Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetic Nanoparticles (MNPs)
2.2. Characterization of MF66-MNPs
2.3. Cell Culture
2.4. Murine Xenograft Model, Tumor Implantation and In Vivo Magnetic Hyperthermia
2.5. In Vivo Effects of Magnetic Hyperthermia on Tumor Volume, Temperature Distribution in Tumor Tissue and Blood Count
2.6. MNPs Distribution In Vivo
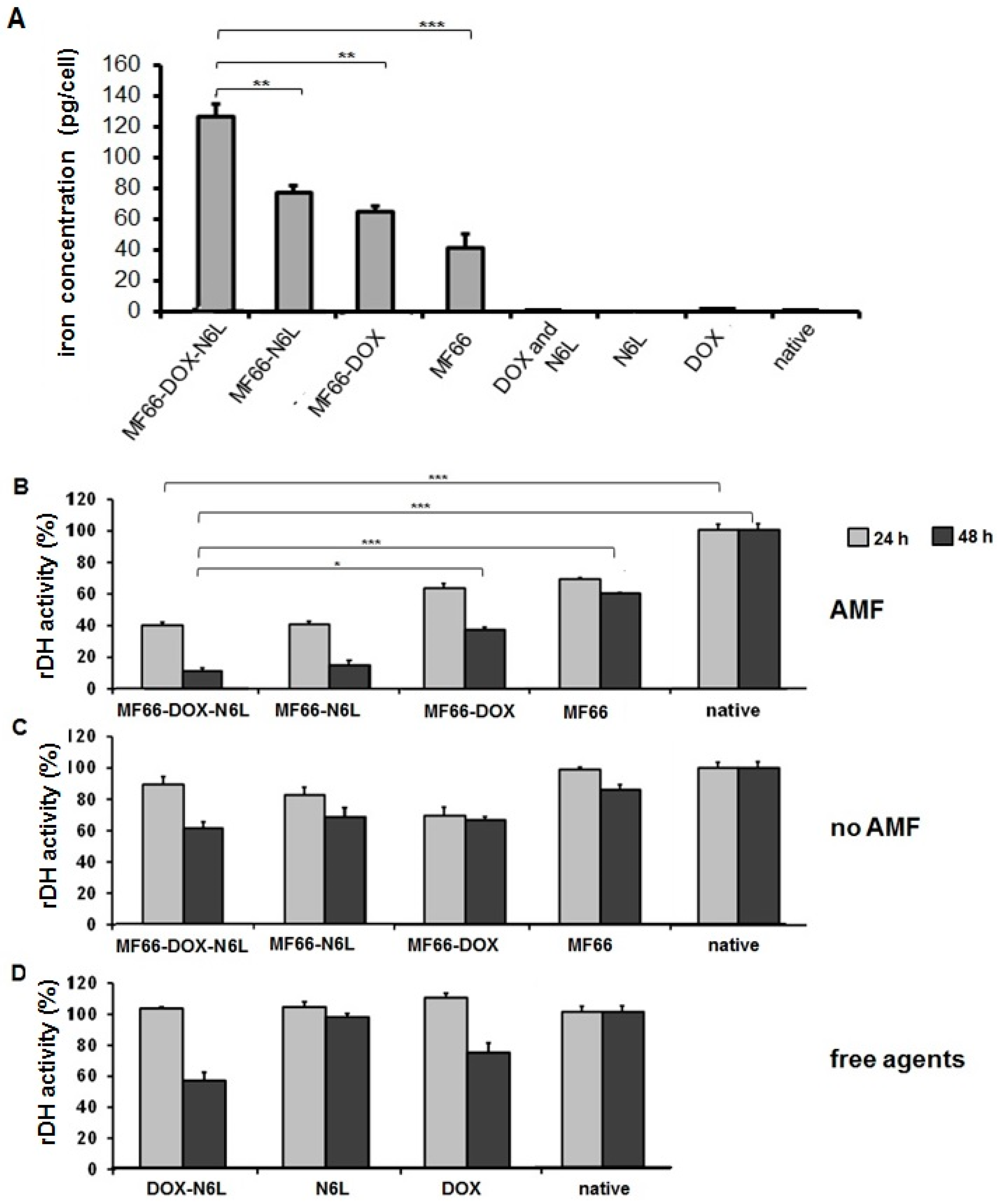
2.7. Determination of Cellular Uptake of MNP Formulations
2.8. In Vitro Magnetic Hyperthermia
2.9. Analysis of Cell Viability
2.10. Microscopy
2.11. Prussian Blue Staining for Iron Detection in Cells in Vitro
2.12. Statistics
3. Results
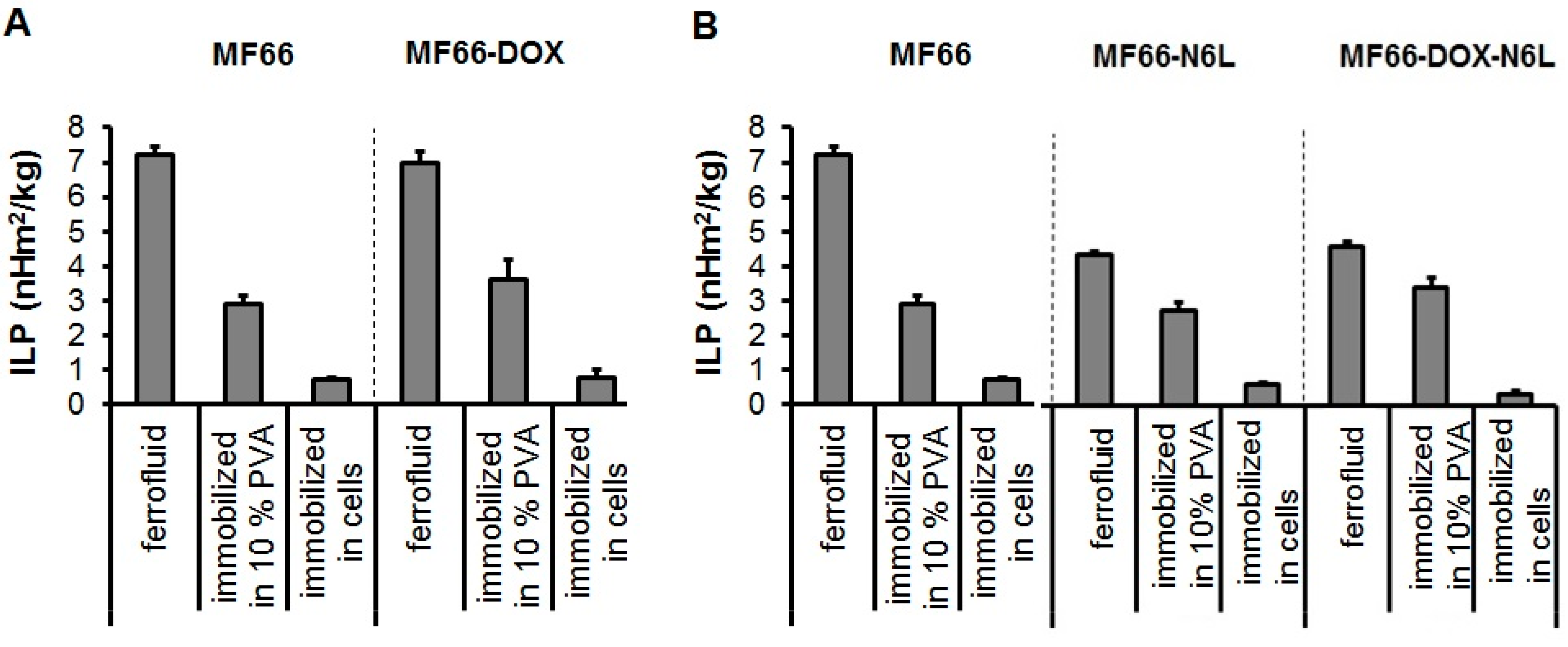
3.1. MF66-MNP Formulations Show Good Heating Potential
3.2. DOX-Functionalized MNPs with Magnetic Hyperthermia Cause Tumor Regression in a BT474 Xenograft Model
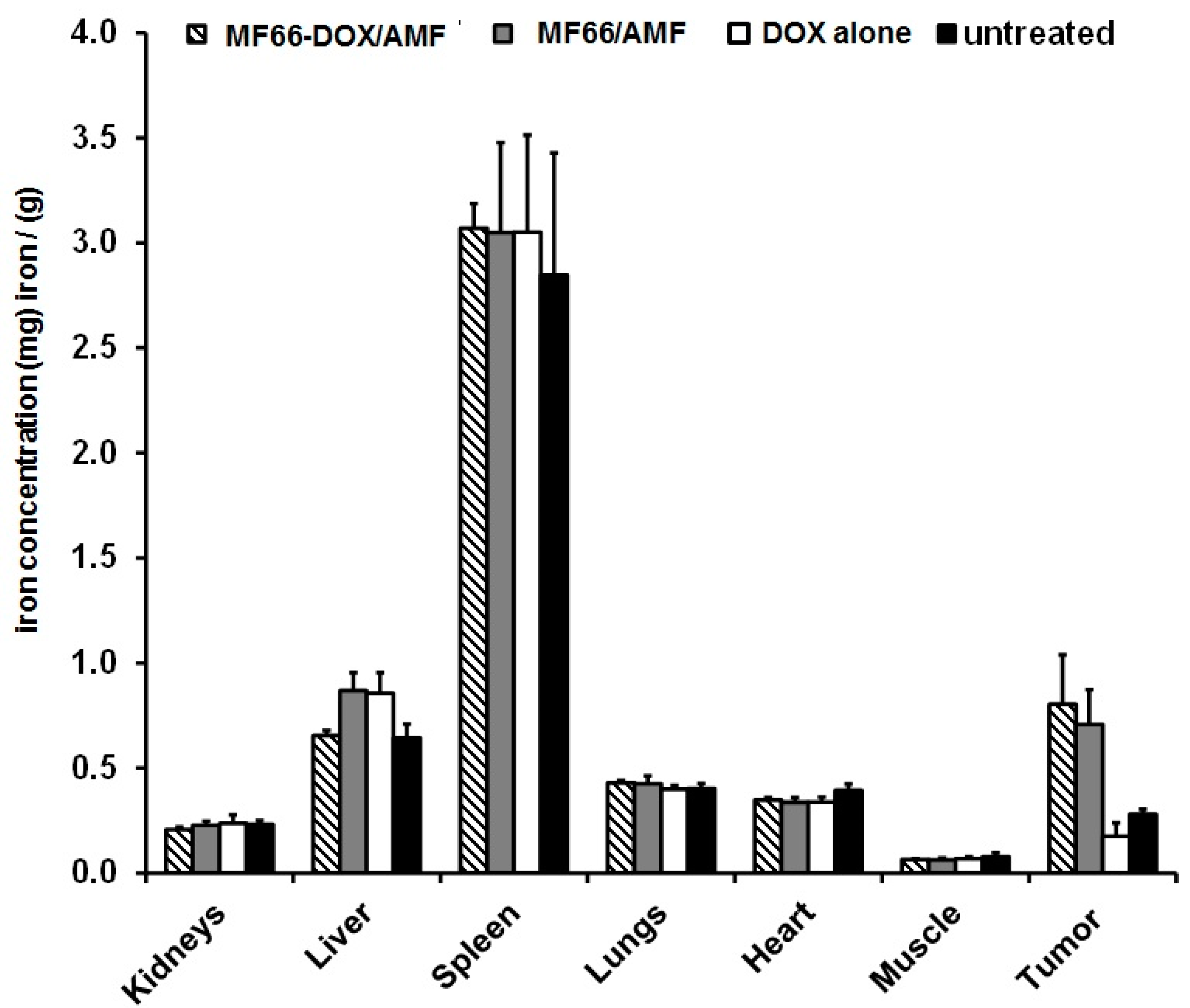
3.3. When Functionalized to MNPs, DOX Does not Exert any Transient Effect on Blood Cells and the Intratumorally Administered MNP Formulations Remained in the BT474 Tumors
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ibrahim, M.K.; Taghour, M.S.; Metwaly, A.M.; Belal, A.; Mehany, A.B.M.; Elhendawy, M.A.; Radwan, M.M.; Yassin, A.M.; El-Deeb, N.M.; Hafez, E.E.; et al. Design, synthesis, molecular modeling and anti-proliferative evaluation of novel quinoxaline derivatives as potential DNA intercalators and topoisomerase II inhibitors. Eur. J. Med. Chem. 2018, 155, 117–134. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silva, J.; Miranda, S.E.M.; Leite, E.A.; de Paula Sabino, A.; Borges, K.B.G.; Cardoso, V.N.; Cassali, G.D.; Guimaraes, A.G.; Oliveira, M.C.; de Barros, A.L.B. Toxicological study of a new doxorubicin-loaded pH-sensitive liposome: A preclinical approach. Toxicol. Appl. Pharmacol. 2018, 352, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Swift, L.P.; Rephaeli, A.; Nudelman, A.; Phillips, D.R.; Cutts, S.M. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 2006, 66, 4863–4871. