Controllable Electrically Guided Nano-Al/MoO3 Energetic-Film Formation on a Semiconductor Bridge with High Reactivity and Combustion Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
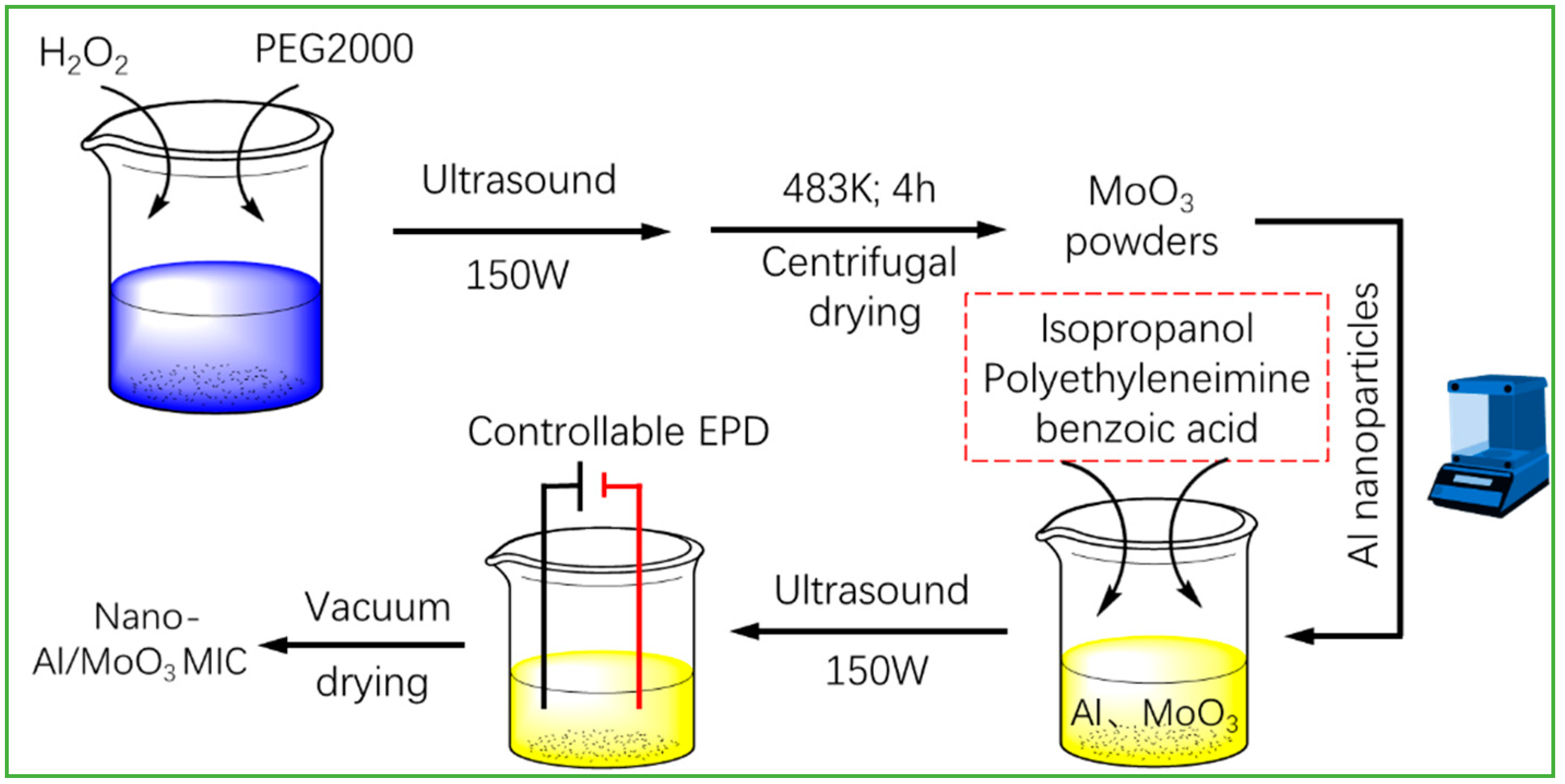
2.2. Controllable Design of Nano-Al/MoO3 MIC
2.3. Material Characterization
3. Results and Discussion
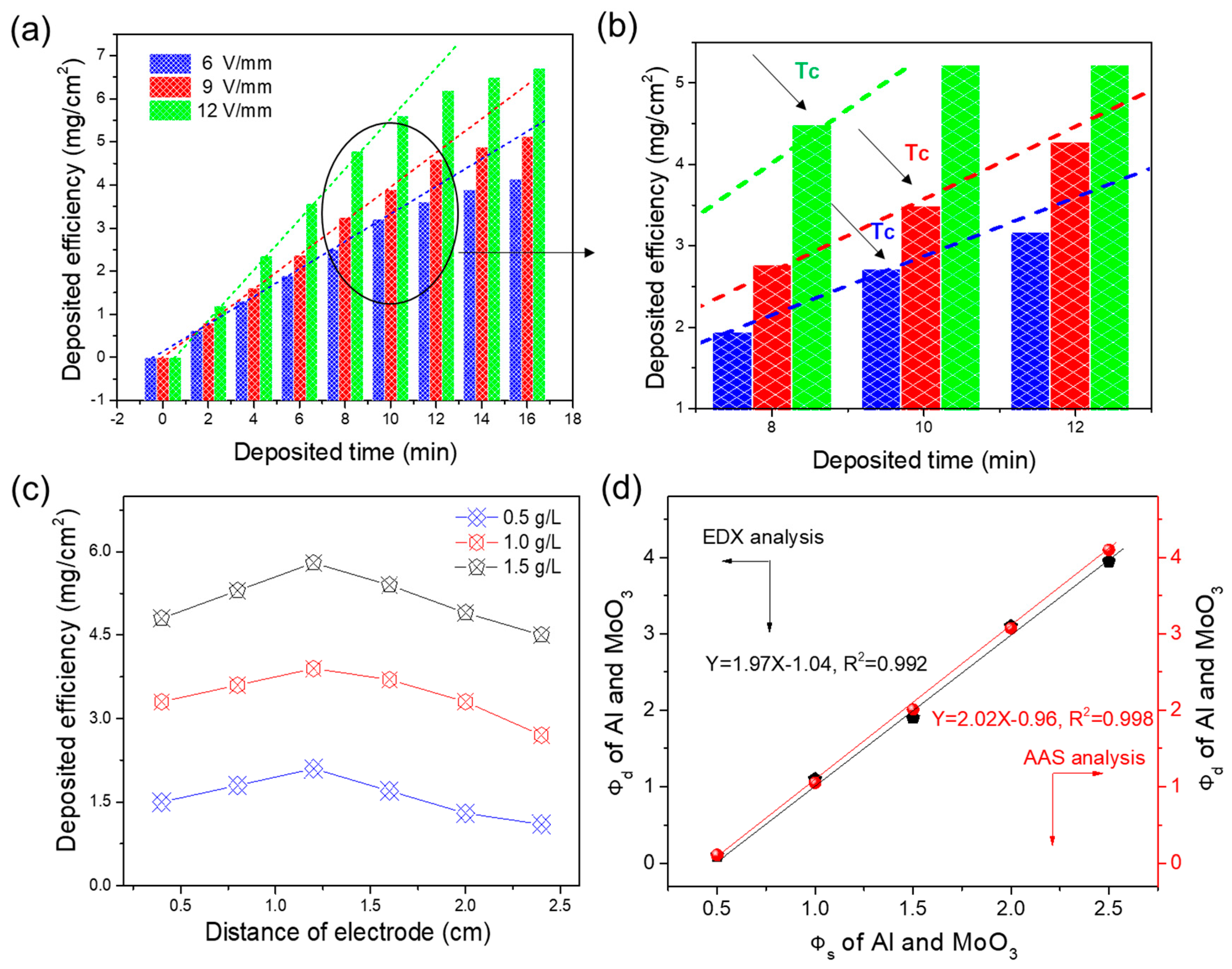
3.1. EPD Dynamic Studies
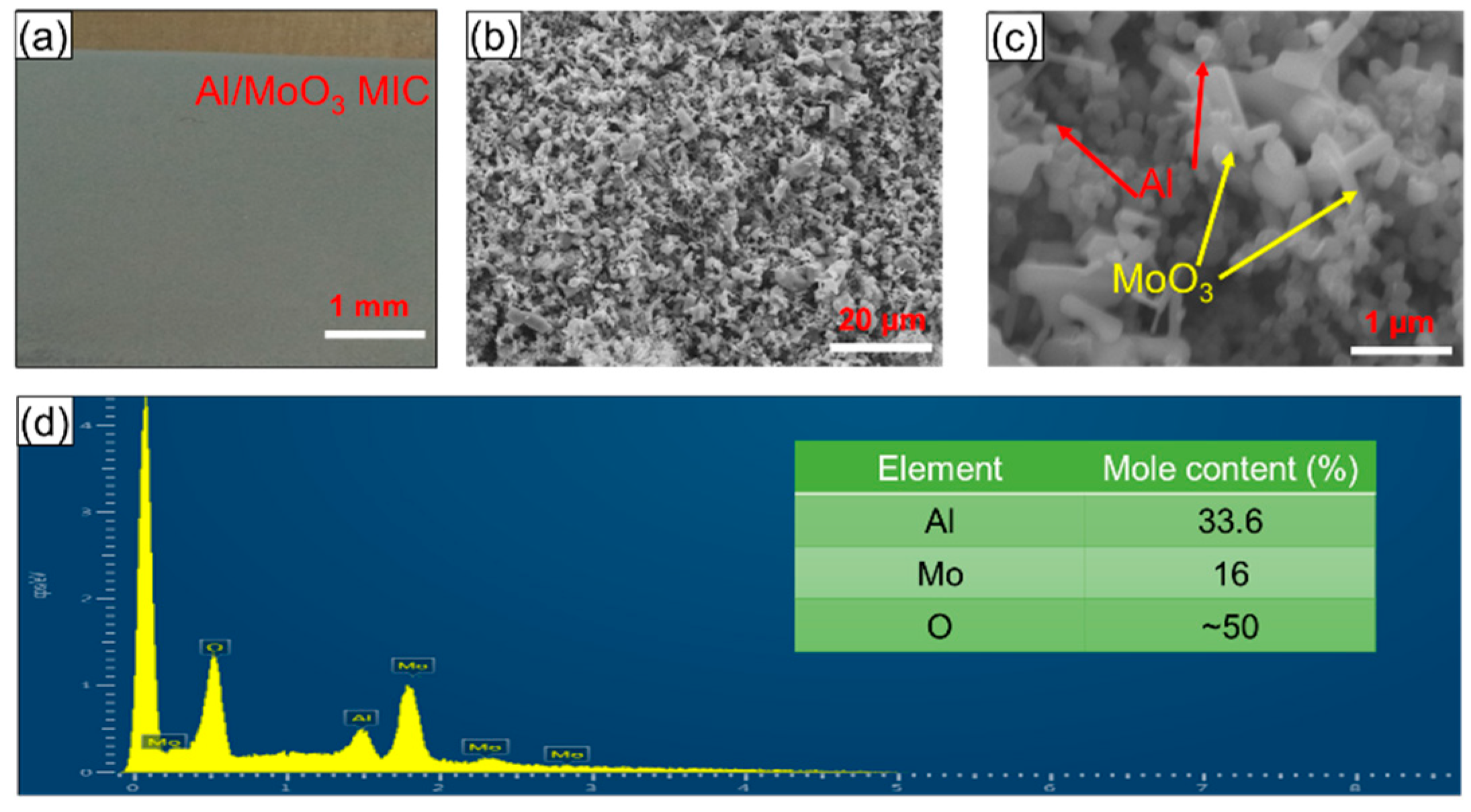
3.2. Characteristics of Nano-Al/MoO3 MIC
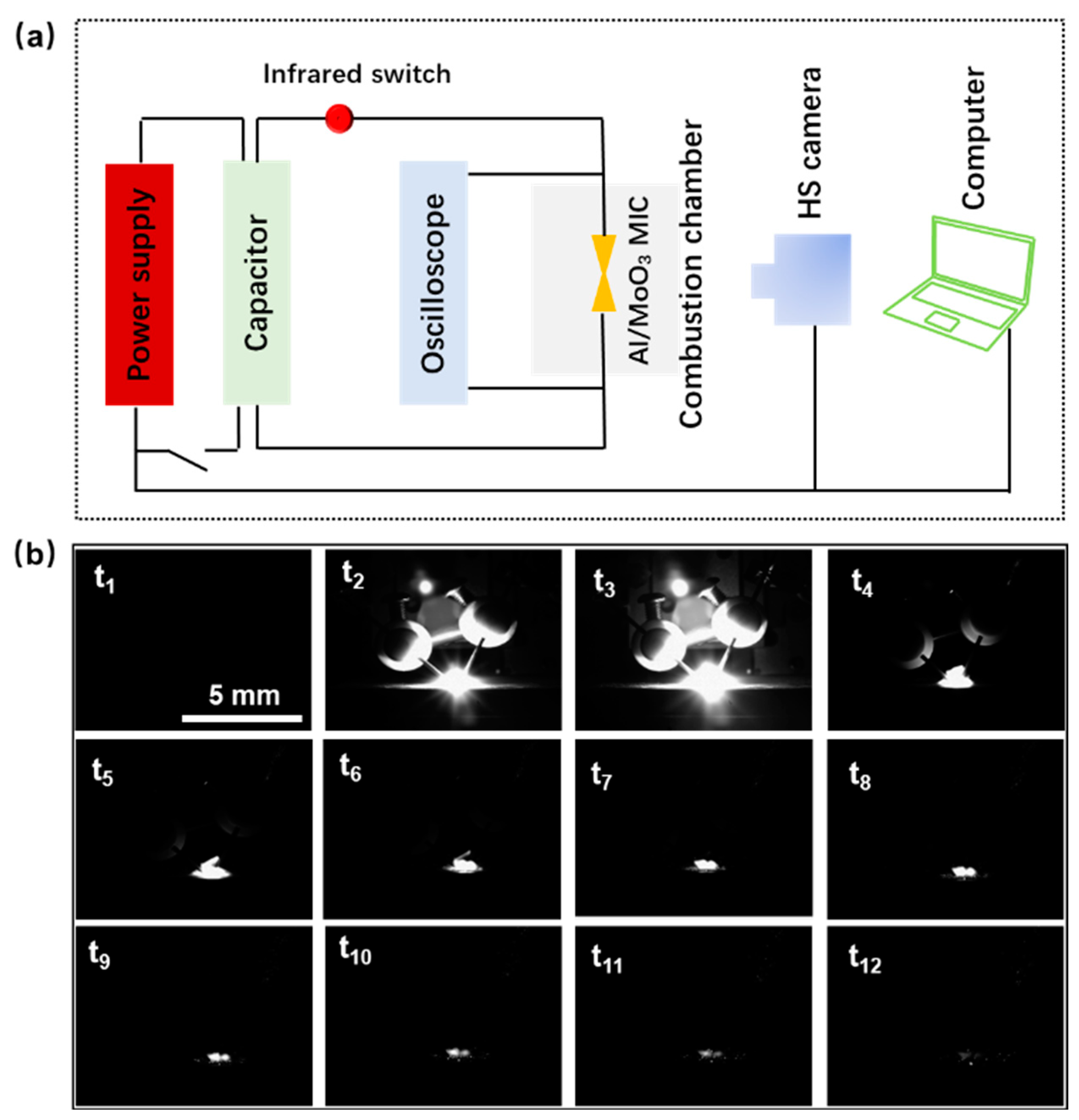
3.3. Thermal Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Sun, C.; Hu, B.; Yu, C.; Lu, M. Synthesis and characterization of the pentazolate anion cyclo-N5− in (N5)6(H3O)3(NH4)4Cl. Science 2017, 355, 374–376. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Zhu, C.; Duoss, E.B.; Gash, A.E.; Kolesky, D.B.; Kuntz, J.D.; Lewis, J.A.; Spadaccini, C.M. 3D Printing: Controlling Material Reactivity Using Architecture (Adv. Mater. 10/2016). Adv. Mater. 2016, 28, 1901. [Google Scholar] [CrossRef]
- He, W.; Liu, P.-J.; He, G.-Q.; Gozin, M.; Yana, Q.-L. Highly Reactive Metastable Intermixed Composites (MICs): Preparation and Characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Deng, P.; Hu, S.; Ren, L.; Li, X.; Xiao, P.; Liu, Y. Fabrication and Characterization of Nanoenergetic Hollow Spherical Hexanitrostibene (HNS) Derivatives. Nanomater. 2018, 8, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Shao, S.; Fang, G.; Meng, B.; Xie, Z.; Wang, L. High-Efficiency Inverted Polymer Solar Cells with Transparent and Work-Function Tunable MoO3-Al Composite Film as Cathode Buffer Layer. Adv. Mater. 