Hybrid Sol–Gel Silica Coatings Containing Graphene Nanosheets for Improving the Corrosion Protection of AA2024-T3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Chemical Modification and Characterization of Graphene Nanosheets
2.3. Synthesis and Characterization of Hybrid Silica Sols
2.4. Deposition and Characterization of Hybrid Silica Sol–Gel Coatings
3. Results and Discussion
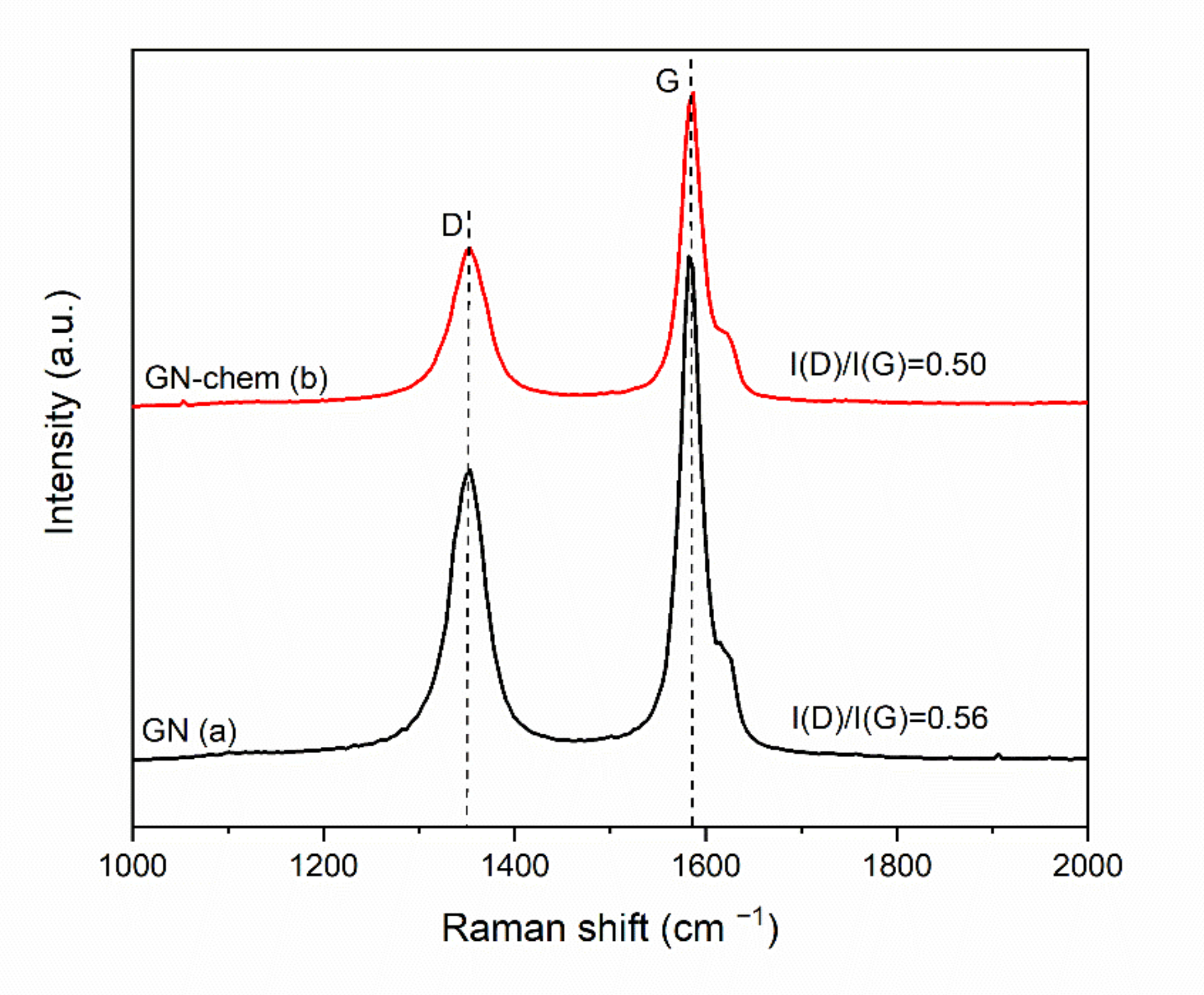
3.1. Characterization of Modified Graphene Nanosheets
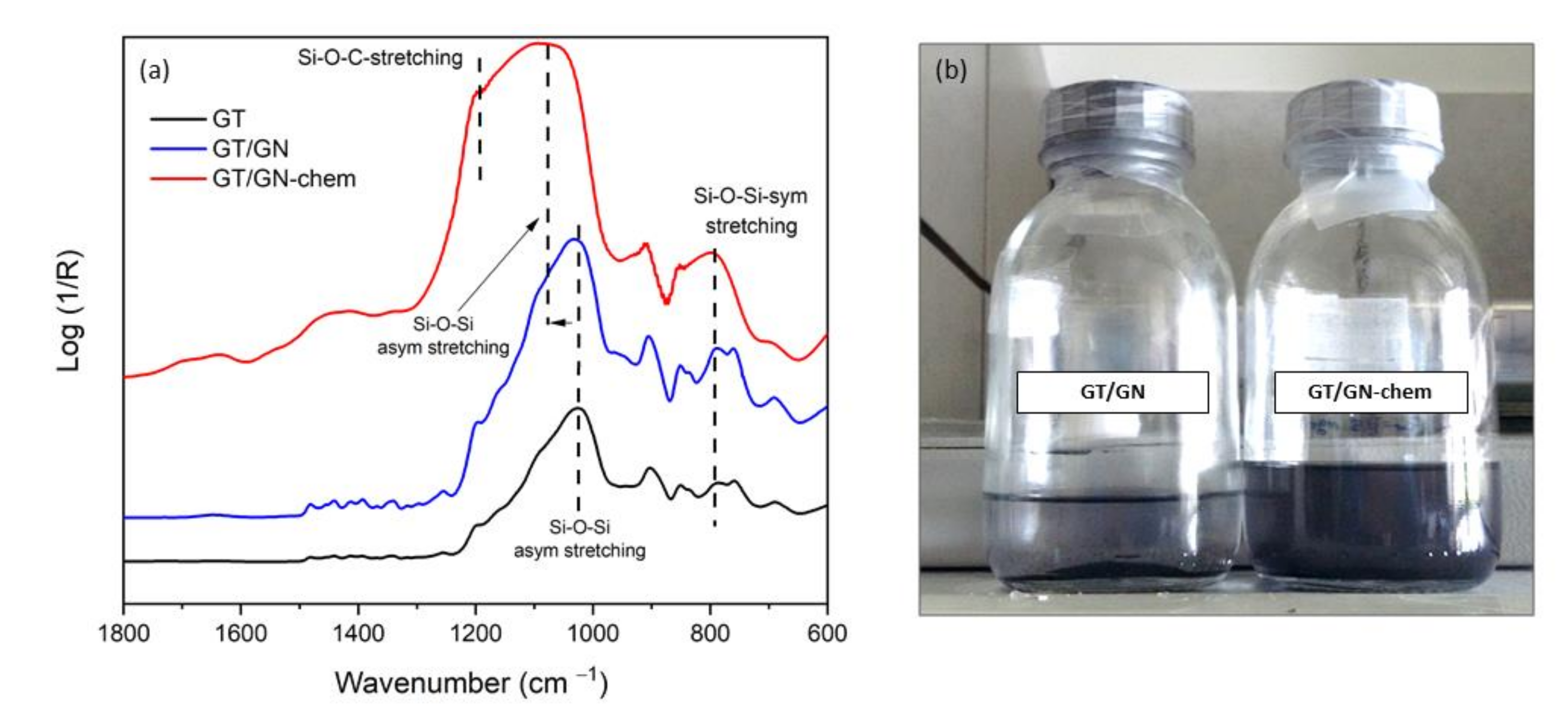
3.2. Synthesis and Characterization of the Sols
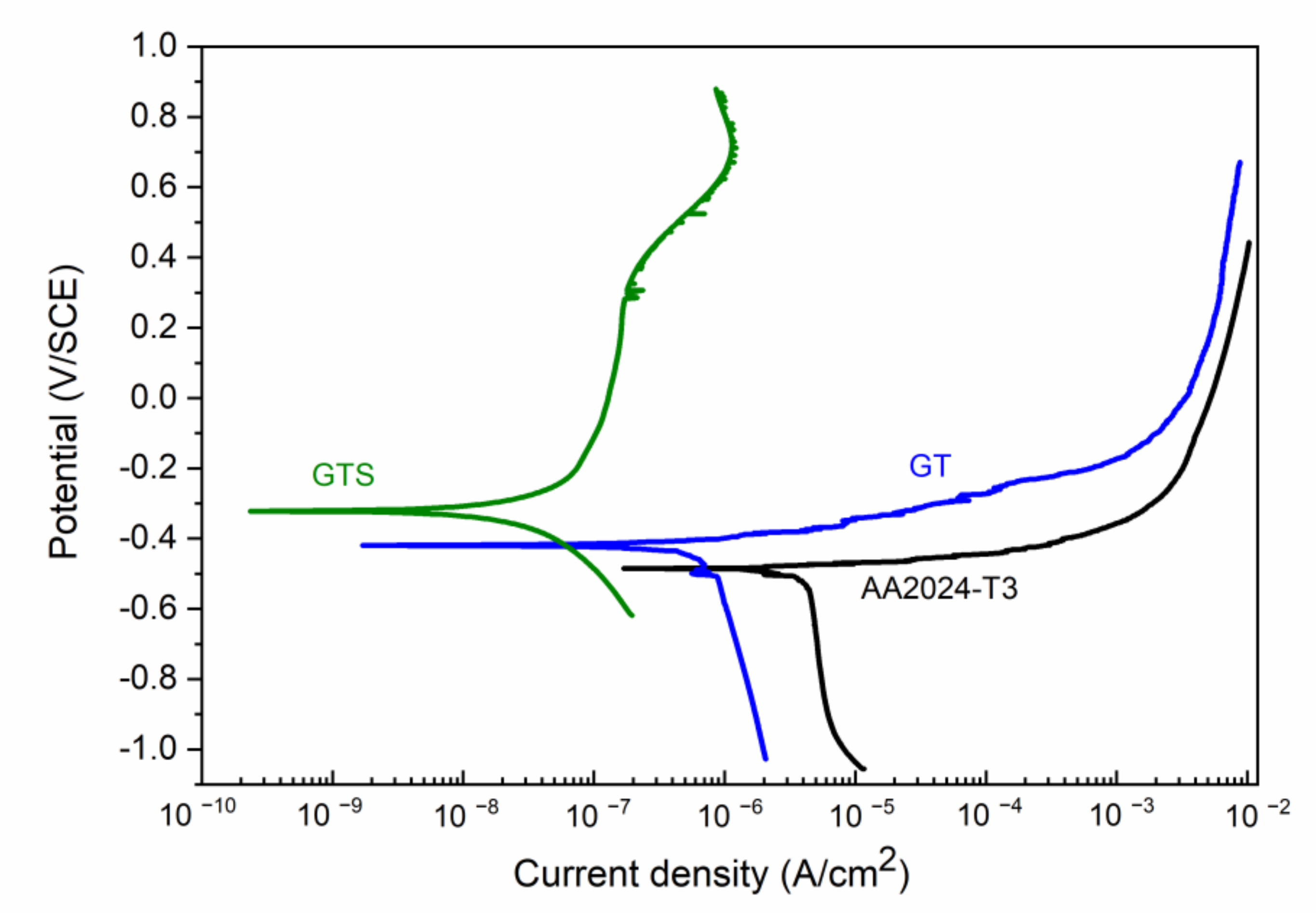
3.3. Characterization of the Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140. [Google Scholar] [CrossRef] [Green Version]
- Buchheit, R.G.; Grant, R.P.; Hlava, P.F.; McKenzie, B.; Zender, G.L. Local dissolution phenomena associated with S Phase (Al2CuMg) particles in aluminum alloy 2024-T3. J. Electrochem. Soc. 1997, 144, 2621–2627. [Google Scholar] [CrossRef]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Mohamadzadeh Moghadam, M.H. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ polymerization approach to graphene-reinforced nylon-6 composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Santos, C.M.; Tria, M.C.R.; Vergara, R.A.M.V.; Ahmed, F.; Advincula, R.C.; Rodrigues, D.F. Antimicrobial graphene polymer (PVK-GO) nanocomposite films. Chem. Commun. 2011, 47, 8892–8894. [Google Scholar] [CrossRef]
- Fujimoto, H. Theoretical X-ray scattering intensity of carbons with turbostratic stacking and AB stacking structures. Carbon 2003, 41, 1585–1592. [Google Scholar] [CrossRef]
