Enhanced Photocatalytic Degradation of the Imidazolinone Herbicide Imazapyr upon UV/Vis Irradiation in the Presence of CaxMnOy-TiO2 Hetero-Nanostructures: Degradation Pathways and Reaction Intermediates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the Photocatalyst
2.3. Catalyst Characterization
2.4. Photocatalytic Degradation of Pollutants
2.5. Radical Scavenging Investigation
2.6. Analytical Methods
2.6.1. High Performance Liquid Chromatography (HPLC)
2.6.2. Mass Spectrometry-Electrospray Ionisation (MS-ESI)
2.6.3. Total Organic Carbon (TOC) Analysis
3. Results and Discussion
3.1. Characterization of Materials
3.2. Photocatalytic Activity of CaxMnOy-TiO2
3.2.1. Detection of ROS under UV–Vis and Vis Irradiation
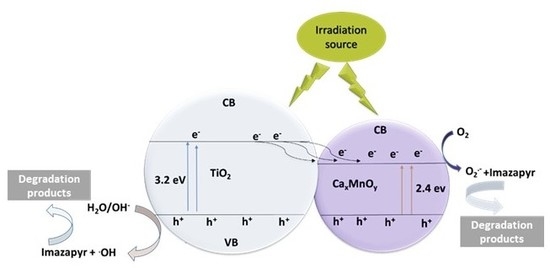
3.2.2. Investigation of the Reaction Products and Reaction Mechanism
3.2.3. Recyclability of CaxMnOy-TiO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fu, H.; Zhang, S.; Xu, T.; Zhu, Y.; Chen, J. Photocatalytic degradation of RhB by fluorinated Bi2WO6 and distributions of the intermediate products. Environ. Sci. Technol. 2008, 42, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- di Corcia, A.; Crescenzi, C.; Guerriero, E.; Samperi, R. Ultratrace determination of atrazine and its six major degradation products in water by solid-phase extraction and liquid chromatography-electrospray/mass spectrometry. Environ. Sci. Technol. 1997, 31, 1658–1663. [Google Scholar] [CrossRef]
- Pizarro, P.; Guillard, C.; Perol, N.; Herrmann, J.M. Photocatalytic degradation of Imazapyr in water: Comparison of activities of different supported and unsupported TiO2-based catalysts. Catal. Today 2005, 101, 211–218. [Google Scholar] [CrossRef]
- Martínez, C.; Vilariño, S.; Fernández, M.I.; Faria, J.; Canle, M.L.; Santaballa, J.A. Mechanism of degradation of ketoprofen by heterogeneous photocatalysis in aqueous solution. Appl. Catal. B Environ. 2013, 142–143, 633–646. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navio, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Zhou, Y.; Zhang, Z.; He, M. Facile photochemical synthesis of Au/Pt/g-C3N4 with plasmon-enhanced photocatalytic activity for antibiotic degradation. ACS Appl. Mater. Interfaces 2015, 7, 9630–9637. [Google Scholar] [CrossRef]
- Quivet, E.; Faure, R.; Georges, J.; Païssé, J.O.; Herbreteau, B. Kinetic studies of Imazapyr photolysis and characterization of the main photoproducts. Toxicol. Environ. Chem. 2004, 86, 195–204. [Google Scholar] [CrossRef]
- López, M.C.; Fernández, M.I.; Martínez, C.; Santaballa, J.A. (Re)Greening photochemistry: Using light for degrading persistent organic pollutants. Rev. Environ. Sci. Biotechnol. 2012, 11, 213–221. [Google Scholar]
- López, M.C.; Fernández, M.I.; Martínez, C.; Santaballa, J.A. Photochemistry for pollution abatement. Pure Appl. Chem. 2013, 85, 1437–1449. [Google Scholar] [CrossRef]
- Romeiro, A.; Freitas, D.; Azenha, M.E.; Canle, M.; Burrows, H.D. Effect of the calcination temperature on the photocatalytic efficiency of acidic sol–gel synthesized TiO2 nanoparticles in the degradation of alprazolam. Photochem. Photobiol. Sci. 2017, 16, 935–945. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. B 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Konstantinou, I.; Sakkas, V.; Albanis, T. Photocatalytic degradation of the herbicides propanil and molinate over aqueous Ti O2 suspensions: Identification of intermediates and the reaction pathway. Appl. Catal. B 2001, 34, 227–239. [Google Scholar] [CrossRef]
