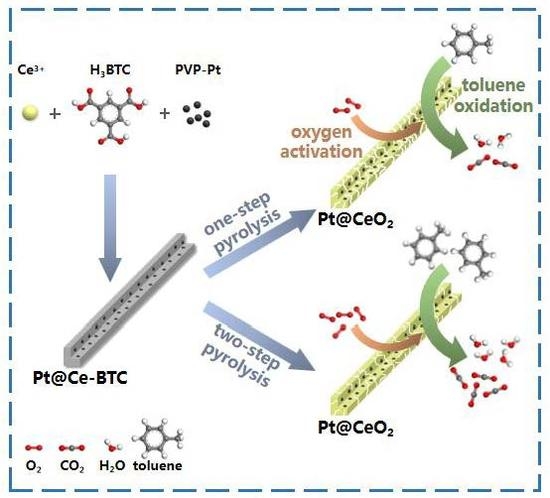
CeO2-Supported Pt Catalysts Derived from MOFs by Two Pyrolysis Strategies to Improve the Oxygen Activation Ability
Abstract
:1. Introduction
2. Materials and Method
2.1. Catalyst Preparation
2.2. Activity Measurement
2.3. Catalyst Characterization
3. Results and Discussion
3.1. Structural Analysis
3.2. Surface Chemical Properties
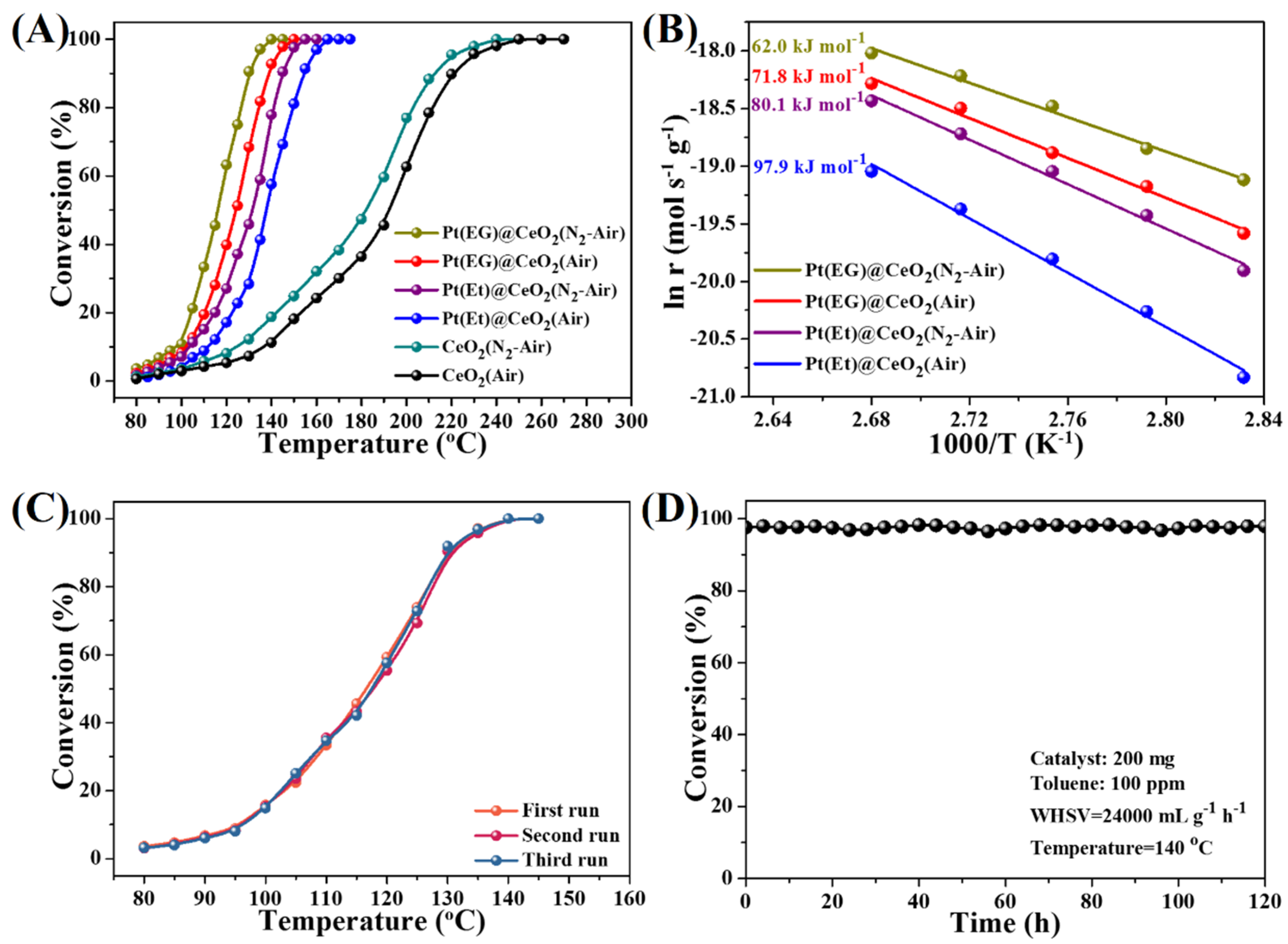
3.3. Catalytic Activity, Reusability and Durability
3.4. Effect of Oxygen Activation Ability on Toluene Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lai, Y.; Chen, T.; Lan, Y.; Chen, B.; You, J.; Yang, C.; Lai, N.; Wu, J.; Chen, C. Pt/SBA-15 as a Highly Efficient Catalyst for Catalytic Toluene Oxidation. ACS Catal. 2014, 4, 3824–3836. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size Effect of Pt Nanoparticles on the Catalytic Oxidation of Toluene over Pt/CeO2 Catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- White, R.; Luque, R.; Budarin, V.; Clark, J.; Macquarrie, D. Supported Metal Nanoparticles on Porous Materials. Methods and Applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef]
- Cho, H.; Deng, H.; Miyasaka, K.; Dong, Z.; Cho, M.; Neimark, A.; Kang, J.; Yaghi, O.; Terasaki, O. Extra Adsorption and Adsorbate Superlattice Formation in Metal-Organic Frameworks. Nature 2015, 527, 503–507. [Google Scholar]
- Li, J.; Kuppler, R.; Zhou, H. Selective Gas Adsorption and Separation in Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous Metal-Organic-Framework Nanoscale Carriers as a Potential Platform for Drug Delivery and Imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.; Roberts, J.; Scheidt, K.; Nguyen, S.; Hupp, J. Metal-Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.; Hauser, B.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting Functionality to a Metal-Organic Framework Material by Controlled Nanoparticle Encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, J.; Xu, Q. Immobilizing Metal Nanoparticles to Metal-Organic Frameworks with Size and Location Control for Optimizing Catalytic Performance. J. Am. Chem. Soc. 2013, 135, 10210–10213. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H. Metal-Organic Frameworks Meet Metal Nanoparticles Synergistic Effect for Enhanced Catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, G.; Cui, C.; Liu, Y.; Li, S.; Yan, W.; Xing, C.; Chi, Y.; Yang, Y.; Huo, F. A Family of Metal-Organic Frameworks Exhibiting Size-Selective Catalysis with Encapsulated Noble-Metal Nanoparticles. Adv. Mater. 2014, 26, 4056–4060. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xiao, C.; Maligal-Ganesh, R.; Zhou, L.; Goh, T.; Li, X.; Tesfagaber, D.; Thiel, A.; Huang, W. Pt Nanoclusters Confined within Metal-Organic Framework Cavities for Chemoselective Cinnamaldehyde Hydrogenation. ACS Catal. 2014, 4, 1340–1348. [Google Scholar] [CrossRef]
