Enhanced Photocatalytic Properties of PET Filaments Coated with Ag-N Co-Doped TiO2 Nanoparticles Sensitized with Disperse Blue Dyes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Simultaneous Dyeing and Depositing Ag–N Co-Doped TiO2 Particles on PET Filaments
2.3. Characterization Techniques
2.4. Evaluation of the Photocatalytic Properties
3. Results and Discussion
3.1. Morphologies, Compositions and Structures
3.2. Chemical States
3.3. Separation Efficiency of Photo-Generated Electron-Hole Pairs
3.4. Optical Properties and Electronic Band Structures
3.5. Photocatalytic Performances
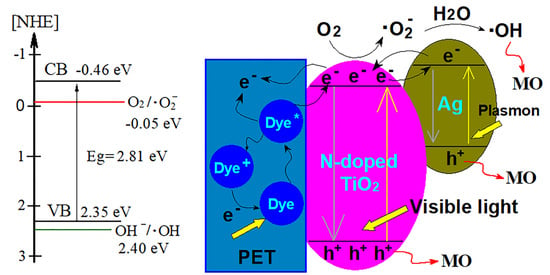
3.6. Photocatalytic Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saeed, M.; Muneer, M.; Khosa, M.K.K.; Akram, N.; Khalid, S.; Adeel, M.; Nisar, A.; Sherazi, S. Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium. Green Process. Synth. 2019, 8, 659–666. [Google Scholar] [CrossRef]
- Yuan, J.X.; Wang, E.J.; Chen, Y.M.; Yang, W.S.; Yao, J.H.; Cao, Y.A. Doping mode, band structure and photocatalytic mechanism of B-N-codoped TiO2. Appl. Surf. Sci. 2011, 257, 7335–7342. [Google Scholar] [CrossRef]
- Naraginti, S.; Thejaswini, T.V.L.; Prabhakaran, D.; Sivakumar, A.; Satyanarayana, V.S.; Prasad, A.S.A. Enhanced photo-catalytic activity of Sr and Ag co-doped TiO2 nanoparticles for the degradation of Direct Green-6 and Reactive Blue-160 under UV & visible light. Spectrochim. Acta A 2015, 149, 571–579. [Google Scholar]
- Murcia, J.J.; Avila-Martinez, E.G.; Rojas, H.; Cubillos, J.; Ivanova, S.; Penkova, A.; Laguna, O.H. Powder and nanotubes titania modified by dye sensitization as photocatalysts for the organic pollutants elimination. Nanomaterials 2019, 9, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xi, J.H.; Ji, Z.G. Mo plus N codoped TiO2 sheets with dominant {001} facets for enhancing visible-light photocatalytic activity. J. Mater. Chem. 2012, 22, 17700–17708. [Google Scholar] [CrossRef]
- Malligavathy, M.; Iyyapushpam, S.; Nishanthi, S.T.; Padiyan, D.P. Photoreduction synthesis of silver on Bi2O3/TiO2 nanocomposites and their catalytic activity for the degradation of methyl orange. J. Mater. Sci. Mater. El. 2017, 28, 18307–18321. [Google Scholar] [CrossRef]
- Gao, H.T.; Lu, B.; Liu, W.C.; Ding, C.H.; Liu, F.F.; Liu, G.J. The energetic and electronic properties of 4d transition metals doped TiO2 from first-principles. J. Comput. Theor. Nanos. 2012, 9, 2198–2207. [Google Scholar] [CrossRef]
- Liu, G.L.; Han, C.; Pelaez, M.; Zhu, D.W.; Liao, S.J.; Likodimos, V.; Kontos, A.G.; Falaras, P.; Dionysiou, D.D. Enhanced visible light photocatalytic activity of C-N-codoped TiO2 films for the degradation of microcystin-LR. J. Mol. Catal. A Chem. 2013, 372, 58–65. [Google Scholar] [CrossRef]
- Vukoje, I.D.; Tomasevic-Ilic, T.D.; Zarubica, A.R.; Dimitrijevic, S.; Budimir, M.D.; Vranjes, M.R.; Saponjic, Z.V.; Nedeljkovic, J.M. Silver film on nanocrystalline TiO2 support: Photocatalytic and antimicrobial ability. Mater. Res. Bull. 2014, 60, 824–829. [Google Scholar] [CrossRef]
- Li, M.J.; Yu, Z.B.; Liu, Q.; Sun, L.; Huang, W.Y. Photocatalytic decomposition of perfluorooctanoic acid by noble metallic nanoparticles modified TiO2. Chem. Eng. J. 2016, 286, 232–238. [Google Scholar] [CrossRef]
- Peng, C.C.; Wang, W.Z.; Zhang, W.W.; Liang, Y.J.; Zhuo, L. Surface plasmon-driven photoelectrochemical water splitting of TiO2 nanowires decorated with Ag nanoparticles under visible light illumination. Appl. Surf. Sci. 2017, 420, 286–295. [Google Scholar] [CrossRef]
