Electrospun PCL/PGS Composite Fibers Incorporating Bioactive Glass Particles for Soft Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution Preparation
2.2. Electrospinning Process
2.3. Characterization
2.3.1. Microstructure and Composition
2.3.2. Mechanical Characterization
2.3.3. Wettability
2.3.4. In Vitro Acellular Bioactivity and Degradation Study
2.3.5. Cell Culture
2.3.6. Statistics
3. Results and Discussion
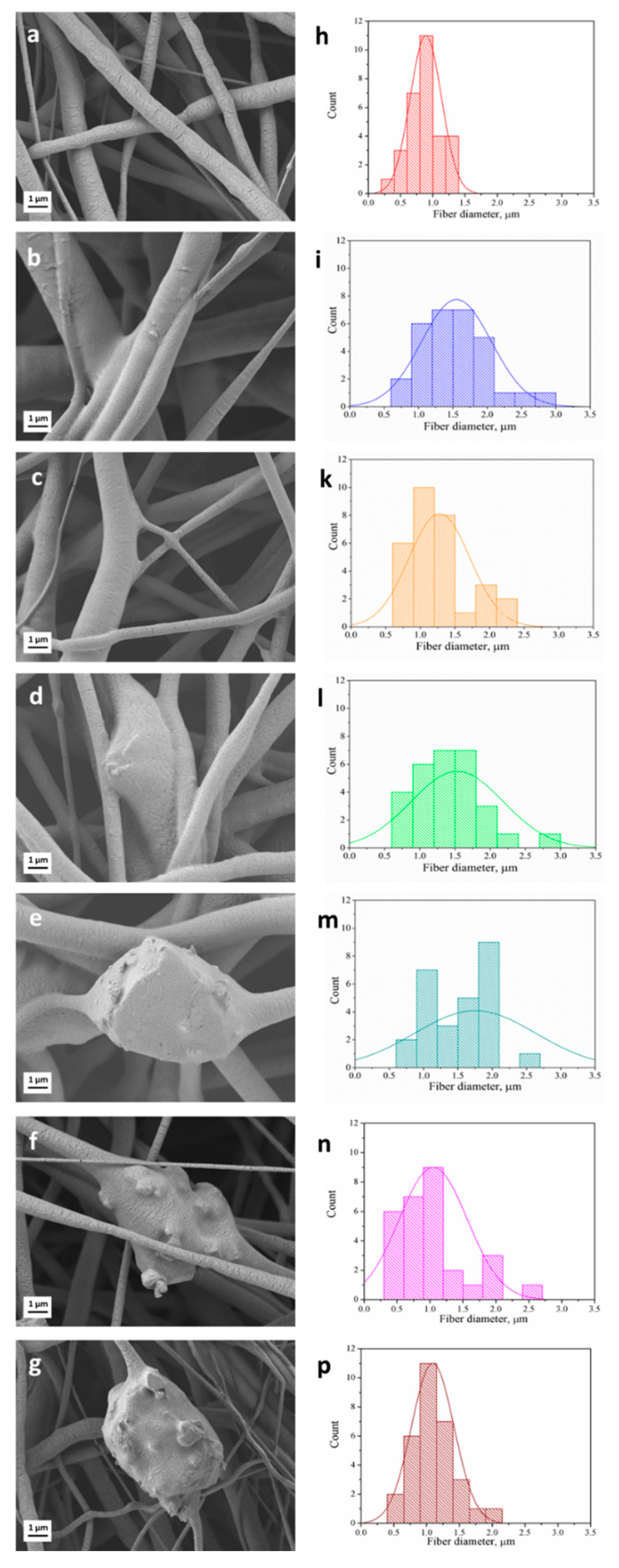
3.1. Fiber Morphology
3.2. Chemical Characterization
3.3. Wettability
3.4. Degradation in PBS Solution
3.5. In Vitro Acellular Bioactivity
3.6. Mechanical Properties
3.7. Cell Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subia, B.; Kundu, J.; Kundu, S.C. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. Tissue Eng. 2010. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008, 317–352. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Du Toit, L.C.; Ndesendo, V.M.K. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 789289. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. [Google Scholar] [CrossRef]
- Vogt, L.; Rivera, L.R.; Liverani, L.; Piegat, A.; El Fray, M.; Boccaccini, A.R. Poly(ε-caprolactone)/poly(glycerol sebacate) electrospun scaffolds for cardiac tissue engineering using benign solvents. Mater. Sci. Eng. C 2019, 103, 109712. [Google Scholar] [CrossRef]
- Masoumi, N.; Johnson, K.L.; Howell, M.C.; Engelmayr, G.C. Valvular interstitial cell seeded poly(glycerol sebacate) scaffolds: Toward a biomimetic in vitro model for heart valve tissue engineering. Acta Biomater. 2013, 9, 5974–5988. [Google Scholar] [CrossRef]
- Kemppainen, J.M.; Hollister, S.J. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J. Biomed. Mater. Res. Part A 2010, 94A, 9–18. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, K.; Hao, J.; Yang, T.; Geng, X.; Zhang, W. Biomimetic poly(glycerol sebacate)/polycaprolactone blend scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2019, 30, 53. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Yang, K.; Tang, W.; Liu, Y.; Yuan, Y.; Liu, C. A poly(glycerol sebacate)-coated mesoporous bioactive glass scaffold with adjustable mechanical strength, degradation rate, controlled-release and cell behavior for bone tissue engineering. Colloids Surfaces B Biointerfaces 2015, 131, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Czugala, M.; Stafiej, P.; Fathi, M.; Bahners, T.; Gutmann, J.S.; Singer, B.B.; Fuchsluger, T.A. Poly (glycerol sebacate)-poly (ε-caprolactone) blend nanofibrous scaffold as intrinsic bio- and immunocompatible system for corneal repair. Acta Biomater. 