- Na, K.; Choi, K.; Yaghi, O.; Somorjai, G. Metal Nanocrystals Embedded in Single Nanocrystals of MOFs Give Unusual Selectivity as Heterogeneous Catalysts. Nano Lett. 2014, 14, 5979–5983. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Ji, W.; Xu, Z.; Liu, P.; Zhang, S.; Zhou, W.; Li, H.; Zhang, T.; Li, L.; Lu, X.; Wu, J.; et al. Metal-Organic Framework Derivatives for Improving the Catalytic Activity of the CO Oxidation Reaction. ACS Appl. Mater. Interfaces 2017, 9, 15394–15398. [Google Scholar] [CrossRef]
- Cao, X.; Tan, C.; Sindoro, M.; Zhang, H. Hybrid Micro-Nano-Structures Derived from Metal-Organic Frameworks: Preparation and Applications in Energy Storage and Conversion. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Liu, H.; Zheng, H.; Gao, T.; Shen, M.; Qu, Q.; Zheng, H. Metal Organic Frameworks-Derived Co3O4 Hollow Dodecahedrons with Controllable Interiors as Outstanding Anodes for Li Storage. J. Mater. Chem. A 2014, 2, 12194–12200. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, W.; Weng, X.; Liu, Y.; Wang, H.; Wu, Z. Active Oxygen Species in Lan+1NinO3n+1 Layered Perovskites for Catalytic Oxidation of Toluene and Methane. J. Phys. Chem. C 2016, 120, 3259–3266. [Google Scholar] [CrossRef]
- Yang, C.; Yu, X.; Heiβler, S.; Weidler, P.; Nefedov, A.; Wang, Y.; Wöll, C.; Kropp, T.; Paier, J.; Sauer, J. O2 Activation on Ceria Catalysts The Importance of Substrate Crystallographic Orientation. Angew. Chem. Int. Ed. 2017, 56, 16399–16404. [Google Scholar] [CrossRef]
- Gustafsson, M.; Bartoszewicz, A.; Martín-Matute, B.; Sun, J.; Grins, J.; Zhao, T.; Li, Z.; Zhu, G.; Zou, X. A Family of Highly Stable Lanthanide Metal-Organic Frameworks: Structural Evolution and Catalytic Activity. Chem. Mater. 2010, 22, 3316–3322. [Google Scholar] [CrossRef]
- Ji, P.; Sawano, T.; Lin, Z.; Urban, A.; Boures, D.; Lin, W. Cerium Hydride Secondary Building Units in a Porous Metal Organic Framework for Catalytic Hydroboration and Hydrophosphination. J. Am. Chem. Soc. 2016, 138, 14860–14863. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, J. Hollow-Structured Mesoporous Materials: Chemical Synthesis, Functionalization and Applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ Pyrolysis of Ce MOFs to Prepare CeO2 Catalyst with Obviously Improved Catalytic Performance for Toluene Combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zhao, W.; Gao, D.; Li, C.; Ye, C.; Sun, G. Design of Poroushollow Structured Ceria by Partial Thermal Decomposition of Ce-MOF and Selective Etching. ACS Appl. Mater. Interfaces 2017, 9, 39594–39601. [Google Scholar] [CrossRef]
