1. Introduction
Increased number of skeletal system disorders, due to the increase of the human lifespan, imposes a need for a new and more efficient bone healing procedure, since conventional treatments with auto- and allografts have many drawbacks, such as infections, pain, long-term recovery, and rejection by a host immune system [
1,
2]. Tissue engineering, as an interdisciplinary approach integrating biology, medicine, and engineering, offers an alternative by developing synthetic bone substitutes, which are able to provide bone repair and regeneration, even at the large scale bone defects [
1,
3,
4].
Biocompatible porous three-dimensional (3D) scaffolds that mimic the natural bone structure have given the most promising results due to their biostimulative effect on cells proliferation and neovascularization [
1,
2,
3,
4,
5]. Besides, these scaffolds have to mimic bone in all biological aspects and disintegrate at a rate that is similar to the new bone production to achieve the most efficient healing treatment [
6,
7]. Among the wide range of biomaterials used for the synthesis of bone scaffolds [
8], hydroxyapatite [HAP, Ca
10(PO
4)
6(OH)
2] is one of the most widely utilized, since it possesses structural and chemical similarity to natural bone. It is biocompatible, bioresorbable, and bioactive, and it enables excellent cell adhesiveness [
4,
9,
10,
11].
The main drawbacks of HAP represent its lower rate of degradation in comparison to natural bone, and its mechanical strength, which mostly depends on the microstructure. To overcome it, HAP was modified due to available nanotechnology and widely combined with synthetic polymers. These composite scaffolds exhibited better mechanical properties and bioactivity, in comparison to ceramics or polymer alone [
9,
10,
12]. Bearing all that in mind, novel porous bone substitute that is based on nanostructured HAP covered with thin polymeric film of poly(lactide-co-glycolide) (PLGA) has been synthesized recently and showed to present excellent biocompatibility through many in vitro and in vivo investigations, as well as numerous superior characteristics in comparison to commercially available product [
13,
14,
15].
However, after being implanted in the tissue, the degradation process of the material in local tissue occurs, and dissolved products obtain to systematic circulation. The chronical presence of scaffolds’ products in circulation could lead to toxicity, and this aspect of nanostructured HAP has been poorly investigated so far.
Systemic subchronic toxicity studies, usually conducted in rodents by repeated administration of tested compounds for more than 90 days (defined by the international standard ISO 10993: Biological evaluation of medical devices), are necessary before clinical application of bone substitute, especially at the large scale bone defects. A combined investigation of genotoxicity in vitro and systemic chronic toxicity could provide new insights in safety evaluation and potential long-term adverse effects of investigated materials and its leachable products [
16].
The aim of this study was to provide toxicological profile based on in vitro genotoxicity and in vivo systemic subchronic toxicity. A range of endpoints was evaluated, including biochemical analyses, and histological and stereological measurements on liver, kidney, and spleen tissues, after 120 days of oral administration. This study contributes valuable understanding of the novel three-dimensional (3D) nanohydroxyapatite-PLGA scaffold (ALBO-OS) interaction on the systemic level to organism under subchronic exposure scenario.
2. Materials and Methods
2.1. Test Material
A novel composite scaffold that was based on calcium hydroxyapatite and poly(lactide-co-glycolide) (PLGA), named ALBO-OS, was used for in vivo toxicity study. The detailed procedure of material’s synthesis and characterization is explained in our previous investigations [
13,
14,
15], and a summary is given in this paper.
Synthesis and Characterization
Briefly, HAP powder was hydrothermally synthesized from an approximately stoichiometric mixture of calcium hydroxide (Ca(OH)2) and (NH4)2HPO4 (p.a. Merck KGaA, Darmstadt, Germany). The obtained hydroxyapatite powder was further used for the production of HAP granules. Further, ceramic slurry was made by mixing HAP granules and starch with water, and then poured over polyurethane foam with the required pore size distribution and dried at room temperature. Finally, it was heated in an oven at 600 °C and sintered at 1200 °C for 4 h. The obtained porous HAP compact was further disintegrated into 300 μm–1 mm granules. Finally, PLGA (Durect Corporation, Birmingham, AL, USA, 50:50, M = 45,000–70,000) thin film was deposited onto the surface of HA granules to obtain final product.