2017, 50, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Sundback, C.A.; Shyu, J.Y.; Wu, A.J.; Sheahan, T.P.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. In vitro and in vivo biocompatibility analysis of poly (glycerol sebacate) as a potential nerve guide material. Mater. Res. Soc. Symp. Proc. 2004, 26, 5454–5464. [Google Scholar]
- Saudi, A.; Rafienia, M.; Zargar Kharazi, A.; Salehi, H.; Zarrabi, A.; Karevan, M. Design and fabrication of poly (glycerol sebacate)-based fibers for neural tissue engineering: Synthesis, electrospinning, and characterization. Polym. Adv. Technol. 2019, 30, 1427–1440. [Google Scholar] [CrossRef]
- Pritchard, C.D.; Arnér, K.M.; Neal, R.A.; Neeley, W.L.; Bojo, P.; Bachelder, E.; Holz, J.; Watson, N.; Botchwey, E.A.; Langer, R.S.; et al. The use of surface modified poly(glycerol-co-sebacic acid) in retinal transplantation. Biomaterials 2010, 31, 2153–2162. [Google Scholar] [CrossRef]
- Ghosh, F.; Neeley, W.L.; Arnér, K.; Langer, R. Selective removal of photoreceptor cells in vivo using the biodegradable elastomer poly(glycerol sebacate). Tissue Eng. Part A 2011, 17, 1675–1682. [Google Scholar] [CrossRef]
- Wieland, A.M.; Sundback, C.A.; Hart, A.; Kulig, K.; Masiakos, P.T.; Hartnick, C.J. Poly(glycerol sebacate)-engineered plugs to repair chronic tympanic membrane perforations in a chinchilla model. Otolaryngol. Head Neck Surg. 2010, 143, 127–133. [Google Scholar] [CrossRef]
- Sundback, C.A.; McFadden, J.; Hart, A.; Kulig, K.M.; Wieland, A.M.; Pereira, M.J.N.; Pomerantseva, I.; Hartnick, C.J.; Masiakos, P.T. Behavior of poly(glycerol sebacate) plugs in chronic tympanic membrane perforations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1943–1954. [Google Scholar] [CrossRef]
- Sant, S.; Hwang, C.M.; Lee, S.H.; Khademhosseini, A. Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties. J. Tissue Eng. Regen. Med. 2011, 5, 283–291. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Coombes, A.G.A.; Rizzi, S.C.; Williamson, M.; Barralet, J.E.; Downes, S.; Wallace, W.A. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]
- Chim, H.; Hutmacher, D.W.; Chou, A.M.; Oliveira, A.L.; Reis, R.L.; Lim, T.C.; Schantz, J.T. A comparative analysis of scaffold material modifications for load-bearing applications in bone tissue engineering. Int. J. Oral Maxillofac. Surg. 2006, 35, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 2003, 66, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Iyer, D.; Gaharwar, A.K.; Patel, A.; Khademhosseini, A. Effect of biodegradation and de novo matrix synthesis on the mechanical properties of valvular interstitial cell-seeded polyglycerol sebacate-polycaprolactone scaffolds. Acta Biomater. 2013, 9, 5963–5973. [Google Scholar] [CrossRef]