- Yang, C.; Yu, X.; Heiβler, S.; Nefedov, A.; Colussi, S.; Llorca, J.; Trovarelli, A.; Wang, Y.; Wöll, C. Surface Faceting and Reconstruction of Ceria Nanoparticles. Angew, Chem. Int. Ed. 2017, 56, 375–379. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Wang, L.; Wang, B.; Li, Y. Oxygen Vacancy Clusters Promoting Reducibility and Activity of Ceria Nanorods. J. Am. Chem. Soc. 2009, 131, 3140–3141. [Google Scholar] [CrossRef]
- Kopelent, R.; Bokhoven, J.; Szlachetko, J.; Edebeli, J.; Paun, C.; Nachtegaal, M.; Safonova, O. Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal Catalysts. Angew. Chem. Int. Ed. 2015, 54, 8728–8731. [Google Scholar] [CrossRef]
- Soler, L.; Casanovas, A.; Escudero, C.; Pérez-Dieste, V.; Aneggi, E.; Trovarelli, A.; Llorca, J. Ambient Pressure Photoemission Spectroscopy Reveals the Mechanism of Carbon Soot Oxidation in Ceria-Based Catalysts. ChemCatChem 2016, 8, 2748–2751. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Liu, X.; Meng, D.; Guo, Y.; Guo, Y.; Lu, G. Effect of Ceria Crystal Plane on the Physicochemical and Catalytic Properties of Pd/Ceria for CO and Propane Oxidation. ACS Catal. 2016, 6, 2265–2279. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer ΙΙΙ, H.; Overbury, S. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Wu, Z.; Dai, S. Shape-Controlled Ceria-based Nanostructures for Catalysis Applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Zhu, X.; Hensen, E.; Lefferts, L.; Mojet, B. Defect Chemistry of Ceria Nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. [Google Scholar] [CrossRef]
- Pu, Z.; Lu, J.; Luo, M.; Xie, Y. Study of Oxygen Vacancies in Ce0.9Pr0.1O2-δ Solid Solution by in Situ X-ray Diffraction and in Situ Raman Spectroscopy. J. Phys. Chem. C 2007, 111, 18695–18702. [Google Scholar] [CrossRef]
- López, J.; Gilbank, A.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The Prevalence of Surface Oxygen Vacancies over the Mobility of Bulk Oxygen in Nanostructured Ceria for the Total Toluene Oxidation. Appl. Catal. B Environ. 2015, 174, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Freund, H.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Hetergeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Allian, A.; Takanabe, K.; Fujdala, K.; Hao, X.; Truex, T.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and Mechanism of CO Oxidation on Supported Platinum Nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Li, T.; Zhao, Z.; Gong, X.; Chen, Y.; Duan, A.; Jiang, G.; Wei, Y. Interfacial Effects of CeO2-Supported Pd Nanorod in Catalytic CO Oxidation: A Theoretical Study. J. Phys. Chem. C 2015, 119, 12923–12934. [Google Scholar] [CrossRef]