2.2. Genotoxicity Investigations In Vitro
2.2.1. Cell Exposure and Viability Evaluation
THP-1 cells were seeded in 12-well plates in concentration 15 × 10
4 cells per well. The next day cells were exposed to ALBO-OS extract. Extract contained maximal concentrations of Ca
2+ ions, which exact value, was previously exactly ordered by ICP. The concentration corresponded to concentration of released Ca
2+ ions in saturated solution obtained after immersion of 0.05, 5, 10, and 50 mg/mL ALBO OS in 10 mL distilled water with previously adjusted pH at 7.37, during 120 h. For negative control, the cells were not treated with material, while cells exposed to methyl methanesulfonate (MMS) solution (40 µM) were used as a positive control. The cells were exposed to various treatments for 1 h, after which they were centrifuged (200×
g, 5 min.) and resuspended in Dulbecco’s phosphate-buffered saline (DPBS, Sigma-Aldrich, Saint Louis, MO, USA). Prior to alkaline comet assay, Trypan blue exclusion assay [
17] was used for cell viability test. Cells’ viability evaluation was performed by microscopic observation (AXIO Vert.A1; Carl Zeiss, Jena, Germany) at 400× magnification (with minimum 200 cells/sample).
2.2.2. Alkaline Comet Assay
The comet assay (single cell gel electrophoresis assay) was used to detect possible single- strand DNA breaks, double-strand DNA breaks, alkali labile site, and incomplete excision repair sites [
18]. The microscopic slides were pre-coated with 0.5% normal-melting point (NMP) agarose. Cells were mixed with 1% low-melting point (LMP) agarose and then applied onto slides. After agarose solidification, the additional layer of 1% LMP agarose was applied, and followed by the slides immersion for 60 min. in alkaline lysis solution (1 M NaCl, 0.1 M EDTA, 10 mM Tris-HCl, 0.1% N-lauroylsarcosine, 1% Triton X-100, 30 mM NaOH). The slides were further gently washed in distilled water, and immersed for 20 min. in cold electrophoresis buffer (0.3 M NaOH, 1 mM EDTA, pH > 13), followed by the electrophoresis (30 min. at 0.7 V/cm) in the same buffer. At the end of electrophoresis, the slides were neutralized with 0.4 M Tris-HCl (pH 7.5) and stained with ethidium bromide (10 µg/mL). An epifluorescent microscope (Axio Imager.Z1; Carl Zeiss, Jena, Germany) at 400× magnification was used for slides analysis. CometAssay software (developed by Prof. Igor Kononenko research group, Laboratory for Cognitive Modeling, Faculty of Computer and Information Science, University of Ljubljana) was used to analyze 50 randomly selected comets per slide. As the DNA damage parameter, the percentage of DNA presence in the comet’s tail (tail DNA (%)) was used. The measurements were done in three independent repeats.
2.3. Systemic Subchronic Toxicity Investigation
The Ethical Committee of the Faculty of Veterinary Medicine, University of Belgrade and Ministry of Agriculture and Environment Protection of the Republic of Serbia approved the experimental protocol (decision number 01-831/2), and realized in accordance with recommendations of European Good Laboratory Practice (86/609 EEC) related to protection and use of laboratory animals.
2.3.1. Experimental Protocol
The investigation included 21 Wistar rats (Ratus norvegicus), at the age 8–12 weeks, and body weight 240–260 g. Animals were placed in individual cages. During the experiment, the animals were kept under following conditions: temperature 23 ± 3 °C, air humidity 55 ± 5%, 8–12 air changes/h, and artificial lighting at intervals of 12 h day/night. The animals had free access to food and water. The animal health status was monitored daily during the experiment.
