J. Alloys Compd. 2012, 516, 172–178. [Google Scholar] [CrossRef]
- Gao, B.; Fan, H.; Zhang, X. Hydrothermal synthesis of single crystal MoO3 nanobelts and their electrochemical properties as cathode electrode materials for rechargeable lithium batteries. J. Phys. Chem. Solids 2012, 73, 423–429. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Anandhababu, G.; Mahalingam, T.; Ravi, G. Photoelectrochemical study of MoO3 assorted morphology films formed by thermal evaporation. J. Energy Chem. 2016, 25, 798–804. [Google Scholar] [CrossRef]
- Hui, B.; Li, G.; Zhao, X.; Wang, L.; Wu, D.; Li, J.; Via, B.K. h-MoO3 microrods grown on wood substrates through a low-temperature hydrothermal route and their optical properties. J. Mater. Sci. Mater. Electron. 2017, 28, 3264–3271. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Saukko, S.; Mizsei, J.; Tóth, A.L.; Madarász, J.; Pokol, G. Gas sensing selectivity of hexagonal and monoclinic WO3 to H2S. Solid State Sci. 2010, 12, 1857–1860. [Google Scholar] [CrossRef]
- Chaudhari, G.N.; Bende, A.M.; Bodade, A.B.; Patil, S.S.; Sapkal, V.S. Structural and gas sensing properties of nanocrystalline TiO2:WO3-based hydrogen sensors. Sens. Actuators B Chem. 2006, 115, 297–302. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, Á.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Varga-Josepovits, K.; László, K.; et al. WO3 photocatalysts: Influence of structure and composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef] [Green Version]






| Sample | Additive | T (°C) | t (h) |
|---|---|---|---|
| MoO3-1 | - | 90 | 3 |
| MoO3-2 | - | 90 | 6 |
| MoO3-3 | - | 210 | 3 |
| MoO3-4 | - | 210 | 6 |
| MoO3-5 | - | 240 | 3 |
| MoO3-6 | - | 240 | 6 |
| MoO3-7 | CTAB | 240 | 3 |
| MoO3-8 | CTAB | 240 | 6 |
| MoO3-9 | CrCl3 | 240 | 3 |
| MoO3-10 | CrCl3 | 240 | 6 |
| Sample | SBET (m2/g) | EDX (Atom %) | |
|---|---|---|---|
| Mo (Average) | O (Average) | ||
| MoO3-1, hexagonal | 0.21 | 24.3 | 75.7 |
| MoO3-8, orthorhombic | 9.7 | 24.8 | 75.2 |
| MoO3-10, monoclinic | 2.9 | 25.7 | 74.3 |
| Sample | Response Time (min) | Recovery Time (min) |
|---|---|---|
| MoO3-10, monoclinic | 1.2 | 22.5 |
| MoO3-8, orthorhombic | 1.2 | 10.1 |
| MoO3-1, hexagonal | 1.2 | 20.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagyné-Kovács, T.; Studnicka, L.; Lukács, I.E.; László, K.; Pasierb, P.; Szilágyi, I.M.; Pokol, G. Hydrothermal Synthesis and Gas Sensing of Monoclinic MoO3 Nanosheets. Nanomaterials 2020, 10, 891. https://doi.org/10.3390/nano10050891
Nagyné-Kovács T, Studnicka L, Lukács IE, László K, Pasierb P, Szilágyi IM, Pokol G. Hydrothermal Synthesis and Gas Sensing of Monoclinic MoO3 Nanosheets. Nanomaterials. 2020; 10(5):891. https://doi.org/10.3390/nano10050891
Chicago/Turabian StyleNagyné-Kovács, Teodóra, Levente Studnicka, István Endre Lukács, Krisztina László, Pawel Pasierb, Imre Miklós Szilágyi, and György Pokol. 2020. "Hydrothermal Synthesis and Gas Sensing of Monoclinic MoO3 Nanosheets" Nanomaterials 10, no. 5: 891. https://doi.org/10.3390/nano10050891