Dispersibility and Size Control of Silver Nanoparticles with Anti-Algal Potential Based on Coupling Effects of Polyvinylpyrrolidone and Sodium Tripolyphosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AgNPs
2.3. Characterization of AgNPs
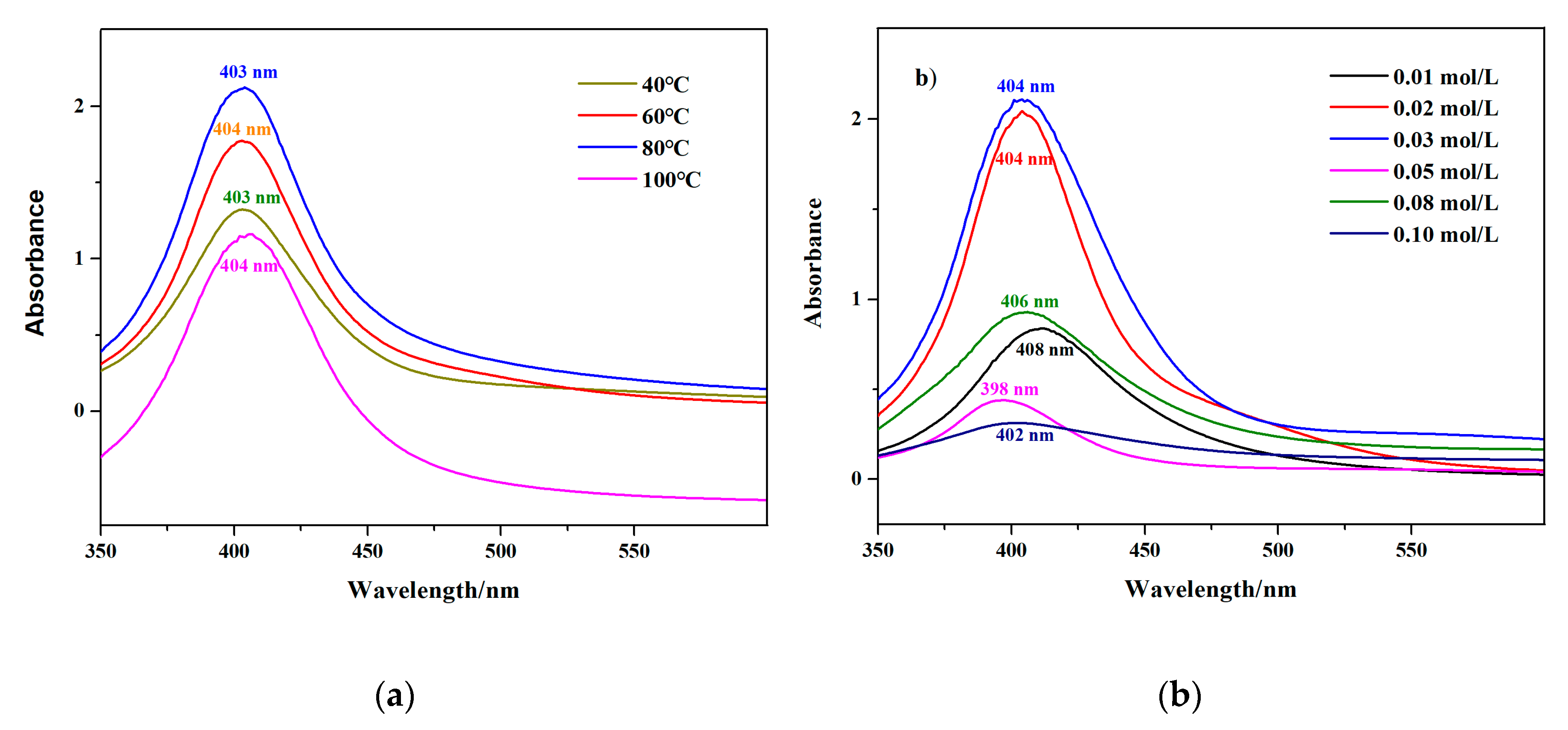
2.3.1. Ultraviolet-Visible (UV-Vis) Spectroscopy
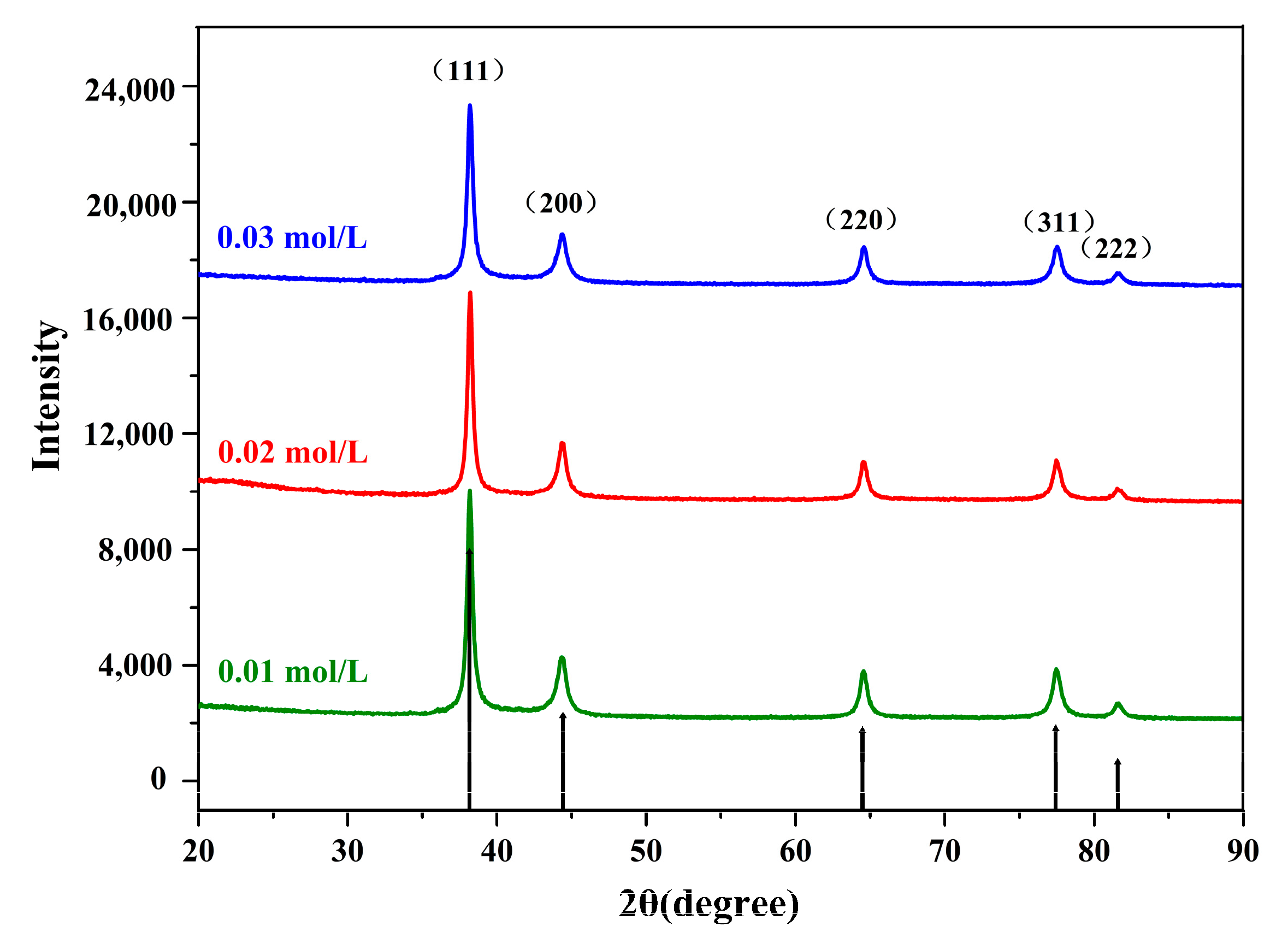
2.3.2. X-Ray Diffraction (XRD)
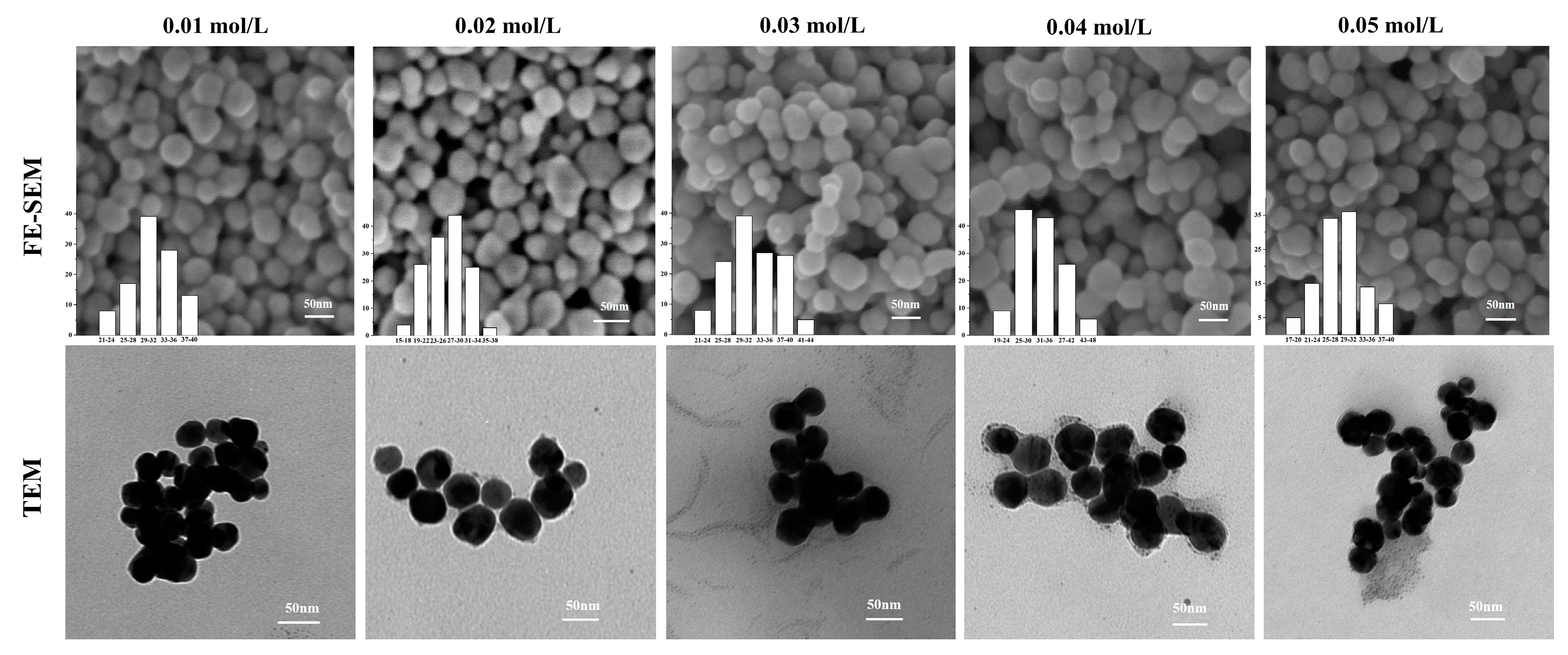
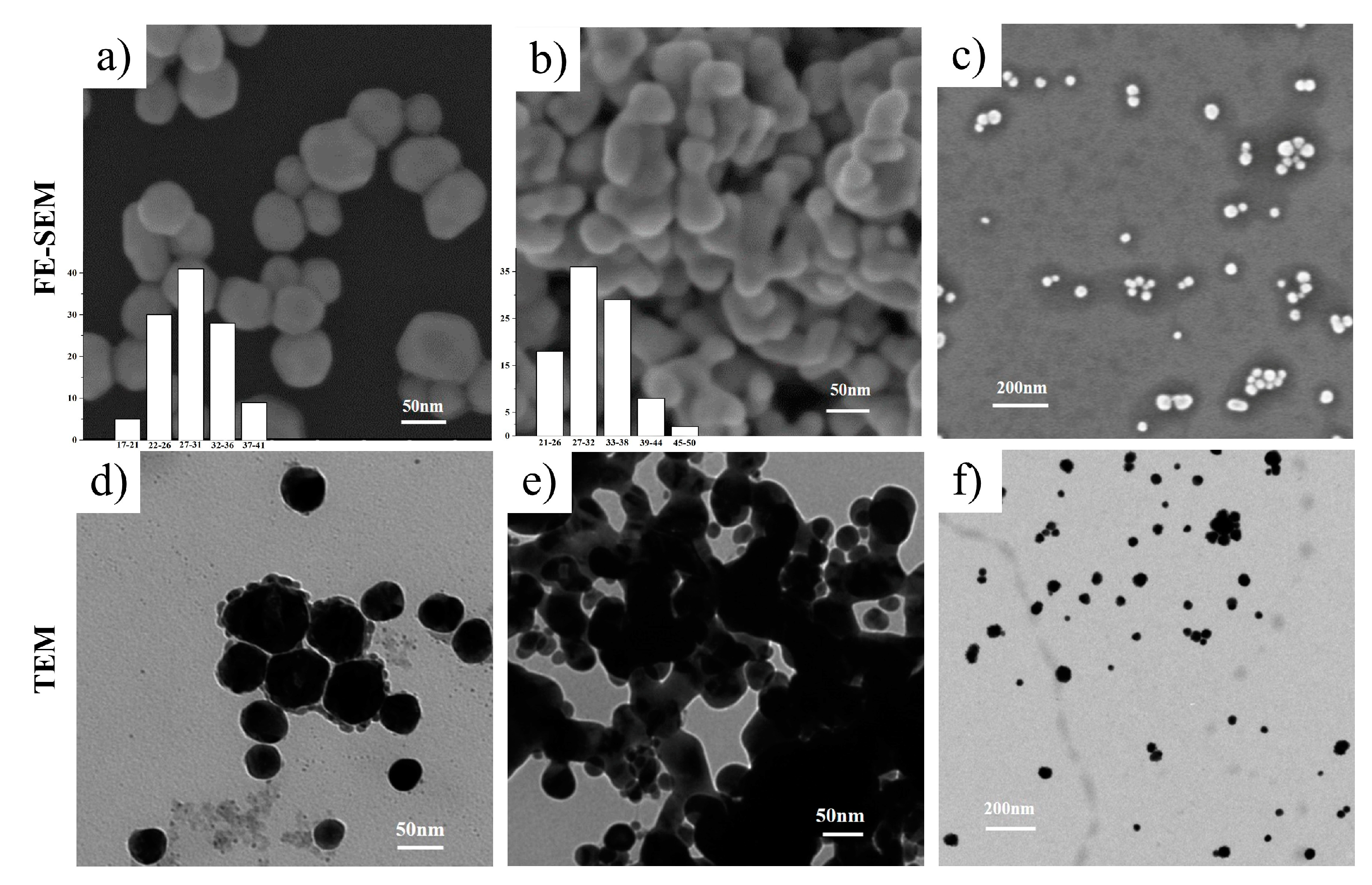
2.3.3. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. Zeta Potential
2.3.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Anti-Algal Activity of AgNPs
2.4.1. Algae Cultivation
2.4.2. Photosynthetic Pigment Measurement
3. Results and Discussion
3.1. Synthesis and Parameter Control
3.1.1. Individual Protective Agents versus Combined Protective Agents
3.1.2. Effects of Temperature and Silver Nitrate Concentration
3.2. Structural Characterization
3.2.1. XRD Analysis
3.2.2. FE-SEM and TEM Analysis
3.2.3. Zeta Potential
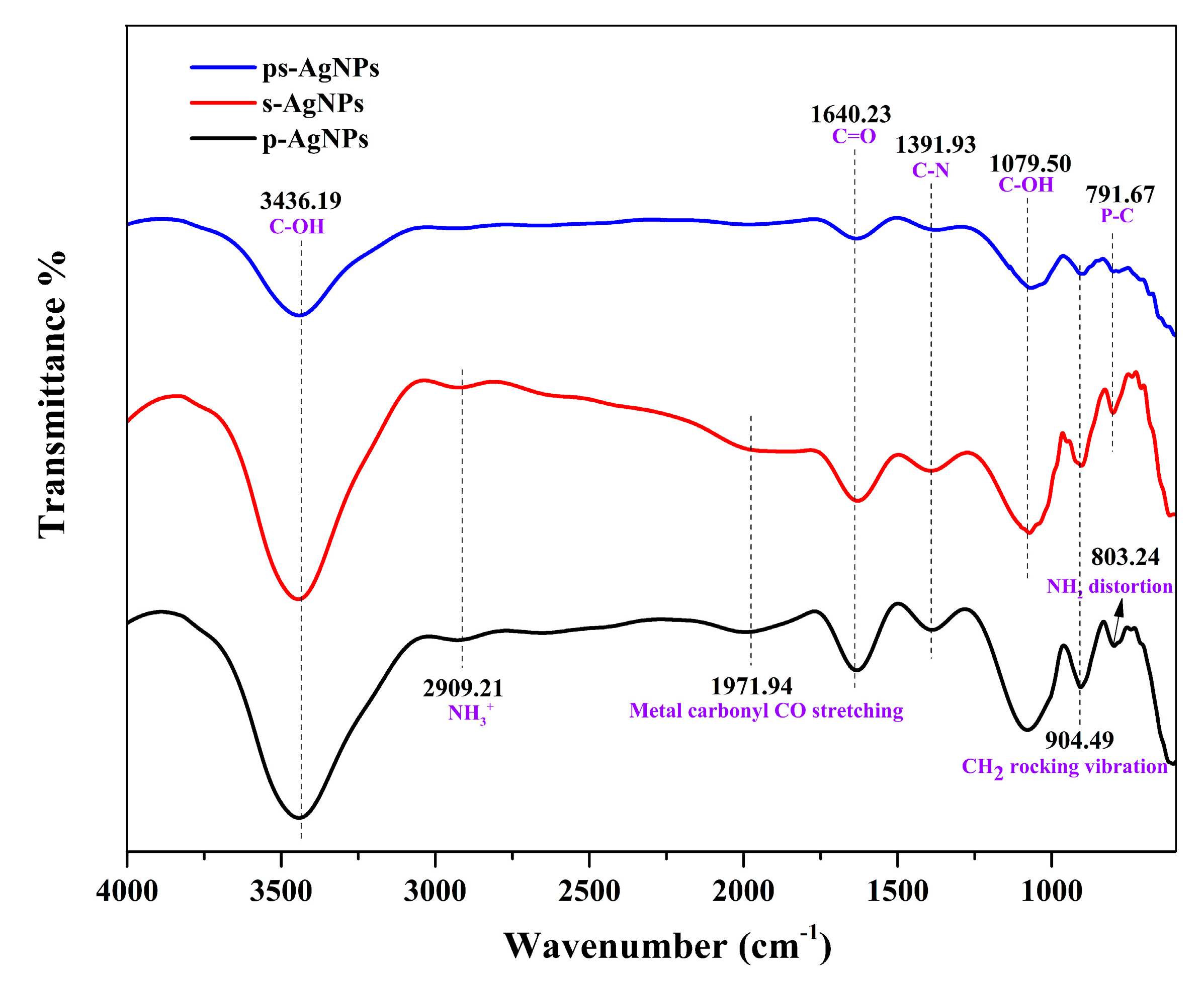
3.2.4. FTIR Analysis
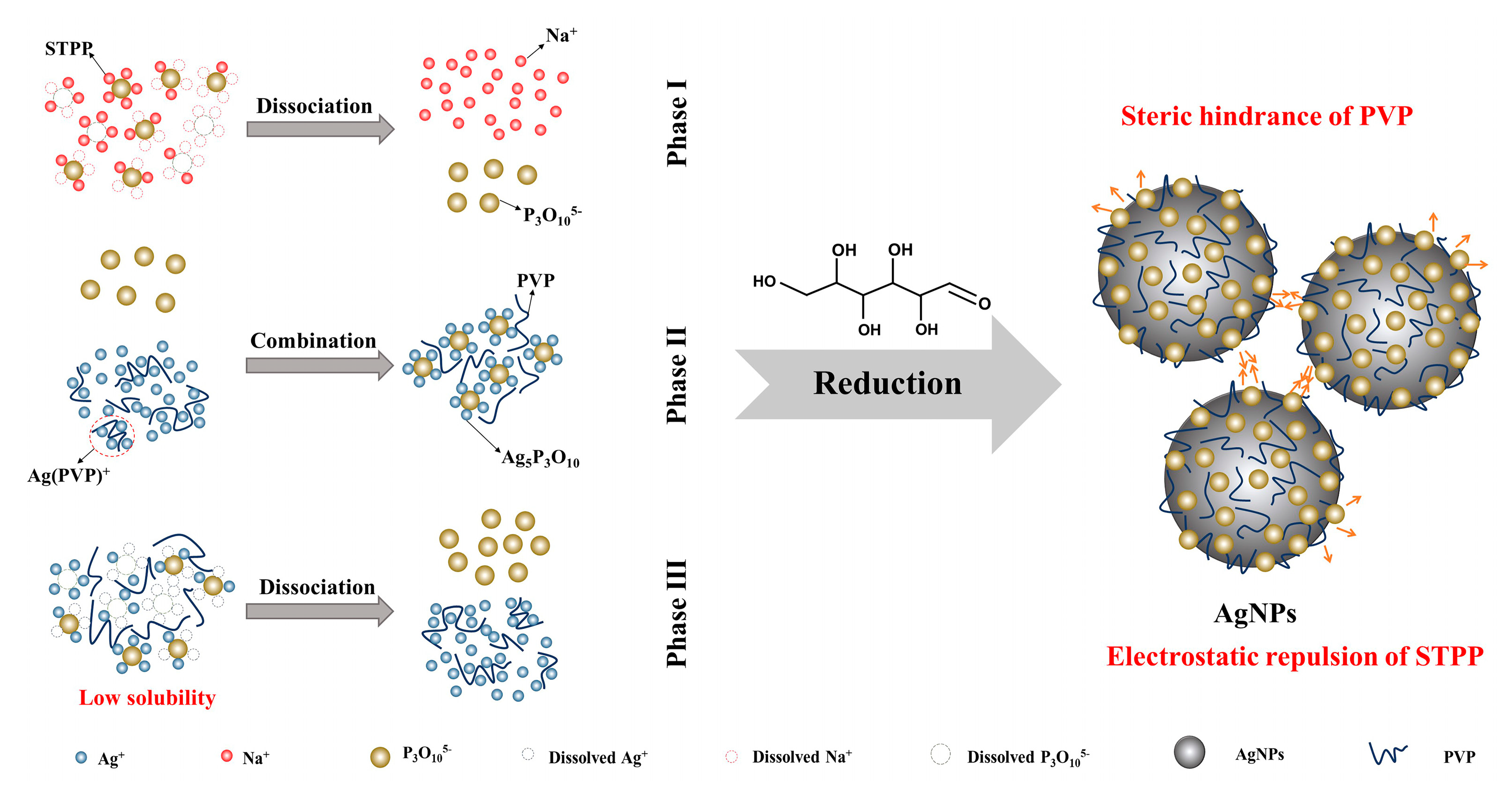
3.3. Formation Mechanism