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Huun, J.; Lonning, P.E.; Knappskog, S. Effects of concomitant inactivation of p53 and pRb on response to doxorubicin treatment in breast cancer cell lines. Cell Death Discov. 2017, 3, 17026. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, Y.S.; Kim, D.K. Doxorubicin exerts cytotoxic effects through cell cycle arrest and Fas-mediated cell death. Pharmacology 2009, 84, 300–309. [Google Scholar] [CrossRef]
- Kabel, A.M.; Alzahrani, A.A.; Bawazir, N.M.; Khawtani, R.O.; Arab, H.H. Targeting the proinflammatory cytokines, oxidative stress, apoptosis and TGF-beta1/STAT-3 signaling by irbesartan to ameliorate doxorubicin-induced hepatotoxicity. J. Infect. Chemother. 2018, 24, 623–631. [Google Scholar] [CrossRef]
- Kratz, F.; Ehling, G.; Kauffmann, H.M.; Unger, C. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin-binding prodrug of the anticancer agent doxorubicin. Hum. Exp. Toxicol. 2007, 26, 19–35. [Google Scholar] [CrossRef]
- Bielack, S.S.; Erttmann, R.; Winkler, K.; Landbeck, G. Doxorubicin: Effect of different schedules on toxicity and anti-tumor efficacy. Eur. J. Cancer Clin. Oncol. 1989, 25, 873–882. [Google Scholar] [CrossRef]
- Hu, J.; Xie, L.; Zhao, W.; Sun, M.; Liu, X.; Gao, W. Design of tumor-homing and pH-responsive polypeptide-doxorubicin nanoparticles with enhanced anticancer efficacy and reduced side effects. Chem. Commum. 2015, 51, 11405–11408. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Bahadur, D. Doxorubicin-loaded dendritic-Fe3O4 supramolecular nanoparticles for magnetic drug targeting and tumor regression in spheroid murine melanoma model. Nanomedicine 2018, 14, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, Y.; Zhou, M.; Wang, W.; Shi, M.; Xing, M.; Liao, W. Theranostic pH-sensitive nanoparticles for highly efficient targeted delivery of doxorubicin for breast tumor treatment. Int. J. Nanomed. 2018, 13, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I. In vivo applications of magnetic nanoparticle hyperthermia. Int. J. Hyperth. 2013, 29, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Kaiser, W.A. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine (London) 2012, 7, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperth. 2003, 19, 252–266. [Google Scholar] [CrossRef]