2012, 24, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Grapes, M.; Reeves, R.V.; Fezzaa, K.; Sun, T.; Densmore, J.M.; Sullivan, K.T. In situ observations of reacting Al/Fe2O3 thermite: Relating dynamic particle size to macroscopic burn time. Combust. Flame 2019, 201, 252–263. [Google Scholar] [CrossRef]
- Wang, J.; Qu, Y.; Gong, F.; Shen, J.; Zhang, L. A promising strategy to obtain high energy output and combustion properties by self-activation of nano-Al. Combust. Flame 2019, 204, 220–226. [Google Scholar] [CrossRef]
- Wang, N.; Hu, Y.; Ke, X.; Xiao, L.; Zhou, X.; Peng, S.; Hao, G.; Jiang, W. Enhanced-absorption template method for preparation of double-shell NiO hollow nanospheres with controllable particle size for nanothermite application. Chem. Eng. J. 2020, 379, 122330. [Google Scholar] [CrossRef]
- Dai, J.; Wang, F.; Ru, C.; Xu, J.; Wang, C.; Zhang, W.; Ye, Y.; Shen, R. Ammonium Perchlorate as an Effective Additive for Enhancing the Combustion and Propulsion Performance of Al/CuO Nanothermites. J. Phys. Chem. C 2018, 122, 10240–10247. [Google Scholar] [CrossRef]
- Jian, G.; Feng, J.; Jacob, R.J.; Egan, G.C.; Zachariah, M.R. Super-reactive nanoenergetic gas generators based on periodate salts. Angew. Chem. Int. Ed. 2013, 52, 9743–9746. [Google Scholar] [CrossRef]
- Ramachandran, R.; Vuppuluri, V.S.; Fleck, T.J.; Rhoads, J.F.; Gunduz, I.E.; Son, S.F. Influence of Stoichiometry on the Thrust and Heat Deposition of On-Chip Nanothermites. Propellants Explos. Pyrotech. 2018, 43, 258–266. [Google Scholar] [CrossRef]