- Vazhappilly, T.; de Lara-Castells, M.P.; Micha, D.A. Model studies of the structure and optical properties of the TiO2(110) surface with an adsorbed Ag atom. Mol. Phys. 2019, 117, 2267–2274. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Wei, W.; Dai, Y.; Huang, B.B. Tuning electronic structure and photocatalytic properties by Ag incorporated on (001) surface of anatase TiO2. Appl. Surf. Sci. 2012, 258, 4806–4812. [Google Scholar] [CrossRef]
- Khan, M.; Xu, J.N.; Chen, N.; Cao, W.B.; Asadullah; Usman, Z.; Khan, D.F. Effect of Ag doping concentration on the electronic and optical properties of anatase TiO2: A DFT-based theoretical study. Res. Chem. Intermediat. 2013, 39, 1633–1644. [Google Scholar] [CrossRef]
- Nanaji, K.; Janardhana, R.K.S.K.; Rao, T.N.; Anandan, S. Energy level matching for efficient charge transfer in Ag doped-Ag modified TiO2 for enhanced visible light photocatalytic activity. J. Alloy. Compd. 2019, 794, 662–671. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Novel integrated approach of adsorption and photo-oxidation using Ag-TiO2/PU for bioaerosol removal under visible light. Chem. Eng. J. 2015, 275, 357–365. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M.; Dillert, R.; Bahnemann, D.W. TiO2/Fe3O4/Ag nanophotocatalysts in solar fuel production: New approach to using a flexible lightweight sustainable textile fabric. J. Clean. Prod. 2018, 196, 688–697. [Google Scholar] [CrossRef]
- De Leon-Martinez, P.A.; Soriano-Corral, F.; Avila-Orta, C.A.; Gonzalez-Morones, P.; Hernandez-Hernandez, E.; Ledezma-Perez, A.S.; Covarrubias-Gordillo, C.A.; Espinosa-Lopez, A.C.; Gomez, R.E.D.D. Surface modification of nTiO2/Ag hybrid nanoparticles using microwave-assisted polymerization in the presence of bis(2-hydroxyethyl) terephthalate. J. Nanomater. 2017, 2017, 7079497. [Google Scholar]
- Bezerra, P.C.S.; Cavalcante, R.P.; Garcia, A.; Wender, H.; Martines, M.A.U.; Casagrande, G.A.; Gimenez, J.; Marco, P.; Oliveira, S.C.; Machulek, A. Synthesis, characterization, and photocatalytic activity of pure and N-, B-, or Ag- doped TiO2. J. Brazil. Chem. Soc. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.D.; Lei, Y.P.; Wang, B. Preparation and photocatalytic activity of N-Ag co-doped TiO2/C porous ultrafine fibers mat. Ceram. Int. 2014, 40, 2017–2022. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W.B.; Li, J.; Zaman, M.I.; Manan, A. Density functional theory calculations for the investigation of (Ag, N) codoping effect on the electronic and optical properties of anatase TiO2. Int. J. Mod. Phys. B 2014, 28, 1450112. [Google Scholar] [CrossRef]
- Khan, M.; Zeng, Y. Improving the photo-response of TiO2 by tri-doping: A DFT based atomistic study. Mater. Res. Express 2019, 6, 115510. [Google Scholar] [CrossRef]
- Carre, G.; Garnier, L.; Moeller-Siegert, J.; Gies, J.P.; Keller, V.; Andre, P.; Keller, N. Antibacterial textiles functionalized by layer-by-layer assembly of polyelectrolytes and TiO2 photocatalyst. RSC Adv. 2015, 5, 38859–38867. [Google Scholar] [CrossRef]
- Zhang, F.L.; Cheng, Z.Q.; Cui, L.Y.; Duan, T.T.; Anan, A.; Zhang, C.F.; Kang, L.J. Controllable synthesis of Ag@TiO2 heterostructures with enhanced photocatalytic activities under UV and visible excitation. RSC Adv. 2016, 6, 1844–1850. [Google Scholar] [CrossRef]