3.4. Anti-Algal Activity of AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.Y.; Huang, M.T.; Pender, S.L.F.; Ruslin, M.; Chou, H.H.; Qu, K.L. The application of silver nano-particles on developing potential treatment for chronic rhinosinusitis: Antibacterial action and cytotoxicity effect on human nasal epithelial cell model. Mater. Sci. Eng. C 2017, 80, 624–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Han, J.W.; Kwon, D.N.; Kim, J.H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; He, D.; Gao, S.; Wang, D.; Yokoyama, K.; Wang, L. Biosynthesis of silver nanoparticles by the fungus Arthroderma Fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016, 11, 1899–1906. [Google Scholar]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of silver nanoparticles on growth of eukaryotic green algae. Nano-Micro Lett. 2012, 4, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Barsainya, M.; Singh, D.P. Biogenic synthesis of silver nanoparticle by using secondary metabolites from Pseudomonas aeruginosa DM1 and its anti-algal effect on Chlorella vulgaris and Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. Int. 2017, 24, 4645–4654. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, Y.; Li, M.; Liu, J. Surfactantless synthesis and the surface-enhanced Raman spectra and catalytic activity of differently shaped silver nanomaterials. Eur. J. Inorg. Chem. 2010, 12, 1806–1812. [Google Scholar] [CrossRef]
- Duan, C.; Wang, H.; Ou, X.; Li, F.; Zhang, X. Efficient visible light photocatalyst fabricated by depositing plasmonic Ag nanoparticles on conductive polymer-protected Si nanowire arrays for photoelectrochemical hydrogen generation. ACS Appl. Mater. Inter. 2014, 6, 9742–9750. [Google Scholar] [CrossRef]
- Yu, A.; Wang, Q.; Wang, J.; Chang, C. Rapid synthesis of colloidal silver triangular nanoprisms and their promotion of TiO2 photocatalysis on methylene blue under visible light. Catal. Commun. 2017, 90, 75–78. [Google Scholar] [CrossRef]
- Akaighe, N.; Maccuspie, R.I.; Navarro, D.A.; Aga, D.S.; Banerjee, S.; Sohn, M.; Sharma, V.K. Humic acid-induced silver nanoparticle formation under environmentally relevant conditions. Environ. Sci. Technol. 2011, 45, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Lv, L.; Wang, H. A facile approach to synthesize silver nanorods capped with sodium tripolyphosphate. Mater. Lett. 2009, 63, 2698–2700. [Google Scholar] [CrossRef]