Extracts that were obtained after the highest concentration of ALBO OS, obtained during 5 days immersion of material (100 mg/mL) in distilled water (in accordance with the standard ISO 10993-12:2012—Biological evaluation of medical devices—Part 12: Sample preparation and reference materials) was used for per oral rats feeding. After seven days of adaptation period, the animals were randomly divided into experimental (n = 12) and control group (n = 9). To the animals in the experimental group, 1 mL of ALBO-OS extract was per oral fed for 120 days, every day at the same time, while, for the control group, distilled water was used. During the experiment, control of the health status, behavior, changes in skin and haircut, food and water consummation, urination, and defecation, was performed and recorded daily. The body weight of animals was checked after the adaptation period, and afterward three times through the experiment.
2.3.2. Blood Analysis
At the end of the experiment, the blood samples were taken from lateral tail vein for hematological (leukocytes, thrombocytes, hemoglobin) and biochemical analyses (alanine aminotransaminase (ALT), aspartate aminotransaminase (AST), urea, creatinine, bilirubin, serum protein, glucose, and alkaline phosphatase (ALP)).
2.4. Histological and Stereological Analysis
The animals were sacrificed by intravenous administration of thiopentone sodium solution (170 mg/kg). The rats’ livers, kidneys, and spleens were removed, immersed in Buoin’s fixative solution for 24 h, and, for better absorption, further cut into smaller pieces and then immersed into fresh Buoin’s solution. Subsequently, all of the dissected organs were prepared for the light microscopy using the standard tissue preparation procedure. Tissue was embedded in paraffin. Tissue sections of 5 µm thickness were cut using rotary microtome (Leica rotary Microtome RM 2165, Leica Microsystems, Wetzlar, Germany). Every fifth section was used for the analysis in order not to repeat the analyzed structures. Tissue was stained with hematoxylin-eosin (H&E) (Merck KGaA, Darmstadt, Germany). The qualitative analysis of the microscopic slides was performed while using the light microscope (Leica DM8000 M with MEGA VIEW camera and software system for digital image transfer, Wetzlar, Germany). For quantitative, stereological analysis, micrographs were acquired in the RGB layout and converted to binary format. Measurements were made using a stereo-universal test system according to Cavalier’s principle, while using 16.0 point-counting system (MBF software system Application Suite 3.0.0, MBF Bioscience, Williston, VT, USA), with P2 spacing grid at the maximum 400× magnification.
Stained tissue sections of liver, kidney, and spleen were used to measure the number and volume density (Vv) of epithelial cells. Volume density was calculated through the following formula: Vv = Pf/Pt (mm
0), where Pf represents the number of the desired phase on the test system (epithelial cells) and Pt represents the total number of points of the test system [
19].
The following principle was used for the determination of the number of matches on the test system: all of the endothelial cells’ nuclei were marked as reference point, while the cells surfaces were calculated according to the principles: (i) all cells that have a clearly visible contour and nucleus, (ii) the cells contour do not touch the test system frame, and (iii) the cells contour is clearly visible without a visible nucleus. The numerical density (Nv) of all epithelial cells was calculated based on the cell count (Q) in the volume of analyzed tissues (Vo). The volume of analyzed tissue was evaluated as the product of the number of counted frames (ΣPi), the space of counted frame (a = 25,002), and the height factor (h) (the histological section), as presented in the following formula [
20]:
MBF software system was used to measure the surface and volume of epithelial cells and nuclei, by the thickness of their diameters. Measuring the surface of epithelial cells and nuclei enabled the measurement of the cells, cytoplasm, and nuclei volumes. The ratio between the cells’ nuclei and cytoplasm volumes was used to determine the nucleocytoplasmic ratio (NCO). The mitotic index was determined as the ratio between the number of cells in mitosis and the total number of cells in 10 visible optical fields per slide, with maximum 200× magnification.