- Liu, J.; Su, Y.; Filot, I.; Hensen, E. A Linear Scaling Relation for CO Oxidation on CeO2-Supported Pd. J. Am. Chem. Soc. 2018, 140, 4580–4587. [Google Scholar] [CrossRef]
- Mao, M.; Lv, H.; Li, Y.; Yang, Y.; Zeng, M.; Li, N.; Zhao, X. Metal Support Interaction in Pt Nanoparticles Partially Confined in the Mesopores of Microsized Mesoporous CeO2 for Highly Efficient Purification of Volatile Organic Compounds. ACS Catal. 2016, 6, 418–427. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape Effect of Pt/CeO2 Catalysts on the Catalytic Oxidation of Toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Lee, Y.; He, G.; Akey, A.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I. Raman Analysis of Mode Softening in Nanoparticle CeO2-δ and Au-CeO2-δ During CO Oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Shang, Q.; Xiong, Y.; Zhang, Q.; Luo, Y.; Yu, S.; Jiang, H. Boosting Photocatalytic Hydrogen Production of a Metal Organic Framework Decorated with Platinum Nanoparticles: The Platinum Location Matters. Angew. Chem. 2016, 128, 9535–9539. [Google Scholar] [CrossRef]
- Teranishi, T.; Hosoe, M.; Tanaka, T.; Miyake, M. Size Control of Monodispersed Pt Nanoparticles and Their 2D Organization by Electrophoretic Deposition. J. Phys. Chem. B 1999, 103, 3818–3827. [Google Scholar] [CrossRef]
- Almáši, M.; Zeleňák, V.; Opanasenko, M.; Císařová, I. Ce(ΙΙΙ) and Lu(ΙΙΙ) Metal-Organic Frameworks with Lewis Acid Metal Sites Preparation Sorption Properties and Catalytic Activity. Catal. Today 2015, 243, 184–194. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; He, H.; Li, Y.; Wu, H.; Fu, M.; Ye, D.; Wu, J.; Huang, H.; Hu, Y.; Niu, X. CeO2-Supported Pt Catalysts Derived from MOFs by Two Pyrolysis Strategies to Improve the Oxygen Activation Ability. Nanomaterials 2020, 10, 983. https://doi.org/10.3390/nano10050983
Zhu X, He H, Li Y, Wu H, Fu M, Ye D, Wu J, Huang H, Hu Y, Niu X. CeO2-Supported Pt Catalysts Derived from MOFs by Two Pyrolysis Strategies to Improve the Oxygen Activation Ability. Nanomaterials. 2020; 10(5):983. https://doi.org/10.3390/nano10050983
Chicago/Turabian StyleZhu, Xueqing, Hui He, Yanxia Li, Haoyuan Wu, Mingli Fu, Daiqi Ye, Junliang Wu, Haomin Huang, Yun Hu, and Xiaojun Niu. 2020. "CeO2-Supported Pt Catalysts Derived from MOFs by Two Pyrolysis Strategies to Improve the Oxygen Activation Ability" Nanomaterials 10, no. 5: 983. https://doi.org/10.3390/nano10050983
APA StyleZhu, X., He, H., Li, Y., Wu, H., Fu, M., Ye, D., Wu, J., Huang, H., Hu, Y., & Niu, X. (2020). CeO2-Supported Pt Catalysts Derived from MOFs by Two Pyrolysis Strategies to Improve the Oxygen Activation Ability. Nanomaterials, 10(5), 983. https://doi.org/10.3390/nano10050983