- Hea, W.; Lib, Z.-H.; Chena, S.; Yangc, G.; Yang, Z.; Liu, P.; Yana, Q.-L. Energetic metastable n-Al@PVDF/EMOF composite nanofibers with improved combustion performances. Chem. Eng. J. 2020, 383, 123146. [Google Scholar] [CrossRef]
- Liu, P.; Liu, P.; Wang, M. Ignition and combustion of nano-sized aluminum particles: A reactive molecular dynamics study. Combust. Flame 2019, 201, 276–289. [Google Scholar] [CrossRef]
- Suhaimi, B.F.; Palale, S.; Teh, L.K.; Mathews, N.; Mhaisalkar, S. Energy band and optical modeling of charge transport mechanism and photo-distribution of MoO3/Al-doped MoO3 in organic tandem cells. Funct. Mater. Lett. 2020, 13, 2051003. [Google Scholar] [CrossRef]
- Xu, J.; Tai, Y.; Ru, C.; Dai, J.; Ye, Y.; Shen, R.; Zhu, P. Tuning the Ignition Performance of a Microchip Initiator by Integrating Various Al/MoO3 Reactive Multilayer Films on a Semiconductor Bridge. ACS Appl. Mater. Interfaces 2017, 9, 5580–5589. [Google Scholar] [CrossRef]
- Xu, J.B.; Tai, Y.; Shen, Y.; Dai, J.; Xu, W.; Ye, Y.H.; Shen, R.Q.; Hua, Y. Characteristics of energetic semiconductor bridge initiator based ondifferent stoichiometric ratios of Al/MoO3 reactive multilayer filmsunder capacitor discharge conditions. Sensor. Actuator A 2019, 296, 241–248. [Google Scholar] [CrossRef]
- Weir, C.; Pantoya, M.L.; Daniels, M.A. The role of aluminum particle size in electrostatic ignition sensitivity of composite energetic materials. Combust. Flame 2013, 160, 2279–2281. [Google Scholar] [CrossRef]