- Masoumi, N.; Larson, B.L.; Annabi, N.; Kharaziha, M.; Zamanian, B.; Shapero, K.S.; Cubberley, A.T.; Camci-Unal, G.; Manning, K.B.; Mayer, J.E.; et al. Electrospun PGS: PCL Microfibers Align Human Valvular Interstitial Cells and Provide Tunable Scaffold Anisotropy. Adv. Healthc. Mater. 2014, 3, 929–939. [Google Scholar] [CrossRef]
- Tallawi, M.; Dippold, D.; Rai, R.; D’Atri, D.; Roether, J.A.; Schubert, D.W.; Rosellini, E.; Engel, F.B.; Boccaccini, A.R. Novel PGS/PCL electrospun fiber mats with patterned topographical features for cardiac patch applications. Mater. Sci. Eng. C 2016, 69, 569–576. [Google Scholar] [CrossRef]
- Kalakonda, P.; Aldhahri, M.A.; Abdel-Wahab, M.S.; Tamayol, A.; Moghaddam, K.M.; Ben Rached, F.; Pain, A.; Khademhosseini, A.; Memic, A.; Chaieb, S. Microfibrous silver-coated polymeric scaffolds with tunable mechanical properties. RSC Adv. 2017, 7, 34331–34338. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- ICH. Impurities: Guideline for Residual Solvents Q3C (R5). 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-14.pdf (accessed on 8 April 2020).
- Liverani, L.; Killian, M.S.; Boccaccini, A.R. Fibronectin Functionalized Electrospun Fibers by Using Benign Solvents: Best Way to Achieve Effective Functionalization. Front. Bioeng. Biotechnol. 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Lepry, W.C.; Smith, S.; Liverani, L.; Boccaccini, A.R.; Nazhat, S.N. Acellular bioactivity of sol-gel derived borate glass-polycaprolactone electrospun scaffolds. Biomed. Glas. 2016, 2. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Büttner, T.; Miguez Pacheco, V.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Schuhladen, K.; Wang, X.; Hupa, L.; Boccaccini, A.R. Dissolution of borate and borosilicate bioactive glasses and the influence of ion (Zn, Cu) doping in different solutions. J. Non. Cryst. Solids 2018, 502, 22–34. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Liverani, L.; Boccaccini, A. Versatile Production of Poly(Epsilon-Caprolactone) Fibers by Electrospinning Using Benign Solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, M.J.; Ryu, S.J.; Ki, C.S.; Park, Y.H. Effect of fiber diameter on surface morphology, mechanical property, and cell behavior of electrospun poly(ε-caprolactone) mat. Fibers Polym. 2016, 17, 1033–1042. [Google Scholar] [CrossRef]