- Liu, J.; Hua, L.; Li, S.; Yu, M. Graphene dip coatings: An effective anticorrosion barrier on aluminum. Appl. Surf. Sci. 2015, 327, 241–245. [Google Scholar] [CrossRef]
- Sun, W. Inhibiting the Corrosion-Promotion Activity of Graphene. Chem. Mater. 2015, 27, 2367–2373. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Xue, B.; Yu, M.; Liu, J.; Liu, J.; Li, S.; Xiong, L. Corrosion protection of AA2024-T3 by sol-gel film modified with graphene oxide. J. Alloys Compd. 2017, 725, 84–95. [Google Scholar] [CrossRef]
- Pfaffeneder-Kmen, M.; Casas, I.F.; Naghilou, A.; Trettenhahn, G.; Kautek, W. A Multivariate Curve Resolution evaluation of an in-situ ATR-FTIR spectroscopy investigation of the electrochemical reduction of graphene oxide. Electrochim. Acta 2017, 255, 160–167. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Enhancement of the Corrosion Protection Properties of a Hybrid Sol-Gel Based Silane Film through Impregnation of Functionalized Graphene Oxide Nanosheets. J. Electrochem. Soc. 2017, 164, C1044–C1058. [Google Scholar] [CrossRef]
- Lee, C.Y.; Bae, J.; Kim, T.; Chang, S.; Young, S. Composites: Part A Using silane-functionalized graphene oxides for enhancing the interfacial bonding strength of carbon / epoxy composites. Compos. Part A 2015, 75, 11–17. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. The epoxy coating interfacial adhesion and corrosion protection properties enhancement through deposition of cerium oxide nanofilm modified by graphene oxide. J. Ind. Eng. Chem. 2018, 64, 402–419. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, B.; Ren, Y.; Yu, M.; Qu, Y.; Xie, T.; Zhang, Y.; Wu, Y. Designed nitrogen doping of few-layer graphene functionalized by selective oxygenic groups. Nanoscale Res. Lett. 2014, 9, 646. [Google Scholar] [CrossRef] [Green Version]