- Bao, N.; Miao, X.H.; Hu, X.D.; Zhang, Q.Z.; Jie, X.Y.; Zheng, X.Y. Novel synthesis of plasmonic Ag/AgCl@TiO2 continues fibers with enhanced broadband photocatalytic performance. Catalysts 2017, 7, 117. [Google Scholar] [CrossRef] [Green Version]
- Abid, M.; Bouattour, S.; Ferraria, A.M.; Conceicao, D.S.; Carapeto, A.P.; Feira, L.F.V.; do Rego, A.M.B.; Chehimi, M.M.; Vilar, M.R.; Boufi, S. Facile functionalization of cotton with nanostructured silver/titania for visible-light plasmonic photocatalysis. J. Colloid Interf. Sci. 2017, 507, 83–94. [Google Scholar] [CrossRef]
- Shahini, P.; Ashkarran, A.A. Immobilization of plasmonic Ag-Au NPs on the TiO2 nanofibers as an efficient visible-light photocatalyst. Colloid Surface. A 2018, 537, 155–162. [Google Scholar] [CrossRef]
- Kizildag, N.; Ucar, N.; Onen, A. Nanocomposite polyacrylonitrile filaments with titanium dioxide and silver nanoparticles for multifunctionality. J. Ind. Text. 2018, 47, 1716–1738. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A.; Afrin, T.; Sun, L.; Wang, X.G. Self-cleaning wool: Effect of noble metals and silica on visible-light-induced functionalities of nano TiO2 colloid. J. Text. I 2015, 106, 1348–1361. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.D.; Hao, B.; Ruan, J.M.; Ma, P.C. Preparation of fiber-based plasmonic photocatalyst and its photocatalytic performance under the visible light. Appl. Catal. B Environ. 2015, 166, 287–294. [Google Scholar] [CrossRef]
- Liu, M.Q.; Zhao, J.; Xiao, C.F.; Quan, Q.; Li, X.F. PPy-assisted fabrication of Ag/TiO2 visible-light photocatalyst and its immobilization on PAN fiber. Mater. Des. 2016, 104, 428–435. [Google Scholar] [CrossRef]
- Aksit, A.; Camlibel, N.O.; Zeren, E.T.; Kutlu, B. Development of antibacterial fabrics by treatment with Ag-doped TiO2 nanoparticles. J. Text. I 2017, 108, 2046–2056. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, J.B.; Ding, C.F.; Sun, Y.L.; Lin, Y.N.; Sun, W.Y.; Luo, C.N. Synthesis of surface plasma photocatalyst Ag loaded TiO2 nanowire arrays/graphene oxide coated carbon fiber composites and enhancement of the photocatalytic activity for tetracycline hydrochloride degradation. J. Photoch. Photobio. A 2017, 342, 94–101. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.J.; Wang, W.J.; Chen, J.H.; Cao, Y.X.; Wang, J.; Ji, Z.J. Ternary Ag-deposited TiO2/palygorskite composites with synergistic effect for enhanced photocatalytic activity. Solid State Sci. 2019, 97, 106015. [Google Scholar] [CrossRef]
- Markovic, D.; Vasiljevic, J.; Golja, B.; Tomsic, B.; Simoncic, B.; Radetic, M. Biodegradation of cotton fabric impregnated with TiO2 nanoparticles. J. Serb. Chem. Soc. 2019, 84, 743–755. [Google Scholar] [CrossRef] [Green Version]
- Mihailovic, D.; Saponjic, Z.; Vodnik, V.; Potkonjak, B.; Jovancic, P.; Nedeljkovic, J.M.; Radetic, M. Multifunctional PES fabrics modified with colloidal Ag and TiO2 nanoparticles. Polym. Adv. Technol. 2011, 22, 2244–2249. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. Photo-, bio-, and magneto-active colored polyester fabric with hydrophobic/hydrophilic and enhanced mechanical properties through synthesis of TiO2/Fe3O4/Ag nanocomposite. Ind. Eng. Chem. Res. 2014, 53, 1119–1129. [Google Scholar] [CrossRef]