- Lepock, J.R. Involvement of membranes in cellular responses to hyperthermia. Radiat Res. 1982, 92, 433–438. [Google Scholar] [CrossRef]
- Coss, R.A.; Linnemans, W.A. The effects of hyperthermia on the cytoskeleton: A review. Int. J. Hyperth. 1996, 12, 173–196. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Taratula, O.; Dani, R.K.; Schumann, C.; Xu, H.; Wang, A.; Song, H.; Dhagat, P.; Taratula, O. Multifunctional nanomedicine platform for concurrent delivery of chemotherapeutic drugs and mild hyperthermia to ovarian cancer cells. Int. J. Pharm. 2013, 458, 169–180. [Google Scholar] [CrossRef]
- Le, T.T.H.; Bui, T.Q.; Ha, T.M.T.; Le, M.H.; Pham, H.N.; Ha, P.T. Optimizing the alginate coating layer of doxorubicin-loaded iron oxide nanoparticles for cancer hyperthermia and chemotherapy. J. Mater. Sci. 2018, 53, 13826–13842. [Google Scholar] [CrossRef]
- Destouches, D.; Page, N.; Hamma-Kourbali, Y.; Machi, V.; Chaloin, O.; Frechault, S.; Birmpas, C.; Katsoris, P.; Beyrath, J.; Albanese, P.; et al. A Simple Approach to Cancer Therapy Afforded by Multivalent Pseudopeptides that Target Cell-Surface Nucleoproteins. Cancer Res. 2011, 71, 3296–3305. [Google Scholar] [CrossRef] [PubMed]
- Destouches, D.; Huet, E.; Sader, M.; Frechault, S.; Carpentier, G.; Ayoul, F.; Briand, J.-P.; Menashi, S.; Courty, J. Multivalent pseudopeptides targeting cell surface nucleoproteins inhibit cancer cell invasion through tissue inhibitor of metalloproteinases 3 (TIMP-3) release. J. Biol. Chem. 2012, 287, 43685–43693. [Google Scholar] [CrossRef] [PubMed]
- Destouches, D.; El Khoury, D.; Hamma-Kourbali, Y.; Krust, B.; Albanese, P.; Katsoris, P.; Guichard, G.; Briand, J.-P.; Courty, J.; Hovanessian, A.G. Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin. PLoS ONE 2008, 3, e2518. [Google Scholar]
- Dhez, A.C.; Benedetti, E.; Antonosante, A.; Panella, G.; Ranieri, B.; Florio, T.M.; Cristiano, L.; Angelucci, F.; Giansanti, F.; Di Leandro, L.; et al. Targeted therapy of human glioblastoma via delivery of a toxin through a peptide directed to cell surface nucleolin. J. Cell Physiol. 2018, 233, 4091–4105. [Google Scholar]