- Muthoosamy, K.; Bai, R.G.; Abubakar, I.B.; Sudheer, S.; Lim, H.N.; Loh, H.; Huang, N.M.; Chia, C.H.; Manickam, S. Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int. J. Nanomed. 2015, 10, 1505–1519. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A Mater. Sci. Process. 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Junga, I.; Field, D.A.; Ventrice, C.A., Jr. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visolyfisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Reddy, G.B.; Pasricha, R. Thermal deoxygenation causes photoluminescence shift from UV to blue region in lyophilized graphene oxide. RSC Adv. 2015, 5, 74342–74346. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Chen, J.; Bian, Y.; Zhu, L.; Zhou, Y.; Xiang, Y.; Xia, D. Synthesis, characterization and desulfurization performance of MCM-41 functionalized with Cu by direct synthesis and organosilanes by grafting. J. Porous Mater. 2015, 22, 379–385. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ramezanzadeh, B.; Mahdavian, M. Hybrid silane coating reinforced with silanized graphene oxide nanosheets with improved corrosion protective performance. RSC Adv. 2016, 6, 54102–54112. [Google Scholar] [CrossRef]
- Karthik, N.; Sethuraman, M.G. Assessment of the corrosion protection ability of cysteamine and hybrid sol-gel twin layers on copper in 1% NaCl. RSC Adv. 2015, 5, 8693–8705. [Google Scholar] [CrossRef]