- Frey, C.E.; Wiechen, M.; Kurz, P. Water-oxidation catalysis by synthetic manganese oxides—Systematic variations of the calcium birnessite theme. Dalton Trans. 2014, 43, 4370–4379. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.C.T.; Ângelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, A. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B Environ. 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhu, L.; Liu, G.; Yu, H.; Tang, H. Enhanced photocatalytic degradation and selective removal of nitrophenols by using surface molecular imprinted Titania. Environ. Sci. Technol. 2008, 42, 1687–1692. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.J.; Pablos, C.; Díaz-Jiménez, L.; Cortés-Hernández, D.A.; Sharma, P.K.; Castro-Alférez, M.; Fernández-Ibañez, P.; Dunlop, P.S.M.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Miró, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef]
- Sahu, S.; Rout, G.C. Band gap opening in graphene: A short theoretical study. Int. Nano Lett. 2017, 7, 81–89. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Wei, Y.; Vayssieres, L. Stability and performance of sulfide-, nitride-, and phosphide-based electrodes for photocatalytic solar water splitting. Cite J. Phys. Chem. Lett. 2017, 8, 5228–5238. [Google Scholar] [CrossRef]
- Pomilla, F.R.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Molinari, R.; Barbieri, G.; Marcì, G.; Palmisano, L.; Sharma, P.K.; Brown, A.; Byrne, J.A. An investigation into the stability of graphitic c3n4 as a photocatalyst for co2 reduction. J. Phys. Chem. C 2018, 122, 28727–28738. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Chen, Z.; Abram, D.N.; Jaramillo, T.F. Thin films of sodium birnessite-type MnO2: Optical properties, electronic band structure, and solar photoelectrochemistry. J. Phys. Chem. C 2011, 115, 11830–11838. [Google Scholar] [CrossRef]
- Kalathil, S.; Khan, M.M.; Ansari, S.A.; Lee, J.; Cho, M.H. Band gap narrowing of titanium dioxide (TiO2) nanocrystals by electrochemically active biofilms and their visible light activity. Nanoscale 2013, 5, 6323. [Google Scholar] [CrossRef]
- Bougarrani, S.; Skadell, K.; Arndt, R.; el Azzouzi, M.; Gläser, R. Novel CaxMnOy/TiO2 composites for efficient photocatalytic degradation of methylene blue and the herbicide imazapyr in aqueous solution under visible light irradiation. J. Environ. Chem. Eng. 2018, 6, 1934–1942. [Google Scholar] [CrossRef]
- Paudel, P.; Negusse, A.; Jaisi, D.P. Birnessite-catalyzed degradation of glyphosate: A mechanistic study aided by kinetics batch studies and NMR spectroscopy. Soil Sci. Soc. Am. J. 2015, 79, 815–831. [Google Scholar] [CrossRef]
- Devi, L.G.; Kottam, N.; Kumar, S.G. Preparation and characterization of Mn-doped titanates with a bicrystalline framework: Correlation of the crystallite size with the synergistic effect on the photocatalytic activity. J. Phys. Chem. C 2009, 113, 15593–15601. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes—Current status and prospect. Proc. Est. Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar]