- Benedetti, E.; Antonosante, A.; d’Angelo, M.; Cristiano, L.; Galzio, R.; Destouches, D.; Florio, T.M.; Dhez, A.C.; Astarita, C.; Cinque, B.; et al. Nucleolin antagonist triggers autophagic cell death in human glioblastoma primary cells and decreased in vivo tumor growth in orthotopic brain tumor model. Oncotarget 2015, 6, 42091–42104. [Google Scholar]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dahring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015, 17, 66. [Google Scholar] [CrossRef]
- Latorre, A.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Somoza, A. Multifunctionalization of magnetic nanoparticles for controlled drug release: A general approach. Eur. J. Med. Chem. 2014, 82, 355–362. [Google Scholar] [CrossRef]
- Kossatz, S.; Ludwig, R.; Dahring, H.; Ettelt, V.; Rimkus, G.; Marciello, M.; Salas, G.; Patel, V.; Teran, F.J.; Hilger, I. High Therapeutic Efficiency of Magnetic Hyperthermia in Xenograft Models Achieved with Moderate Temperature Dosages in the Tumor Area. Pharm. Res. 2014, 31, 3274–3288. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Janko, C.; Poettler, M.; Tripal, P.; Zaloga, J.; Cicha, I.; Durr, S.; Nowak, J.; Odenbach, S.; Slabu, I.; et al. Flow cytometry for intracellular SPION quantification: Specificity and sensitivity in comparison with spectroscopic methods. Int. J. Nanomed. 2015, 10, 4185–4201. [Google Scholar] [CrossRef]
- Bandekar, A.; Karve, S.; Chang, M.Y.; Mu, Q.; Rotolo, J.; Sofou, S. Antitumor efficacy following the intracellular and interstitial release of liposomal doxorubicin. Biomaterials 2012, 33, 4345–4352. [Google Scholar] [CrossRef]
- Stapf, M.; Teichgraber, U.; Hilger, I. Methotrexate-coupled nanoparticles and magnetic nanochemothermia for the relapse-free treatment of T24 bladder tumors. Int. J. Nanomed. 2017, 12, 2793–2811. [Google Scholar] [CrossRef] [PubMed]
- Valeriote, F.A. The use of cell kinetics in the development of drug combinations. Pharmacol. Ther. 1979, 4, 1–33. [Google Scholar] [CrossRef]