- Mosa, J.; Durán, A.; Aparicio, M. Epoxy-polystyrene-silica sol-gel membranes with high proton conductivity by combination of sulfonation and tungstophosphoric acid doping. J. Memb. Sci. 2010, 361, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Tiringer, U.; Milošev, I.; Durán, A.; Castro, Y. Hybrid sol–gel coatings based on GPTMS/TEOS containing colloidal SiO2and cerium nitrate for increasing corrosion protection of aluminium alloy 7075-T6. J. Sol-Gel Sci. Technol. 2018, 85, 546–557. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Pellice, S.A.; Castro, Y.; Aparicio, M.; Durán, A. Improved corrosion resistance of AA2024 alloys through hybrid organic-inorganic sol-gel coatings produced from sols with controlled polymerisation. Surf. Coat. Technol. 2009, 203, 1897–1903. [Google Scholar] [CrossRef]
- Suegama, P.H.; Recco, A.A.C.; Tschiptschin, A.P.; Aoki, I.V. Influence of silica nanoparticles added to an organosilane film on carbon steel electrochemical and tribological behaviour. Prog. Org. Coat. 2007, 60, 90–98. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, H.; Jiang, D.; Parkin, I.P.; Zhang, X. Reactive silica nanoparticles turn epoxy coating from hydrophilic to super-robust superhydrophobic. RSC Adv. 2019, 9, 12547–12554. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, S.; Sato, N.; Tölle, F.; Mülhaupt, R.; Fiedler, B.; Schulte, K. Fracture toughness and failure mechanism of graphene based epoxy composites. Compos. Sci. Technol. 2014, 97, 90–99. [Google Scholar] [CrossRef]
- Xu, S.; Hartvickson, S.; Zhao, J.X. Increasing surface area of silica nanoparticles with a rough surface. ACS Appl. Mater. Interfaces 2011, 3, 1865–1872. [Google Scholar] [CrossRef] [PubMed]


| Sample | GN (g) | GPTMS (mol) | TEOS (mol) | EtOH (mol) | HCl (1M) (mol) | HNO3 (mol) | LUDOX (mol) | SiO2 Concentration (g/L) |
|---|---|---|---|---|---|---|---|---|