2.5. Immunohistochemical Analysis
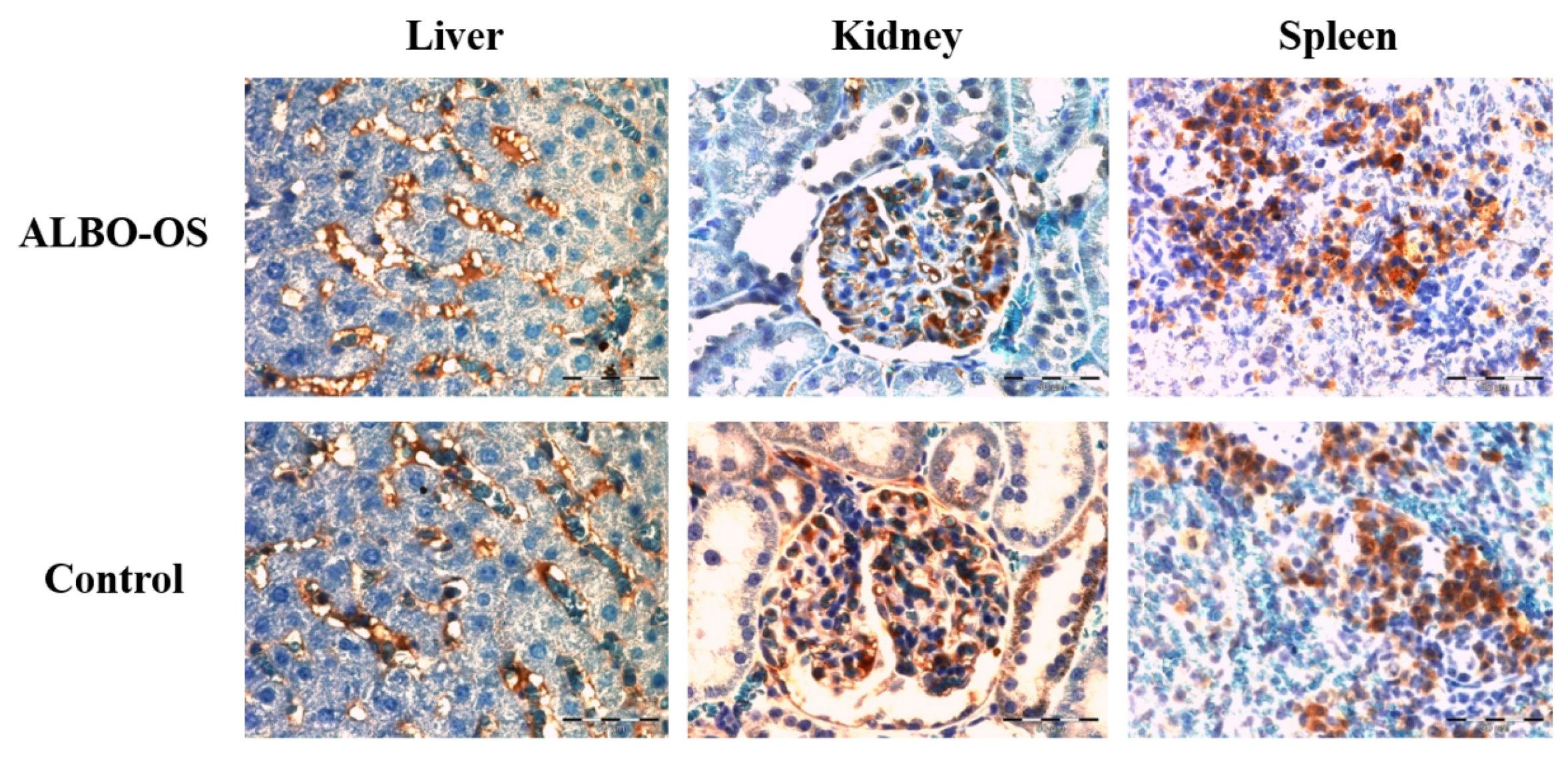
For the immunohistochemical analysis, the paraffin tissue sections of rat liver, spleen, and kidney were analyzed cut at 5 µm, and the slides were heated for 60 min. at 56 °C. Prior to the antigen retrieval step, the sections were deparaffinized and rehydrated through the series of xylenes and alcohols. To block endogenous peroxidase activity, tissue sections were treated with 3% H2O2 solution in PBS. Tor epitope retrieval, tissue sections were heated in microwave oven for 21 min., at 680 W, in 10 mmol/L citrate buffer, pH 6.0. The tissue sections were incubated with appropriate antibodies (against CD68 (RTU-CD68, Leica, LOT 6000698, Wetzlar, Germany) and Ki67 protein (Monoclonal Mouse Anti-human Ki67 Antigen, Clone Ki-S5, Sigma-Aldrich, Saint Louis, MO, USA (dilution 1:100)) over night, at +4 °C, in the humid chamber. Streptavidin-biotin technique was used for immunostaining (LSAB+/HRP Kit, Peroxidase Labeling, K0690, DAKO Cytomation, Glostrup, Denmark). Immunoreactivity complex was visualized with DAKO Liquid DAB+ Substrate/Chromogen System (Dako, CA, USA, Code No. K3468) and then counterstained with Mayer’s hematoxylin (Merck, KGaA, Darmstadt, Germany). As negative control served the tissue sections with omitted primary antibody. For positive control, the tissue sections that were known to express CD68 and Ki67 were used. CD68 and Ki67 positive cells in tissues were analyzed by a light microscope (Olympus AX70, Hamburg, Germany) with 40× magnification.
In all of analyzed sample of tissue, five hot spots were selected for image analysis in ImageJ (The National Institutes of Health, Bethesda, MD, USA). Ki67 and CD68 expressions were calculated by determining the positive Ki67/CD68 areas (brown-colored cells) in microscopic fields (40× magnification), based on the threshold [
21]. Median values of CD68 and Ki67 immunostaining were calculated for each individual tissue sample and then used for further analysis.
2.6. Statistical Analyses