- Wolenski, C.; Wood, A.; Mathai, C.J.; He, X.; McFarland, J.A.; Gangopadhyay, K.; Gangopadhyay, S.; Maschmann, M.R. Nanoscale surface reactions by laser irradiation of Al nanoparticles on MoO3 flakes. Nanotechnol. 2018, 30, 045703. [Google Scholar] [CrossRef]
- Xu, J.; Shen, Y.; Wang, C.; Dai, J.; Tai, Y.; Ye, Y.; Shen, R.; Wang, H.; Zachariah, M.R. Controlling the energetic characteristics of micro energy storage device by in situ deposition Al/MoO3 nanolaminates with varying internal structure. Chem. Eng. J. 2019, 373, 345–354. [Google Scholar] [CrossRef]
- Williams, R.A.; Schoenitz, M.; Ermoline, A.; Dreizin, E.L. Low-temperature exothermic reactions in fully-dense Al/MoO3 nanocomposite powders. Thermochim. Acta 2014, 594, 1–10. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducéré, J.-M.; Baijot, V.; Pinon, S.; Calais, T.; Esteve, A.; Rouhani, M.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, W.; Huang, W.; Liu, B.; Lin, H.; Dong, C. Carbon Nanotube-Graphene Hybrid Electrodes with Enhanced Thermo-Electrochemical Cell Properties. Nanomater. 2019, 9, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokunaga, S.; Itoh, Y.; Yaguchi, Y.; Tanaka, H.; Araoka, F.; Takezoe, H.; Aida, T. Electrophoretic Deposition for Cholesteric Liquid-Crystalline Devices with Memory and Modulation of Reflection Colors. Adv. Mater. 2016, 28, 4077–4083. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Daniels-Race, T. Electrophoretic Deposition of Carbon Nanotubes on 3-Amino-Propyl-Triethoxysilane (APTES) Surface Functionalized Silicon Substrates. Nanomaterials 2013, 3, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.; Boettcher, S.W. Adaptive semiconductor/electrocatalyst junctions in water-splitting photoanodes. Nat. Mater. 