- Salehi, S.; Fathi, M.; Javanmard, S.H.; Bahners, T.; Gutmann, J.S.; Ergün, S.; Steuhl, K.P.; Fuchsluger, T.A. Generation of PGS/PCL blend nanofibrous scaffolds mimicking corneal stroma structure. Macromol. Mater. Eng. 2014, 17, 1033–1042. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Nikkhah, M.; Sant, S.; Khademhosseini, A. Anisotropic poly (glycerol sebacate)-poly (-caprolactone) electrospun fibers promote endothelial cell guidance. Biofabrication 2015, 7, 015001. [Google Scholar] [CrossRef]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Menceloǧlu, Y.Z.; Acatay, K.; Vargel, I.; Pişkin, E. In vitro and in vivo degradation of non-woven materials made of poly(ε-caprolactone) nanofibers prepared by electrospinning under different conditions. J. Biomater. Sci. Polym. Ed. 2005, 16, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, E.M.; Allen, R.A.; Gao, J.; Pesce, M.; Wang, Y. Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds. Acta Biomater. 2015, 18, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Ishii, H.; Thouas, G.A.; Lyon, A.R.; Wright, J.S.; Blaker, J.J.; Chrzanowski, W.; Boccaccini, A.R.; Ali, N.N.; Knowles, J.C.; et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials 2010, 31, 3885–3893. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, I.; Krebs, N.; Hart, A.; Neville, C.M.; Huang, A.Y.; Sundback, C.A. Degradation behavior of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 2009, 91, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Hild, N.; Tawakoli, P.N.; Halter, J.G.; Sauer, B.; Buchalla, W.; Stark, W.J.; Mohn, D. pH-dependent antibacterial effects on oral microorganisms through pure PLGA implants and composites with nanosized bioactive glass. Acta Biomater. 2013, 9, 9118–9125. [Google Scholar] [CrossRef]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167. [Google Scholar] [CrossRef]
- Liverani, L.; Piegat, A.; Niemczyk, A.; El Fray, M.; Boccaccini, A.R. Electrospun fibers of poly(butylene succinate–co–dilinoleic succinate) and its blend with poly(glycerol sebacate) for soft tissue engineering applications. Eur. Polym. J. 2016, 81, 295–306. [Google Scholar] [CrossRef]







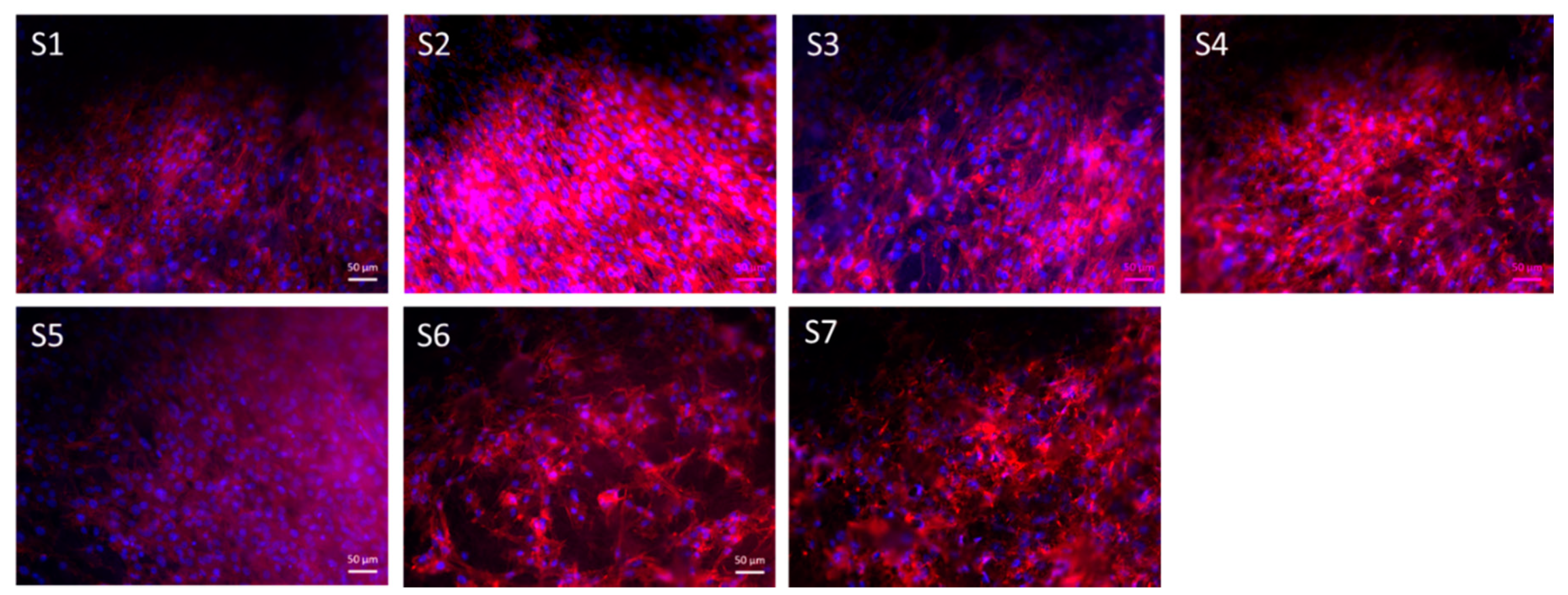
| Sample Name | Label | Solution Concentration [% w/v]/Polymer-BG Ratio PCL:PGS:BG | Applied Voltage [kV] | Distance Tip-Target [cm] | Needle Diameter | Flow Rate [mL/h] | Temperature [oC] | Relative Humidity [%] |
|---|---|---|---|---|---|---|---|---|
| PCL/PGSp | S1 | 20/1:0.5:0 | 15 | 11 | 21G | 0.4 | 24 ± 0.8 | 34 ± 12 |