All of the values were presented as mean ± standard deviation. Group comparisons were performed while using parametric (two-way ANOVA, one-way ANOVA followed by Dunnett’s test and t-test) or nonparametric tests (Kruskal–Wallis and Mann–Whitney U-test), depending on data distribution, with significance set at p < 0.05. Statistical software SPSS 20.0 (IBM corp., Armonk, NY, USA) was used for data processing.
4. Discussion
This study aimed to characterize toxicological potential of ALBO OS scaffold by investigating its potential genotoxicity in vitro and systemic subchronic toxicity after oral exposure, which is very important for its potential application in dental medicine.
Genotoxicity investigation on THP-1 cells using comet assay showed the lack of damaged DNAs in the cells that were cultivated in material’s extracts up to concentration 50 mg/mL which highly suggests that there were no changes inside of the cells on the level of DNA. This is in accordance with previous investigations that also reported low genotoxicity of hydroxyapatite and PLGA alone, which are the main components of the ALBO-OS [
22,
23,
24].
The blood analysis showed that there were no significant changes in the hematological parameters between the experimental and control group. Neither myelotoxic nor autoimmune effects on peripheral blood cells were detected in the experimental groups. This is in agreement with previous reports on the low hazard of these material [
13,
25]. Among the analyzed biochemical parameters, only alkaline phosphatase was significantly higher in the experimental group, compared to the control. This difference indicated the potential of the absorption of calcium and phosphates from ALBO OS, which consequently might led to an increase of ALP serum [
26].
The results of histopathological examination of systemic toxicity on liver, kidney and spleen sacrified rats after their oral exposure to extract of ALBO OS maximal concentration (100 mg/mL) during 120 days per oral feeding were particularly important for the determination of the toxicological profile ALBO OS.