- Lucht, K.P.; Mendoza-Cortes, J.L. Birnessite: A layered manganese oxide to capture sunlight for water-splitting catalysis. J. Phys. Chem. C 2015, 119, 22838–22846. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, P.S.M.; Galdi, A.; McMurray, T.A.; Hamilton, J.W.J.; Rizzo, L.; Byrne, J.A. Comparison of photocatalytic activities of commercial titanium dioxide powders immobilised on glass substratMuaes. J. Adv. Oxid. Technol. 2010, 13, 99–106. [Google Scholar]
- Xu, X.; Zhou, X.; Li, X.; Yang, F.; Jin, B.; Xu, T.; Li, G.; Li, M. Electrodeposition synthesis of MnO2/TiO2 nanotube arrays nanocomposites and their visible light photocatalytic activity. Mater. Res. Bull. 2014, 59, 32–36. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM\n. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Li, B.; Gao, G.; Zhai, D.; Wei, C.; He, Y.; Du, H.; Kang, F. Synthesis, characterization and electrochemical performance of manganese dioxide in a quaternary microemulsion: The role of the co-surfactant and water. Int. J. Electrochem. Sci. 2013, 8, 8740–8751. [Google Scholar]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2017, 6, 3531–3555. [Google Scholar] [CrossRef]
- Lee, S.Y.; Gonzlez-Flores, D.; Ohms, J.; Trost, T.; Dau, H.; Zaharieva, I.; Kurz, P. Screen-printed calcium–birnessite electrodes for water oxidation at neutral pH and an “Electrochemical Harriman Series”. ChemSusChem 2014, 7, 3442–3451. [Google Scholar] [CrossRef]
- Chukhrov, F.V.; Sakharov, B.A.; Gorshkov, A.I.; Drits, V.A.; Dikov, Y.P. Crystal structure of birnessite from the pacific ocean. Int. Geol. Rev. 1985, 27, 1082–1088. [Google Scholar] [CrossRef]
- Jin, Q.; Arimoto, H.; Fujishima, M.; Tada, H. Manganese oxide-surface modified titanium(IV) dioxide as environmental catalyst. Catalysts 2013, 3, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-K.; Chen, Y.-C.; Lin, Y.-G.; Chen, L.-C.; Chen, K.-H. Birnessite-type manganese oxides nanosheets with hole acceptor assisted photoelectrochemical activity in response to visible light. J. Mater. Chem. 2012, 22, 2733–2739. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic degradation of diclofenac using TiO2–SnO2 mixed oxide catalysts. Environ. Technol. 2017, 9, 1–26. [Google Scholar] [CrossRef]
- Liao, D.L.; Badour, C.A.; Liao, B.Q. Preparation of nanosized TiO2/ZnO composite catalyst and its photocatalytic activity for degradation of methyl orange. J. Photochem. Photobiol. A Chem. 2008, 194, 11–19. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Fan, H.; Jiang, T.; Li, X.; Xie, T. Synthesis of ordered multivalent Mn-TiO2 nanospheres with tunable size: A high performance visible-light photocatalyst. Nano Res. 2011, 4, 460–469. [Google Scholar] [CrossRef]
- Atitar, M.F.; Dillert, R.; Bahnemann, D.W. Surface interactions between Imazapyr and the TiO2 surface: An in situ ATR-FTIR study. J. Phys. Chem. C 2017, 121, 4293–4303. [Google Scholar] [CrossRef]