- Ludwig, R.; Teran, F.J.; Teichgraeber, U.; Hilger, I. Nanoparticle-based hyperthermia distinctly impacts production of ROS, expression of Ki-67, TOP2A, and TPX2, and induction of apoptosis in pancreatic cancer. Int. J. Nanomed. 2017, 12, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Dikomey, E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int. J. Radiat. Biol. 2001, 77, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Moorthy, M.S.; Manivasagan, P.; Bharathiraja, S.; Oh, J. Magnetic hyperthermia and pH-responsive effective drug delivery to the sub-cellular level of human breast cancer cells by modified CoFe2O4 nanoparticles. Biochimie 2017, 133, 7–19. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Nguyen Tran, D.H.; Bach, L.G.; Vu-Quang, H.; Nguyen, D.C.; Park, K.D.; Nguyen, D.H. Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery. Pharmaceutics 2019, 11, 120. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Sader, M.; Couleaud, P.; Carpentier, G.; Gilles, M.; Bousserrhine, N.; Livet, A.; Cascone, I.; Destouches, D.; Cortajarena, A.L.; Courty, J. Functionalization of Iron Oxide Magnetic Nanoparticles with the Multivalent Pseudopeptide N6l for Breast Tumor Targeting. Nanomed. Nanotechnol. 2015, 6, 1–8. [Google Scholar]
- Bracci, L.; Mandarini, E.; Brunetti, J.; Depau, L.; Pini, A.; Terzuoli, L.; Scali, S.; Falciani, C. The GAG-specific branched peptide NT4 reduces angiogenesis and invasiveness of tumor cells. PLoS ONE 2018, 13, e0194744. [Google Scholar] [CrossRef]
- Mendoza-Rodriguez, C.A.; Cerbon, M.A. Tumor suppressor gene p53: Mechanisms of action in cell proliferation and death. Rev. Investig. Clin. 2001, 53, 266–273. [Google Scholar]
- Sharpless, N.E.; DePinho, R.A. p53: Good cop/bad cop. Cell 2002, 110, 9–12. [Google Scholar] [CrossRef]
- Bouchard, V.J.; Rouleau, M.; Poirier, G.G. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 2003, 31, 446–454. [Google Scholar] [CrossRef]
- Savitskaya, M.A.; Onishchenko, G.E. Mechanisms of Apoptosis. Biochemistry (Moscow) 2015, 80, 1393–1405. [Google Scholar] [CrossRef]