- Mahdieh, Z.M.; Shekarriz, S.; Taromi, F.A.; Montazer, M. A new method for in situ synthesis of Ag-TiO2 nanocomposite particles on polyester/cellulose fabric by photoreduction and self-cleaning properties. Cellulose 2018, 25, 2355–2366. [Google Scholar] [CrossRef]
- Yu, H.L.; Wu, Q.X.; Wang, J.; Liu, L.Q.; Zheng, B.; Zhang, C.; Shen, Y.G.; Huang, C.L.; Zhou, B.; Jia, J.R. Simple fabrication of the Ag-Ag2O-TiO2 photocatalyst thin films on polyester fabrics by magnetron sputtering and its photocatalytic activity. Appl. Surf. Sci. 2020, 503, in press. [Google Scholar] [CrossRef]
- Shi, H.F.; Yu, Y.C.; Zhang, Y.; Feng, X.J.; Zhao, X.Y.; Tan, H.Q.; Khan, S.U.; Li, Y.G.; Wang, E.B. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl. Catal. B Environ. 2018, 221, 280–289. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M.A. A robust super-paramagnetic TiO2:Fe3O4: Ag nanocomposite with enhanced photo and bio activities on polyester fabric via one step sonosynthesis. Ultrason. Sonochem. 2015, 27, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Manivannan, R.; Heo, G.; Ryu, J.W.; Son, Y.A. Porphyrin dye/TiO2 entrenched in PET to attain self-cleaning property through visible light photocatalytic activity. Res. Chem. Intermediat. 2019, 45, 3655–3671. [Google Scholar] [CrossRef]
- Yu, L.F.; Zhang, S.M.; Zhang, M.; Chen, J.D. Superhydrophobicity construction with dye-sensitised TiO2 on fabric surface for both oil/water separation and water bulk contaminants purification. Appl. Surf. Sci. 2017, 425, 46–55. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H. Preparation of Fe-doped TiO2 nanoparticles immobilized on polyamide fabric. Appl. Surf. Sci. 2012, 258, 10034–10041. [Google Scholar] [CrossRef]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Te. 2018, 15, 2009–2032. [Google Scholar] [CrossRef]
- Hu, L.X.; Wang, F.M.; Xu, G.B. Unique microstructure of kapok fibers in longitudinal microscopic images. Text. Res. J. 2017, 87, 2255–2262. [Google Scholar] [CrossRef]
- Allahyarzadeh, V.; Montazer, M.; Nejad, N.H.; Samadi, N. In situ synthesis of nano silver on polyester using NaOH/Nano TiO2. J. Appl. Polym. Sci. 2013, 129, 892–900. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, H.J.; Mao, N.T. The disappearance of photocatalytic properties of titanium dioxide nanoparticles formed on PET fabrics treated in a simultaneous hydrothermal-dyeing process. J. Text. I 2018, 109, 1510–1520. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Lin, C.P.; Bi, H.J.; Liu, Y.G.; Yan, Q.S. Magnetically separable CuFe2O4/AgBr composite photocatalysts: Preparation, characterization, photocatalytic activity and photocatalytic mechanism under visible light. Appl. Surf. Sci. 2016, 392, 701–707. [Google Scholar] [CrossRef]
- Yang, L.W.; Liang, L.L.; Wang, L.J.; Zhu, J.C.; Gao, S.W.; Xia, X.F. Accelerated photocatalytic oxidation of carbamazepine by a novel 3D hierarchical protonated g-C3N4/BiOBr heterojunction: Performance and mechanism. Appl. Surf. Sci. 2019, 473, 527–539. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Li, X.M.; Zeng, G.M.; Wang, D.B.; Niu, C.G.; Zhao, J.W.; An, H.X.; Xie, T.; Deng, Y.C. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4(040) Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, H.; Wang, Y.Q.; Wu, K.Q.; Huang, H.; Tang, G.G.; Yang, J. Synthesis and characterization of graphene oxide modified AgBr nanocomposites with enhanced photocatalytic activity and stability under visible light. Appl. Surf. Sci. 2014, 319, 306–311. [Google Scholar] [CrossRef]