2013, 13, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.T.; Worsley, M.A.; Kuntz, J.D.; Gash, A.E. Electrophoretic deposition of binary energetic composites. Combust. Flame 2012, 159, 2210–2218. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X. Fabrication and Kinetics Study of Nano-Al/NiO Thermite Film by Electrophoretic Deposition. J. Phys. Chem. A 2015, 119, 4688–4694. [Google Scholar] [CrossRef]
- Guo, X.; Lai, C.; Jiang, X.; Mi, W.; Yin, Y.; Li, X.; Shu, Y. Remarkably facile fabrication of extremely superhydrophobic high-energy binary composite with ultralong lifespan. Chem. Eng. J. 2018, 335, 843–854. [Google Scholar] [CrossRef]
- Jang, N.-S.; Ha, S.-H.; Jung, S.-H.; Kim, S.H.; Lee, H.M.; Kim, J.-M. Fully packaged paper heater systems with remote and selectiveignition capabilities for nanoscale energetic materials. Sensor. Actuator A 2019, 287, 121–130. [Google Scholar] [CrossRef]
- Zhang, H.; Kinnear, C.; Mulvaney, P. Fabrication of single-nanocrystal srrays. Adv. Mater. 2019, 1904551. [Google Scholar]
- Guo, X.; Li, X.; Wei, Z.; Li, X.; Niu, L. Rapid fabrication and characterization of superhydrophobic tri-dimensional Ni/Al coatings. Appl. Surf. Sci. 2016, 387, 8–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Jiang, Z. Electrophoretic Deposition Forming of SiC-TZP Composites in a Nonaqueous Sol Media. J. Am. Ceram. Soc. 1994, 77, 1946–1949. [Google Scholar] [CrossRef]
- Seo, H.-S.; Kim, J.-K.; Kim, J.-W.; Kim, H.-S.; Koo, K.-K. Thermal behavior of Al/MoO3 xerogel nanocomposites. J. Ind. Eng. Chem. 2014, 20, 189–193. [Google Scholar] [CrossRef]