- Romeiro, A.; Azenha, M.E.; Canle, M.; Rodrigues, V.H.N.; da Silva, J.P.; Burrows, H.D. Titanium dioxide nanoparticle photocatalysed degradation of ibuprofen and naproxen in water: Competing hydroxyl radical attack and oxidative decarboxylation by semiconductor holes. Chem Select 2018, 3, 10915–10924. [Google Scholar] [CrossRef]
- Carrier, M.; Perol, N.; Herrmann, J.M.; Bordes, C.; Horikoshi, S.; Paisse, J.O.; Baudot, R.; Guillard, C. Kinetics and reactional pathway of Imazapyr photocatalytic degradation Influence of pH and metallic ions. Appl. Catal. B Environ. 2006, 65, 11–20. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Sayilgan, E.; Kukrer, T.; Yigit, N.O.; Civelekoglu, G.; Kitis, M. Acidic leaching and precipitation of zinc and manganese from spent battery powders using various reductants. J. Hazard. Mater. 2010, 173, 137–143. [Google Scholar] [CrossRef]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]




















| Catalyst | Zeta Potential (mV) | pH | Isoelectric Point |
|---|---|---|---|
| TiO2 | 26.3 ± 0.4 | 4.14 | 6.1 ± 0.2 |
| CaxMnOy-TiO2 | 37.7 ± 0.5 | 4.09 | 6.7 ± 0.2 |
| Product Number | Formula | Molar Mass | MS-ESI | Intensity in the Presence of (TiO2) a.u. | Intensity in the Presence of (CaxMnOy-TiO2) a.u. | |
|---|---|---|---|---|---|---|
| Positive Mode (M+H)+ | Negative Mode (M-H)− | |||||
| 1 (Imazapyr) | C13H15N3O3 | 261.281 | 262.14 | 260.45 | 20,696 | 14,760 |
| 2 | C12H15N3O | 217.272 | 218.7 | 216.2 | 3081 | Nd. |
| 3 | C12H15N3O2 | 233.271 | 234.5 | 232.4 | 2096 | Nd. |
| 4 | C12H15N3O4 | 265.269 | 266.42 | 264.13 | 1230 | Nd. |
| 5 | C11H17N3O3 | 239.275 | 240.3 | 238 | 6051 | Nd. |
| 6 | C8H11N3O3 | 197.194 | 198.2 | 196.5 | 5690 | 9340 |
| 7 | C10H9N3O4 | 235.199 | 236.2 | 234.9 | 3120 | 948 |
| 8 | C9H7N3O3 | 205.173 | 206.18 | 204.3 | 980 | Nd. |
| 9 | C7H5NO5 | 183.119 | Nf. | 182.5 | Nd. | 586 |
| 10 | C7H5NO4 | 167.120 | Nf. | 166.7 | Nd. | Nd. |
| 11 | C6H5NO3 | 139.110 | Nf. | 138.48 | 1986 | 523 |
| 12 | C7H12N2O2 | 156.185 | Nf. | 155.2 | Nd. | 396 |
| 13 | C3H4N2O2 | 100.077 | Nf. | 99.8 | 428 | 291 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougarrani, S.; Sharma, P.K.; Hamilton, J.W.J.; Singh, A.; Canle, M.; El Azzouzi, M.; Byrne, J.A. Enhanced Photocatalytic Degradation of the Imidazolinone Herbicide Imazapyr upon UV/Vis Irradiation in the Presence of CaxMnOy-TiO2 Hetero-Nanostructures: Degradation Pathways and Reaction Intermediates. Nanomaterials 2020, 10, 896. https://doi.org/10.3390/nano10050896
Bougarrani S, Sharma PK, Hamilton JWJ, Singh A, Canle M, El Azzouzi M, Byrne JA. Enhanced Photocatalytic Degradation of the Imidazolinone Herbicide Imazapyr upon UV/Vis Irradiation in the Presence of CaxMnOy-TiO2 Hetero-Nanostructures: Degradation Pathways and Reaction Intermediates. Nanomaterials. 2020; 10(5):896. https://doi.org/10.3390/nano10050896
Chicago/Turabian StyleBougarrani, Salma, Preetam K. Sharma, Jeremy W. J. Hamilton, Anukriti Singh, Moisés Canle, Mohammed El Azzouzi, and John Anthony Byrne. 2020. "Enhanced Photocatalytic Degradation of the Imidazolinone Herbicide Imazapyr upon UV/Vis Irradiation in the Presence of CaxMnOy-TiO2 Hetero-Nanostructures: Degradation Pathways and Reaction Intermediates" Nanomaterials 10, no. 5: 896. https://doi.org/10.3390/nano10050896