- Shu, J.X.; Wang, Z.H.; Xia, G.Q.; Zheng, Y.Y.; Yang, L.H.; Zhang, W. One-pot synthesis of AgCl@Ag hybrid photocatalyst with high photocatalytic activity and photostability under visible light and sunlight irradiation. Chem. Eng. J. 2014, 252, 374–381. [Google Scholar] [CrossRef]
- Ma, S.L.; Zhan, S.H.; Jia, Y.N.; Shi, Q.; Zhou, Q.X. Enhanced disinfection application of Ag-modified g-C3N4 composite under visible light. Appl. Catal. B Environ. 2016, 186, 77–87. [Google Scholar] [CrossRef]
- Kotlhao, K.; Mtunzi, F.M.; Pakade, V.; Ejidike, I.P.; Klink, M.J. Synthesis, characterization and evaluation of Ag-TiO2 nanocomposites for photo-catalytic degradation of selected chlorophenols. Dig. J. Nanomater. Bios. 2018, 13, 835–846. [Google Scholar]
- Wu, L.Z.; Yu, Y.; Song, L.; Zhi, J.F. M\TiO2 (M=Au, Ag) transparent aqueous sols and its application on polymeric surface antibacterial post-treatment. J. Colloid Interf. Sci. 2015, 446, 213–217. [Google Scholar] [CrossRef]
- Liu, W.H.; Wei, C.J.; Wang, G.D.; Cao, X.; Tan, Y.X.; Hu, S.Q. In situ synthesis of plasmonic Ag@AgI/TiO2 nanocomposites with enhanced visible photocatalytic performance. Ceram. Int. 2019, 45, 17884–17889. [Google Scholar] [CrossRef]
- Dong, Y.X.; Wang, X.L.; Jin, E.M.; Jeong, S.M.; Jin, B.; Lee, S.H. One-step hydrothermal synthesis of Ag decorated TiO2 nanoparticles for dye-sensitized solar cell application. Renew. Energ. 2019, 135, 1207–1212. [Google Scholar] [CrossRef]
- Meng, S.G.; Sun, W.T.; Zhang, S.J.; Zheng, X.Z.; Fu, X.L.; Chen, S.F. Insight into the transfer mechanism of photogenerated carriers for WO3/TiO2 heterojunction photocatalysts: Is it the transfer of band-band or Z-scheme? Why? J. Phys. Chem. C. 2018, 122, 26326–26336. [Google Scholar] [CrossRef]
- Yi, S.S.; Yan, J.M.; Wulan, B.R.; Li, S.J.; Liu, K.H.; Jiang, Q. Noble-metal-free cobalt phosphide modified carbon nitride: An efficient photocatalyst for hydrogen generation. Appl. Catal. B Environ. 2017, 200, 477–483. [Google Scholar] [CrossRef]
- Shi, H.Y.; Li, N.; Sun, Z.X.; Wang, T.Q.; Xu, L. Interface modification of titanium dioxide nanoparticles by titanium-substituted polyoxometalate doping for improvement of photoconductivity and gas sensing applications. J. Phys. Chem. Solids 2018, 120, 57–63. [Google Scholar] [CrossRef]
- Jiao, J.Q.; Tang, J.G.; Gao, W.; Kuang, D.B.; Tong, Y.X.; Chen, L.P. Plasmonic silver nanoparticles matched with vertically aligned nitrogen-doped titanium dioxide nanotube arrays for enhanced photoelectrochemical activity. J. Power Sources 2015, 274, 464–470. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Ashokkumar, M.; Neppolian, B. Ultrasound assisted synthesis of reduced graphene oxide (rGO) supported InVO4-TiO2 nanocomposite for efficient hydrogen production. Ultrason. Sonochem. 2019, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Sun, Y.J.; Cai, B.; Gan, S.Y.; Han, D.X.; Niu, L.; Wu, T.S. Hierarchically Z-scheme photocatalyst of Ag@AgCl decorated on BiVO4 (040) with enhancing photoelectrochemical and photocatalytic performance. Appl. Catal. B Environ. 2015, 170, 206–214. [Google Scholar] [CrossRef]
- Xie, Z.R.; Tan, H.L.; Wen, X.M.; Suzuki, Y.; Iwase, A.; Kudo, A.; Amal, R.; Scott, J.; Ng, Y.H. The importance of the interfacial contact: Is reduced graphene oxide always an enhancer in photo(electro)catalytic water oxidation? ACS Appl. Mater. Inter. 2019, 11, 23125–23134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, F.; Zhu, H. Immobilization of TiO2 nanoparticles on PET fabric modified with silane coupling agent by low temperature hydrothermal method. Fiber. Polym. 2013, 14, 43–51. [Google Scholar] [CrossRef]