- Morris, G.; Walker, A.J.; Berk, M.; Maes, M.; Puri, B.K. Cell Death Pathways: A Novel Therapeutic Approach for Neuroscientists. Mol. Neurobiol. 2018, 55, 5767–5786. [Google Scholar] [CrossRef]
- Scarlatti, F.; Maffei, R.; Beau, I.; Codogno, P.; Ghidoni, R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008, 15, 1318. [Google Scholar] [CrossRef]
- Ling, Y.H.; el-Naggar, A.K.; Priebe, W.; Perez-Soler, R. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol. Pharmacol. 1996, 49, 832–841. [Google Scholar]
- Aytac, U.; Sato, K.; Yamochi, T.; Yamochi, T.; Ohnuma, K.; Mills, G.B.; Morimoto, C.; Dang, N.H. Effect of CD26/dipeptidyl peptidase IV on Jurkat sensitivity to G2/M arrest induced by topoisomerase II inhibitors. Br. J. Cancer 2003, 88, 455–462. [Google Scholar] [CrossRef][Green Version]
- Villamarin, S.; Ferrer-Miralles, N.; Mansilla, S.; Priebe, W.; Portugal, J. Induction of G(2)/M arrest and inhibition of c-myc and p53 transcription by WP631 in Jurkat T lymphocytes. Biochem. Pharmacol. 2002, 63, 1251–1258. [Google Scholar] [CrossRef]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef]
- Cui, P.F.; Zhuang, W.R.; Hu, X.; Xing, L.; Yu, R.Y.; Qiao, J.B.; He, Y.-J.; Li, F.; Ling, D.; Jiang, H.-L. A new strategy for hydrophobic drug delivery using a hydrophilic polymer equipped with stacking units. Chem. Commun. 2018, 54, 8218–8221. [Google Scholar] [CrossRef]
- Parker, M.A.; King, V.; Howard, K.P. Nuclear magnetic resonance study of doxorubicin binding to cardiolipin containing magnetically oriented phospholipid bilayers. Biochim. Biophys. Acta 2001, 1514, 206–216. [Google Scholar] [CrossRef]
- Speelmans, G.; Staffhorst, R.W.; De Wolf, F.A.; De Kruijff, B. Verapamil competes with doxorubicin for binding to anionic phospholipids resulting in increased internal concentrations and rates of passive transport of doxorubicin. Biochim. Biophys. Acta 1995, 1238, 137–146. [Google Scholar] [CrossRef][Green Version]