- Yu, C.; Zheng, Z.; Zhang, W.; Hu, B.; Chen, Y.; Chen, J.; Ma, K.; Ye, J.; Zhu, J. Sustainable Electrosynthesis of Porous CuN3 Films for Functional Energetic Chips. ACS Sustain. Chem. Eng. 2020, 8, 3969–3975. [Google Scholar] [CrossRef]
- Hu, L.; Yin, P.; Zhao, G.; He, C.; Imler, G.H.; Parrish, D.A.; Gao, H.; Shreeve, J.M. Conjugated Energetic Salts Based on Fused Rings: Insensitive and Highly Dense Materials. J. Am. Chem. Soc. 2018, 140, 15001–15007. [Google Scholar] [CrossRef]
- Van der Heijdena, A.E.D.M. Developments and challenges in the manufacturing, characterization and scale-up of energetic nanomaterials—A review. Chem. Eng. J. 2018, 350, 939–948. [Google Scholar] [CrossRef]
- Yana, Q.-L.; Zhao, F.-Q.; Kuo, K.K.; Zhang, X.-H.; Zeman, S.; DeLuca, L.T. Catalytic effects of nano additives on decomposition and combustion of RDX-, HMX-, and AP-based energetic compositions. Prog. Energy Combust. Sci. 2016, 57, 75–136. [Google Scholar] [CrossRef]
- Tai, Y.; Xu, J.; Wang, F.; Dai, J.; Zhang, W.; Ye, Y.; Shen, R. Experimental and modeling investigation on the self-propagating combustion behavior of Al-MoO3 reactive multilayer films. J. Appl. Phys. 2018, 123, 235302. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.J.; Zhang, L.; Qiao, Z.Q.; Gao, B.; Yang, G.C.; Huang, H. Design andfabricationofenergetic superlattice like-PTFE/Al with superior performance and application in functional micro-initiator. Nano Energy 2015, 12, 597–605. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Li, H.; Zhang, D.; Lai, C.; Li, W. A Comprehensive Investigation on the Electrophoretic Deposition (EPD) of Nano-Al/Ni Energetic Composite Coatings for the Combustion Application. Surf. Coatings Technol. 2015, 265, 83–91. [Google Scholar] [CrossRef]
- Kim, S.B.; Kim, K.J.; Cho, M.H.; Kim, J.H.; Kim, S.H.; Kim, K.-T. Micro- and Nanoscale Energetic Materials as Effective Heat Energy Sources for Enhanced Gas Generators. ACS Appl. Mater. Interfaces 2016, 8, 9405–9412. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Zhu, Y.; Cheng, S.X.; Zheng, H.X.; Liu, Y.S.; Qiao, Z.Q.; Yang, G.C.; Zhang, K.L. Energetic composites based on nano-Al and energetic coordination polymers (ECPs): The “father-son” effect of ECPs. Chem. Eng. J. 2020, 392, 123719. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Sun, Q.; Liang, T.; Giwa, A.S. Controllable Electrically Guided Nano-Al/MoO3 Energetic-Film Formation on a Semiconductor Bridge with High Reactivity and Combustion Performance. Nanomaterials 2020, 10, 955. https://doi.org/10.3390/nano10050955
Guo X, Sun Q, Liang T, Giwa AS. Controllable Electrically Guided Nano-Al/MoO3 Energetic-Film Formation on a Semiconductor Bridge with High Reactivity and Combustion Performance. Nanomaterials. 2020; 10(5):955. https://doi.org/10.3390/nano10050955
Chicago/Turabian StyleGuo, Xiaogang, Qi Sun, Taotao Liang, and A. S. Giwa. 2020. "Controllable Electrically Guided Nano-Al/MoO3 Energetic-Film Formation on a Semiconductor Bridge with High Reactivity and Combustion Performance" Nanomaterials 10, no. 5: 955. https://doi.org/10.3390/nano10050955