- Maheu, C.; Cardenas, L.; Puzenat, E.; Afanasiev, P.; Geantet, C. UPS and UV spectroscopies combined to position the energy levels of TiO2 anatase and rutile nanopowders. Phys. Chem. Chem. Phys. 2018, 20, 25629–25637. [Google Scholar] [CrossRef]
- Wang, Y.B.; Zhao, X.; Cao, D.; Wang, Y.; Zhu, Y.F. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl. Catal. B Environ. 2017, 211, 79–88. [Google Scholar] [CrossRef]
- Yin, L.X.; Zhang, D.D.; Ma, J.H.; Kong, X.G.; Huang, J.F.; Zhang, H.; Liu, C.Q. Facile synthesis and characterization of ZnS nano_microcrystallites with enhanced photocatalytic activity. Power Technol. 2016, 301, 1085–1091. [Google Scholar] [CrossRef]
- Meng, S.G.; Zhang, J.F.; Chen, S.F.; Zhang, S.J.; Huang, W.X. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Zhang, H.; Han, Y.; Yang, L.M.; Guo, X.L.; Wu, H.L.; Mao, N. Photocatalytic activities of PET filaments deposited with N-doped TiO2 nanoparticles sensitized with disperse blue dyes. Catalysts 2020, 10, 531. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Sheng, C.H.; Ben, D.P.; Wu, H.L.; Mao, N.T. Reactive radical species in photocatalytic activities of PET-Ag-TiO2 nanoparticles composites under visible light irradiation. Submitt. Fiber. Polym. 2020. [Google Scholar]
- Zhang, J.H.; Zhang, L.L.; Zhou, S.Y.; Chen, H.Q.; Zhong, H.; Zhao, Y.J.; Wang, X. Magnetically separable attapulgite-TiO2-FexOy composites with superior activity towards photodegradation of methyl orange under visible light radiation. J. Ind. Eng. Chem. 2014, 20, 3884–3889. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Facile preparation of new nanohybrids for enhancing photocatalytic activity toward removal of organic dyes under visible light irradiation. J. Phys. Chem. Solids. 2020, 140, 109271. [Google Scholar] [CrossRef]
- Ma, L.N.; Wang, G.H.; Jiang, C.J.; Bao, H.L.; Xu, Q.H. Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Yang, G.X.; Yin, H.B.; Liu, W.H.; Yang, Y.P.; Zou, Q.; Luo, L.L.; Li, H.P.; Huo, Y.N.; Li, H.X. Synergistic Ag/TiO2-N photocatalytic system and its enhanced antibacterial activity towards Acinetobacter baumannii. Appl. Catal. B Environ. 2018, 224, 175–182. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, D.; Niu, F.; Wang, S.; Qin, L.S.; Huang, Y.X. Enhanced visible light photocatalytic activity of Gd-doped BiFeO3 nanoparticles and mechanism insight. Sci. Rep. 2016, 6, 26467. [Google Scholar] [CrossRef]
- Norcott, P.L.; Hammill, C.L.; Noble, B.B.; Robertson, J.C.; Olding, A.; Bissember, A.C.; Coote, M.L. EMPO−Me: An electrochemically activated methylating agent. J. Am. Chem. Soc. 2019, 141, 15450–15455. [Google Scholar] [CrossRef]
- Samanta, S.; Khilari, S.; Pradhan, D.; Srivastava, R. An efficient, visible light driven, selective oxidation of aromatic alcohols and amines with O2 using BiVO4/g-C3N4 nanocomposite: A systematic and comprehensive study toward the development of a photocatalytic process. ACS Sustain. Chem. Eng. 2017, 5, 2562–2577. [Google Scholar] [CrossRef]
- Xia, L.; Yang, Y.; Gao, Y.; Liu, B.; Li, X.X.; Chen, X.Y.; Song, H.; Zhang, X.M.; Gao, B.; Fu, J.J. Porous N-doped TiO2 nanotubes arrays by reverse oxidation of TiN and their visible-light photocatalytic activity. Surf. Coat. Tech. 2019, 365, 237–241. [Google Scholar] [CrossRef]