- Sangaonkar, G.M.; Pawar, K.D. Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloid. Surf. B 2018, 164, 210–217. [Google Scholar] [CrossRef]
- Jeon, S.H.; Xu, P.; Zhang, B.; Mack, N.H.; Tsai, H.; Chiang, L.Y.; Wang, H.L. Polymer-assisted preparation of metal nanoparticles with controlled size and morphology. J. Mater. Chem. 2011, 21, 2550–2554. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J.; Walker, H.W. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef]
- Huynh, K.A.; Chen, K.L. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ. Sci. Technol. 2011, 45, 5564–5571. [Google Scholar] [CrossRef] [Green Version]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Tejamaya, M.; Romer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Stankus, D.P.; Lohse, S.E.; Hutchison, J.E.; Nason, J.A. Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ. Sci. Technol. 2011, 45, 3238–3244. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Dorantes-Aranda, J.J.; Waite, T.D. Silver nanoparticle-algae interactions: Oxidative dissolution, reactive oxygen species generation and synergistic toxic effects. Environ. Sci. Technol. 2012, 46, 8731–8738. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Oukarroum, A. Silver nanoparticles toxicity effect on photosystem II photochemistry of the green alga Chlamydomonas Reinhardtii treated in light and dark conditions. Toxicol. Environ. Chem. 2012, 94, 1536–1546. [Google Scholar]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts. Plant. Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kvitek, L.; Panacek, A.; Soukupova, J.; Kolar, M.; Vecerova, R.; Prucek, R.; Holecova, M.; Zboril, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Zook, J.M.; Halter, M.D.; Cleveland, D.; Long, S.E. Disentangling the effects of polymer coatings on silver nanoparticle agglomeration, dissolution, and toxicity to determine mechanisms of nanotoxicity. J. Nanopart. Res. 2012, 14, 1165–1173. [Google Scholar] [CrossRef]
- Arif, S.; Batool, A.; Khalid, N.; Ahmed, I.; Janjua, H.A. Comparative analysis of stability and biological activities of violacein and starch capped silver nanoparticles. RSC Adv. 2017, 7, 4468–4478. [Google Scholar] [CrossRef] [Green Version]