Accordingly, the liver is chosen due to its vital role in organism being reflected by its physiological functions, synthesis and detoxification of various metabolites. In other words, any lesions caused by drugs, chemicals, and pathological status could interrupt its functions [
27]. The ALT level, which is found primarily in the liver, and is released into the circulation from damaged hepatocytes, was slightly higher in the experimental group, while the AST level, found in the erythrocytes, myocytes, and kidney cells also, was higher in the control, without significant differences [
27]. Similar results of biocompatibility investigation of Mg-Zn materials were reported, with the assertion that there was no change in the hepatocyte architecture and morphology of liver lobules [
28].
The increased mitotic index and the number of hepatocytes with two nuclei could be explained by the liver adaption to the presence of new material in the organism [
29]. It could be assumed that, as a reaction to chronical presence of ALBO OS extract in organism, the physiological reaction of increased function occurred. It is interesting to note that immunostaining confirmed parenchymal proliferation over increased expression value of the Ki67, while a number of immunoreactive CD68 positive cells remained in the physiological range. The increase in Ki-67 protein expression might be caused by the fact that posttranscriptional regulatory mechanisms suppress protein synthesis until the cells are at the G1/S boundary playing a critical role in the regulation of mitosis. In this case, like too many similar evidences published in other research papers, it seems that Ki67 is involved in the regulation of cell cycle progression, including DNA replication, higher-order chromatin organization, etc. [
30,
31].
In our previous study, the effect of calcium silicate and calcium aluminate implantation on systematic subchronic toxicity was evaluated [
32]. It was concluded that materials induced normal and reversible response in the liver. Namely, the proliferation of parenchymal cells in this case was also reported, with no visible signs of pathological changes or immunological reaction to the materials [
32].
An increase in the number and density of capillary endothelial cells (
p < 0.05) (obtained as all other histological and immunohistochemical parameters included in this paper, by observation 12 animals in experimental group and nine animals in the control group) indicated a statistically significant increased blood flow through the liver in experimental group, when comparing with control group of rats, which is characteristic for processes with more intense blood filtration. Additionally, significant increase the number of endothelial cells (
p < 0.05) participating in the formation of new blood sinusoids was observed, which indicated that the process of the necessary adaptation of the rats’ liver to ALBO-OS is occurred. As it is well known, the liver sinusoidal endothelial cells (LSEC) constitute the sinusoidal wall, or endothelial lining. Therefore, they can be observed as unique capillaries that differ from other capillaries in the body, because of the presence of open pores or fenestrae lacking a diaphragm and a basal lamina underneath the endothelium. The increasing quantity of the Ca
2+ ions, interacting with lipid soluble ionophores, which transport Ca
2+ ions across liver cell membranes, can induce increased transport Ca
2+ ions inside of the liver sinusoidal endothelial cells, influencing their contraction and, consequently blood flow diminishing through them. Therefore, the balance establishing in blood flow through sinusoids, the new blood sinusoids should be formed, simultaneously leading to the increased number of the endothelial cells [
20,
33,
34,
35]. The connective tissue cells, as stromal cells, did not change their structure or density, suggesting no pathological change on liver tissue occurred during four months of exposure. Similar to our results, in previous studies of systematic toxicity of new endodontic materials that are based on calcium hydroxyapatite and calcium silicates, an increased mitotic activity of parenchymal cells was observed and followed by the increased density of the cells and a moderate increase in the liver sinusoids and hepatocyte surface [
36,
37].
The serum levels of creatinine, as an indicator of kidney function and the change of renal histopathology [
38], were very similar in the experimental and control group, as well as values of urea and bilirubin. In the previous study, the absence of any morphological changes in the kidney tissue due to the implantation of biocompatible Mg-Nd-Zn-Zr material into the bone of the rabbit was reported [
39], which is in accordance with the presented results.