| GT | _ | 0.05 | 0.05 | 0.40 | 0.38 | _ | _ | 109 |
| GT/GN | 0.027 | 0.05 | 0.05 | 0.40 | 0.38 | _ | _ | 109 |
| GT/GN-chem | 0.027 | 0.05 | 0.05 | 0.40 | 0.38 | _ | _ | 109 |
| GTS | _ | 0.05 | 0.05 | 0.40 | _ | 0.01 | 0.14 | 262 |
| GTS/GN-chem | 0.026 | 0.05 | 0.05 | 0.40 | _ | 0.01 | 0.14 | 262 |
| Coating | Thickness (μm) | Contact Angle (°) | Roughness (nm) |
|---|---|---|---|
| GT | 2.1 ± 0.7 | 61 ± 4 | 0.5 ± 0.1 |
| GT/GN-chem | 2.3 ± 0.6 | 72 ± 2 | 0.9 ± 0.2 |
| GTS | 5.3 ± 0.3 | 55 ± 2 | 2.3 ± 0.2 |
| GTS/GN-chem | 5.5 ± 0.2 | 74 ± 2 | 3.6 ± 0.1 |
| Sample | Ecorr (V) | jcorr (µA/cm2) | jp (µA/cm2) | ΔE (V) |
|---|---|---|---|---|
| AA2024-T3 | −0.48 ± 0.03 | 3.0 ± 0.5 | _ | _ |
| GT | −0.42 ± 0.02 | 0.5 ± 0.1 | _ | _ |
| GT/GN-chem | −0.33 ± 0.03 | 0.06 ± 0.01 | _ | _ |
| GTS | −0.31 ± 0.03 | 0.03 ± 0.01 | 0.2 ± 0.1 | 0.37 |
| GTS/GN-chem | −0.32 ± 0.04 | 0.002 ± 0.001 | 0.9 ± 0.1 | 0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afsharimani, N.; Durán, A.; Galusek, D.; Castro, Y. Hybrid Sol–Gel Silica Coatings Containing Graphene Nanosheets for Improving the Corrosion Protection of AA2024-T3. Nanomaterials 2020, 10, 1050. https://doi.org/10.3390/nano10061050
Afsharimani N, Durán A, Galusek D, Castro Y. Hybrid Sol–Gel Silica Coatings Containing Graphene Nanosheets for Improving the Corrosion Protection of AA2024-T3. Nanomaterials. 2020; 10(6):1050. https://doi.org/10.3390/nano10061050
Chicago/Turabian StyleAfsharimani, Nasima, Alicia Durán, Dušan Galusek, and Yolanda Castro. 2020. "Hybrid Sol–Gel Silica Coatings Containing Graphene Nanosheets for Improving the Corrosion Protection of AA2024-T3" Nanomaterials 10, no. 6: 1050. https://doi.org/10.3390/nano10061050