| PCL/PGSmxl | S2 | 20/1:0.5:0 | 15 | 11 | 21G | 0.4 | 24 ± 0.8 | 34 ± 12 |
| PCL/PGSmxl/ 13-93 | S3 | 20/1:0.5:0.3 | 15 | 11 | 18G | 0.4 | 24 ± 0.8 | 34 ± 12 |
| PCL/PGSmxl/ 13-93BS | S4 | 20/1:0.5:0.3 | 15 | 11 | 18G | 0.4 | 24 ± 0.8 | 34 ± 12 |
| PCL | S5 | 20/1:0:0 | 15 | 11 | 21G | 0.4 | 24 ± 0.8 | 34 ± 12 |
| PCL/13-93 | S6 | 20/1:0:0.3 | 15 | 11 | 18G | 0.4 | 24 ± 0.8 | 34 ± 12 |
| PCL/13-93BS | S7 | 20/1:0.5:0.3 | 15 | 11 | 18G | 0.4 | 24 ± 0.8 | 34 ± 12 |
| Bioactive Glass Denomination | SiO2 | B2O3 | CaO | K2O | Na2O | MgO | P2O5 |
|---|---|---|---|---|---|---|---|
| 13-93 | 56.6 | - | 18.5 | 11.1 | 5.5 | 4.6 | 3.7 |
| 13-93BS | 20 | 36.6 | 18.5 | 11.1 | 5.5 | 4.6 | 3.7 |
| Sample Name | Sample Label | Average Fiber Diameter [μm] | Young’s Modulus [MPa] | Ultimate Tensile Strength (UTS) [MPa] | Failure Strain [%] |
|---|---|---|---|---|---|
| PCL | S5 | 0.9 ± 0.4 | 2.4 ± 0.5 | 1.3 ± 0.2 | 447 ± 226 |
| PCL/PGSp | S1 | 1.5 ± 0.5 | 3.8 ± 0.8 | 1.0 ± 0.2 | 219 ± 112 |
| PCL/PGSmxl | S2 | 1.5 ± 0.6 | 4.4 ± 0.3 | 1.2 ± 0.2 | 200 ± 103 |
| PCL/PGSmxl/13-93 | S3 | 1.6 ± 0.7 | 4.5 ± 0.3 | 1.0 ± 0.1 | 117 ± 62 |
| PCL/PGSmxl/13-93BS | S4 | 1.7 ± 0.9 | 1.2 ± 0.4 | 0.6 ± 0.1 | 185 ± 106 |
| PCL/13-93 | S6 | 1.1 ± 0.7 | 2.2 ± 0.4 | 1.1 ± 0.2 | 228 ± 139 |
| PCL/13-93BS | S7 | 1.1 ± 0.7 | 0.5 ± 0.3 | 0.9 ± 0.2 | 115 ± 57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luginina, M.; Schuhladen, K.; Orrú, R.; Cao, G.; Boccaccini, A.R.; Liverani, L. Electrospun PCL/PGS Composite Fibers Incorporating Bioactive Glass Particles for Soft Tissue Engineering Applications. Nanomaterials 2020, 10, 978. https://doi.org/10.3390/nano10050978
Luginina M, Schuhladen K, Orrú R, Cao G, Boccaccini AR, Liverani L. Electrospun PCL/PGS Composite Fibers Incorporating Bioactive Glass Particles for Soft Tissue Engineering Applications. Nanomaterials. 2020; 10(5):978. https://doi.org/10.3390/nano10050978
Chicago/Turabian StyleLuginina, Marina, Katharina Schuhladen, Roberto Orrú, Giacomo Cao, Aldo R. Boccaccini, and Liliana Liverani. 2020. "Electrospun PCL/PGS Composite Fibers Incorporating Bioactive Glass Particles for Soft Tissue Engineering Applications" Nanomaterials 10, no. 5: 978. https://doi.org/10.3390/nano10050978
APA StyleLuginina, M., Schuhladen, K., Orrú, R., Cao, G., Boccaccini, A. R., & Liverani, L. (2020). Electrospun PCL/PGS Composite Fibers Incorporating Bioactive Glass Particles for Soft Tissue Engineering Applications. Nanomaterials, 10(5), 978. https://doi.org/10.3390/nano10050978