- Hebeish, A.A.; El-Rafie, M.H.; Abdel-Mohdy, F.A.; Abdel-Halim, E.S.; Emam, H.E. Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles. Carbohyd. Polym. 2010, 82, 933–941. [Google Scholar] [CrossRef]
- Dai, Y.; Deng, T.; Jia, S.; Jin, L.; Lu, F. Preparation and characterization of fine silver powder with colloidal emulsion aphrons. J. Membr. Sci. 2006, 281, 685–691. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloid. Surf. A 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Ahila, N.K.; Ramkumar, V.S.; Prakash, S.; Manikandan, B.; Ravindran, J.; Dhanalakshmi, P.K.; Kannapiran, E. Synthesis of stable nanosilver particles (AgNPs) by the proteins of seagrass Syringodium isoetifolium and its biomedicinal properties. Biomed. Pharmacother. 2016, 84, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Sharma, S. Green synthesis of silver nanoparticles using extracts of Aananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Handa, T.; Kasai, A.; Takenaka, H.; Lin, S.Y.; Ando, Y. Novel method for the preparation of controlled-release theophylline granules coated with a polyelectrolyte complex of sodium polyphosphate-chitosan. J. Pharm. Sci. 1985, 74, 264–268. [Google Scholar] [CrossRef]
- Weng, S. Fourier Transform Infrared Spectrum Analysis, 2nd ed.; Chemical Industry Press: Beijing, China, 2010; pp. 377–388. [Google Scholar]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhao, B.; Hu, L. PVP protective mechanism of ultrafine silver powder synthesized by chemical reduction processes. J. Solid State Chem. 1996, 112, 105–110. [Google Scholar] [CrossRef]
- Carotenuto, G.; Pepe, G.P.; Nicolais, L. Preparation and characterization of nano-sized Ag/PVP composites for optical applications. Eur. Phys. J. B 2000, 16, 11–17. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Deng, B. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, B.; Zhang, H.; He, Y.; Ong, C.N.; Yang, J. Metabolites change of Scenedesmus Obliquus exerted by AgNPs. J. Environ. Sci. 2019, 76, 310–318. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Legros, S.; von der Kammer, F.; Hofmann, T. Natural organic matter concentration and hydrochemistry influence aggregation kinetics of functionalized engineered nanoparticles. Environ. Sci. Technol. 2013, 47, 4113–4120. [Google Scholar] [CrossRef] [PubMed]