- Mailander, V.; Landfester, K. Interaction of nanoparticles with cells. Biomacromolecules 2009, 10, 2379–2400. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Noh, M.S.; Woo, M.; Kim, B.G.; Lee, K.H.; Sohn, B.H.; Park, S.B.; Lee, J.K.; et al. Cellular uptake of magnetic nanoparticle is mediated through energy-dependent endocytosis in A549 cells. J. Vet. Sci. 2006, 7, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Sanhaji, M.; Goring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Prina-Mello, A.; Stapf, M.; Ludwig, R.; Volkov, Y.; et al. The phenotype of target pancreatic cancer cells influences cell death by magnetic hyperthermia with nanoparticles carrying gemicitabine and the pseudo-peptide NucAnt. Nanomedicine 2019, 20, 101983. [Google Scholar] [CrossRef]



| MF66 | MF66-DOX | MF66-N6L | MF66-DOX-N6L | |
|---|---|---|---|---|
| Core size (nm) | 11.7 | 11.7 | 11.7 | 11.7 |
| Coating | DMSA | DMSA | DMSA | DMSA |
| Functionalization (per g iron) | none | 40 µmol DOX | 3.5 µmol N6L | 40 µmol DOX 3.5 µmol N6L |
| Hydrodynamic diameter (nm) | 89 ± 0.5 | 116 ± 0.6 | 115 ± 0.6 | 143 ± 3.8 |
| PDI | 0.36 | 0.39 | 0.23 | 0.29 |
| Zeta potential (mV) | −46 ± 0.7 | −37 ± 0.3 | −40 ± 0.6 | −33 ± 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, Á.; et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials 2020, 10, 1016. https://doi.org/10.3390/nano10061016
Piehler S, Dähring H, Grandke J, Göring J, Couleaud P, Aires A, Cortajarena AL, Courty J, Latorre A, Somoza Á, et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials. 2020; 10(6):1016. https://doi.org/10.3390/nano10061016
Chicago/Turabian StylePiehler, Susann, Heidi Dähring, Julia Grandke, Julia Göring, Pierre Couleaud, Antonio Aires, Aitziber L. Cortajarena, José Courty, Alfonso Latorre, Álvaro Somoza, and et al. 2020. "Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives" Nanomaterials 10, no. 6: 1016. https://doi.org/10.3390/nano10061016
APA StylePiehler, S., Dähring, H., Grandke, J., Göring, J., Couleaud, P., Aires, A., Cortajarena, A. L., Courty, J., Latorre, A., Somoza, Á., Teichgräber, U., & Hilger, I. (2020). Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials, 10(6), 1016. https://doi.org/10.3390/nano10061016