- Stranak, V.; Quaas, M.; Bogdanowicz, R.; Steffen, H.; Wulff, H.; Hubicka, Z.; Tichy, M.; Hippler, R. Effect of nitrogen doping on TiOxNy thin film formation at reactive high-power pulsed magnetron sputtering. J. Phys. D Appl. Phys. 2010, 43, 285203. [Google Scholar] [CrossRef] [Green Version]
- Dong, P.M.; Cheng, X.D.; Jin, Z.; Huang, Z.F.; Nie, X.X.; Wang, X.Y.; Zhang, X.W. The green synthesis of Ag-loaded photocatalyst via DBD cold plasma assisted deposition of Ag nanoparticles on N-doped TiO2 nanotubes. J. Photoch. Photobio. A 2019, 382, 111971. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M.G. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattice Microst. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Fujisawa, J.I.; Muroga, R.; Hanaya, M. Interfacial charge-transfer transitions in a TiO2-benzenedithiol complex with Ti-S-C linkages. Phys. Chem. Chem. Phys. 2015, 17, 29867–29873. [Google Scholar] [CrossRef]












| PET Filaments | Peak | Binding Energy (eV) | FWHM (eV) | Area (Cps•eV) | Relative Sensitivity Factor (RSF) | Atomic Concentration (%) |
|---|---|---|---|---|---|---|
| P–1 | C1s | 285.3 | 5.168 | 5124.6 | 0.278 | 73.02 |
| N1s | 396.7 | 0.242 | 91.5 | 0.477 | 0.74 | |
| O1s | 532.2 | 4.409 | 5227.8 | 0.780 | 24.43 | |
| Ti2p | 459.0 | 3.170 | 957.4 | 2.001 | 1.81 | |
| P–2 | C1s | 282.6 | 2.421 | 6575.5 | 0.278 | 72.97 |
| N1s | 396.1 | 0.313 | 75.1 | 0.477 | 0.47 | |
| O1s | 529.6 | 4.167 | 6577.4 | 0.780 | 23.95 | |
| Ti2p | 456.3 | 2.196 | 1689.3 | 2.001 | 2.49 | |
| Ag3d | 365.6 | 0.386 | 242.4 | 5.987 | 0.12 | |
| TiO2 coated | C1s | 287.0 | 1.924 | 770.8 | 0.278 | 75.02 |
| O1s | 532.0 | 2.134 | 750.2 | 0.780 | 23.95 | |
| Ti2p | 460.1 | 1.795 | 79.4 | 2.001 | 1.03 | |
| Ag-doped TiO2 coated | C1s | 287.2 | 2.020 | 695.4 | 0.278 | 81.82 |
| O1s | 531.8 | 2.187 | 417.9 | 0.780 | 16.13 | |
| Ti2p | 459.8 | 1.795 | 111.2 | 2.001 | 1.73 | |
| Ag3d | 367.2 | 1.410 | 58.6 | 5.987 | 0.32 |
| PET Filaments | τ1 (ns) | B1 (%) | τ2 (ns) | B2 (%) | τaverage (ns) | χ2 |
|---|---|---|---|---|---|---|
| P–1 | 0.96 | 75.19% | 9.01 | 24.81% | 3.06 | 1.159 |
| P–2 | 0.87 | 72.90% | 6.66 | 27.10% | 2.31 | 1.200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Tang, Q.; Li, Q.; Song, Q.; Wu, H.; Mao, N. Enhanced Photocatalytic Properties of PET Filaments Coated with Ag-N Co-Doped TiO2 Nanoparticles Sensitized with Disperse Blue Dyes. Nanomaterials 2020, 10, 987. https://doi.org/10.3390/nano10050987
Zhang H, Tang Q, Li Q, Song Q, Wu H, Mao N. Enhanced Photocatalytic Properties of PET Filaments Coated with Ag-N Co-Doped TiO2 Nanoparticles Sensitized with Disperse Blue Dyes. Nanomaterials. 2020; 10(5):987. https://doi.org/10.3390/nano10050987
Chicago/Turabian StyleZhang, Hui, Qi Tang, Qingshan Li, Qingwen Song, Hailiang Wu, and Ningtao Mao. 2020. "Enhanced Photocatalytic Properties of PET Filaments Coated with Ag-N Co-Doped TiO2 Nanoparticles Sensitized with Disperse Blue Dyes" Nanomaterials 10, no. 5: 987. https://doi.org/10.3390/nano10050987
APA StyleZhang, H., Tang, Q., Li, Q., Song, Q., Wu, H., & Mao, N. (2020). Enhanced Photocatalytic Properties of PET Filaments Coated with Ag-N Co-Doped TiO2 Nanoparticles Sensitized with Disperse Blue Dyes. Nanomaterials, 10(5), 987. https://doi.org/10.3390/nano10050987