| The Concentration of AgNO3 (mol/L) | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 |
|---|---|---|---|---|---|
| pH before synthesis | 10.58 | 10.35 | 10.17 | 10.22 | 10.14 |
| pH after synthesis | 5.82 | 5.44 | 4.74 | 4.67 | 4.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, H.; Li, Y.; Mo, F.; Li, Z.; Chai, R.; Wang, H. Dispersibility and Size Control of Silver Nanoparticles with Anti-Algal Potential Based on Coupling Effects of Polyvinylpyrrolidone and Sodium Tripolyphosphate. Nanomaterials 2020, 10, 1042. https://doi.org/10.3390/nano10061042
Wang M, Li H, Li Y, Mo F, Li Z, Chai R, Wang H. Dispersibility and Size Control of Silver Nanoparticles with Anti-Algal Potential Based on Coupling Effects of Polyvinylpyrrolidone and Sodium Tripolyphosphate. Nanomaterials. 2020; 10(6):1042. https://doi.org/10.3390/nano10061042
Chicago/Turabian StyleWang, Mingshuai, Haibo Li, Yinghua Li, Fan Mo, Zhe Li, Rui Chai, and Hongxuan Wang. 2020. "Dispersibility and Size Control of Silver Nanoparticles with Anti-Algal Potential Based on Coupling Effects of Polyvinylpyrrolidone and Sodium Tripolyphosphate" Nanomaterials 10, no. 6: 1042. https://doi.org/10.3390/nano10061042