The number of epithelial cells in collecting ducts and the connective tissue cells, as well as other stereological and cytological parameters, were not significant (
p > 0.05), while immunostaining revealed significantly higher number of Ki67 positive cells, indicating cell proliferation in experimental group, increased kidney activity, and blood flow. In the study of mineral trioxide aggregate effect on the liver and kidney function, it was reported that the kidney function had subsequently increased after a month, while the increased liver function was observed during the first week and further increased during the observed period [
29]. Glomeruli, the beneficial components of the kidneys influenced by all matter in the blood reacted by shrinkage in the presence of material’s products, while its morphology was preserved. Besides, the area of renal kidney glomeruli of rats (
p < 0.05) was statistically significantly reduced during the treatment by ALBO-OS, probably as a consequence of the increase of filtration blood flow into the them. Fray has developed a mathematical model suggesting the stretch-dependent influx of extracellular calcium ions into juxtaglomerular cells to account for the pressure control of renin secretion [
40]. In detail, an increase of the renal artery pressure would enhance the circumferential stretch of the juxtaglomerular cells, thereby causing membrane depolarization.
Therefore, a rise of the intracellular calcium concentration inhibits reining secretion from these cells, as a consequence of the renal glomeruli shrinkage [
29,
41,
42]. Additionally, the change was reflected in the slight increase in the surface of Bowman’s space as a sign of increased glomerular filtration. In our study, while the spleen retained a normal physiological architecture, the number of lymphocytes increased in the treated rats, thus indicating an immune response to ALBO OS extract. While proliferation of lymphocytes was induced by material during four months of oral administration, lymphocytes were highly colored, uniformly distributed, with no signs of focal aggregations in spleen tissue, suggesting mature lymphocytes were predominantly in the tissue, with no signs of tissue hyperplasia.
Furthermore, number of CD68 positive immune cells in spleen was similar in the experimental and control group. In previous investigations of systemic toxicity of new endodontic material based on hydroxyapatite and calcium silicates, the slight hypertrophic changes in white pulp were reported, with no adverse effect on the spleen function and its immune response activation [
36]. Statistical analysis showed a significant increase (
p < 0.05) of the number of connective cells in the spleen of rats, during the treatment with ALBO-OS, which is a consequence of the increased production of lymphocytes in the spleen and their increased activity in the blood. Additionally, the increase of the number and numerical density of lymphocytes in the spleen of rats during the treatment by ALBO OS was also statistically significant (
p < 0.05).
Calcium plays an essential role in the activation and maturation of lymphocytes. As a second messenger, calcium ions are involved in most biochemical processes that transduce the external signals into cell responses. An increase in Ca
2+ ions and subsequent protein kinase C activation is indispensable for T-lymphocyte proliferation and an increase of their number [
43]. Previous studies reported that increased Ca
2+ ions promotes IL-2 mRNA gene expression and protein synthesis and that IL-2 is a potent T-lymphocyte growth factor [
28,
44,
45]. Since spleen primarily acts as the main filter for blood-borne pathogens and antigens, an increased number of lymphocytes could be explained by higher blood perfusion through spleen and the presence of the materials’ extract at high concentration in organism during a prolonged time period [
46]. Furthermore, haematological analysis revealed no increase of lymphocytes in systematic circulation in the experimental group, which implied the transient and physiological nature of increased spleen tissue lymphocytes.
An estimate of blood volume in the rat is 4.7 to 8.0 mL per 100 g body volume (BW) (mean value 7.0 mL) [
47]. The BW in our case for all rats were 240–260 g, from which follows that blood quantity following Lee and Blaufox research [
47] was probably between 16.8–18.2 mL. Taking in mind that all of the experimental rats were fed with 1 mL/day of fresh saturated solution of extract obtained by immersion 100 mg/mL for 120 days, the total quantity of used extract for 120 days is 120 mL, which is 6.6–7.1 times higher than the total volume of the blood of rats. Although this is exceptionally high quantity of Ca
2+ ions in rat’s blood, the pathological effect expressed over significant changes of morphology of tissue of the liver, kidney, and spleen were not registered, suggesting that the used nanomaterial is biocompatible.