Strongly Luminescent Composites Based on Carbon Dots Embedded in a Nanoporous Silicate Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. CDs Synthesis
2.3. Fabrication of CD@NSG Composites
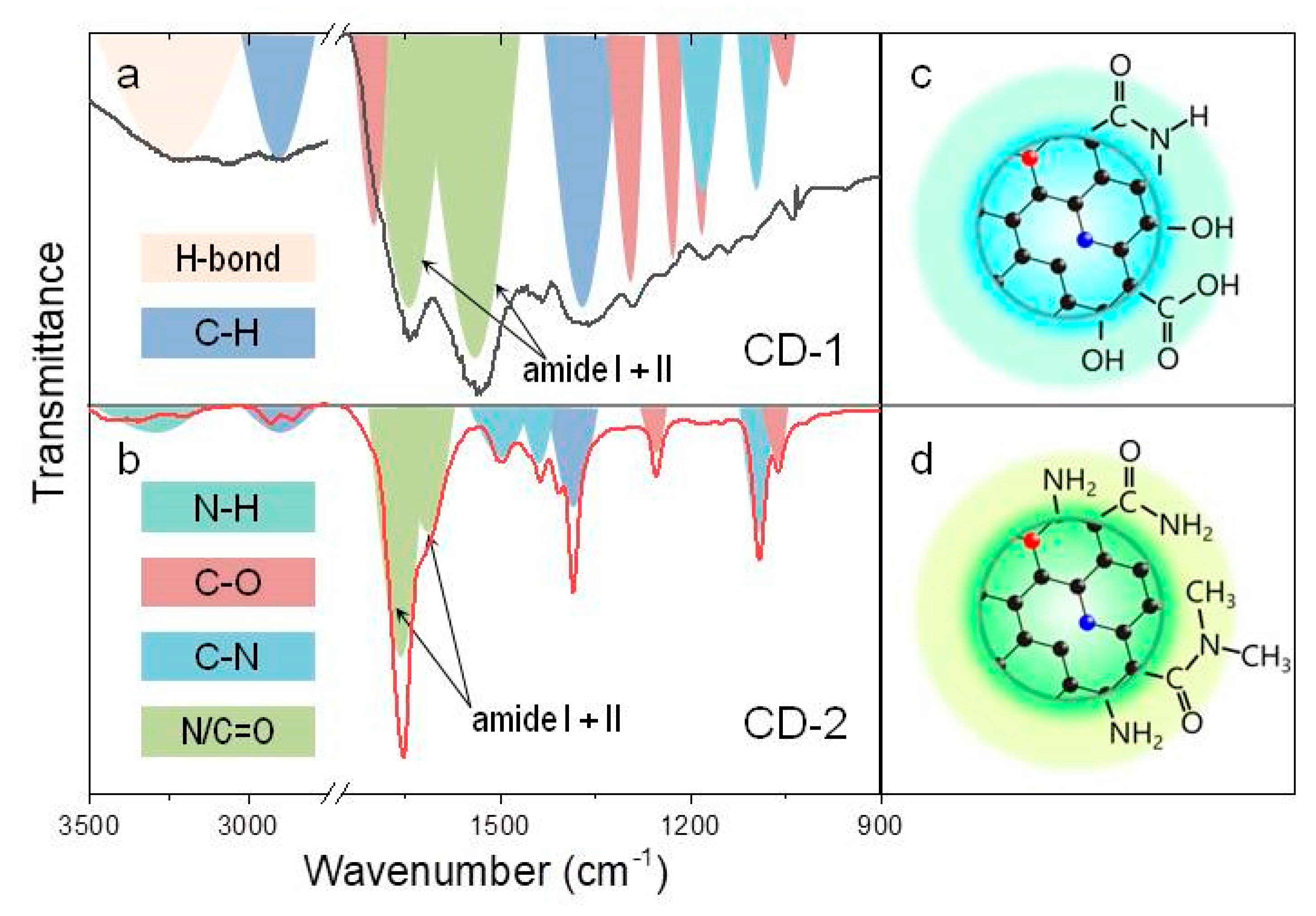
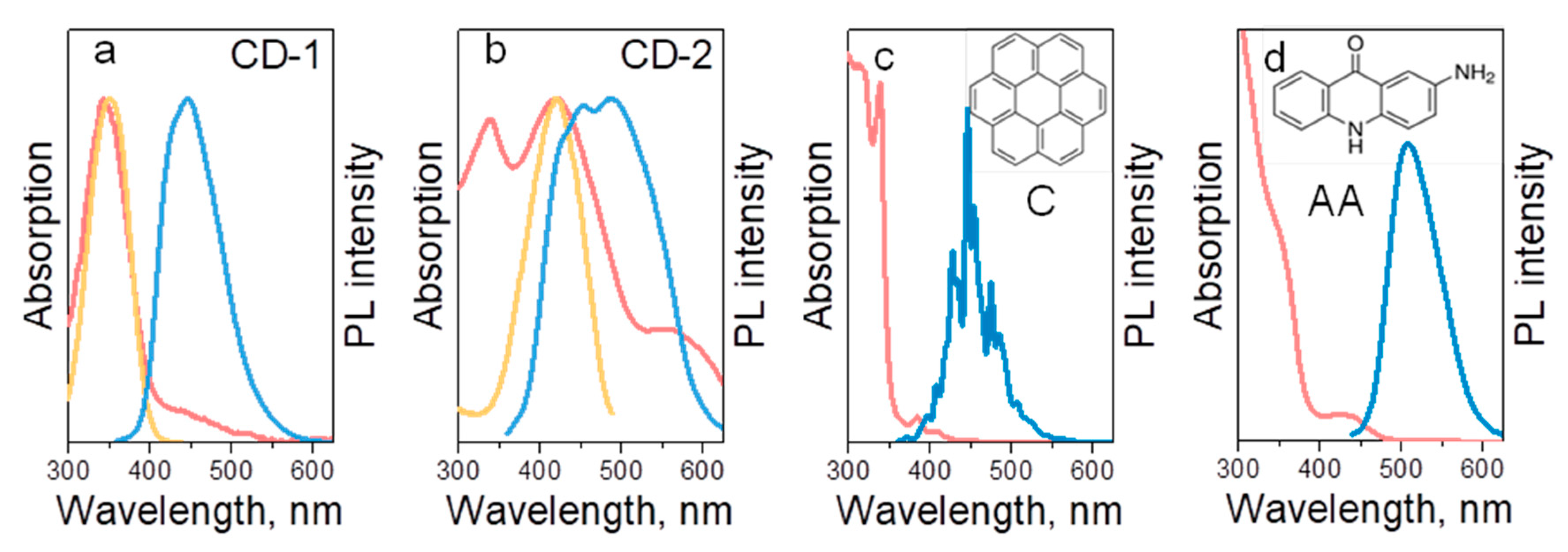
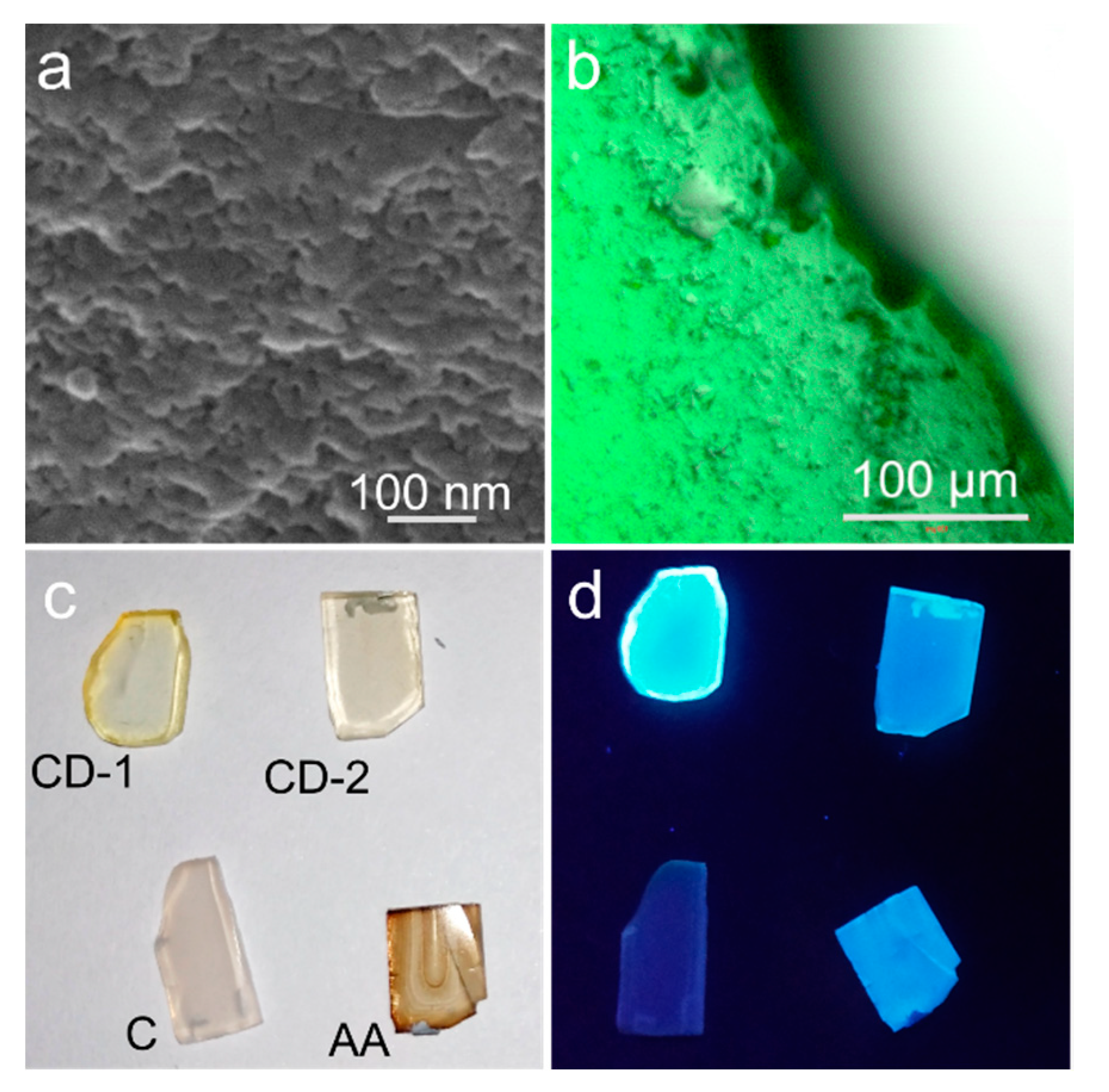
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demchenko, A. Excitons in Carbonic Nanostructures. C J. Carbon Res. 2019, 5, 71. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Reckmeier, C.J.; Schneider, J.; Susha, A.S.; Rogach, A.L. Luminescent colloidal carbon dots: Optical properties and effects of doping. Opt. Express 2016, 24, A312. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Ehrat, F.; Wang, Y.; Milowska, K.Z.; Reckmeier, C.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Carbon Dots: A Unique Fluorescent Cocktail of Polycyclic Aromatic Hydrocarbons. Nano Lett. 2015, 15, 6030–6035. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Embrechts, H.; Damm, C.; Cadranel, A.; Strauss, V.; Distaso, M.; Hinterberger, V.; Guldi, D.M.; Peukert, W. Shedding light on the effective fluorophore structure of high fluorescence quantum yield carbon nanodots. RSC Adv. 2017, 7, 24771–24780. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; Von Seckendorff, M.; Susha, A.S.; Kasak, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence phenomena of carbon dots derived from citric acid and urea—A molecular insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef]
- Vallan, L.; Urriolabeitia, E.P.; Ruipérez, F.; Matxain, J.M.; Canton-Vitoria, R.; Tagmatarchis, N.; Benito, A.M.; Maser, W.K. Supramolecular-Enhanced Charge Transfer within Entangled Polyamide Chains as the Origin of the Universal Blue Fluorescence of Polymer Carbon Dots. J. Am. Chem. Soc. 2018, 140, 12862–12869. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Shao, J.; Zhao, X.; Yang, B. Non-Conjugated Polymer Dots with Crosslink-Enhanced Emission in the Absence of Fluorophore Units. Angew. Chemie Int. Ed. 2015, 54, 14626–14637. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Arefina, I.A.; Khavlyuk, P.D.; Dubavik, A.; Bogdanov, K.V.; Bondarenko, D.P.; Cherevkov, S.A.; Kundelev, E.V.; Fedorov, A.V.; Baranov, A.V.; et al. Influence of the solvent environment on luminescent centers within carbon dots. Nanoscale 2020, 12, 602–609. [Google Scholar] [CrossRef]
- Reckmeier, C.J.; Wang, Y.; Zboril, R.; Rogach, A.L. Influence of Doping and Temperature on Solvatochromic Shifts in Optical Spectra of Carbon Dots. J. Phys. Chem. C 2016, 120, 10591–10604. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Cong, R.; Zhu, S.; Zhao, X.; Liu, J.; S.tse, J.; Meng, S.; Yang, B. PH-Dependent Synthesis of Novel Structure-Controllable Polymer-Carbon NanoDots with High Acidophilic Luminescence and Super Carbon Dots Assembly for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2016, 8, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Miao, X.; Nie, B.; Sun, Z.; Fan, H.; Zhao, Y.; Yang, D. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2017, 30, 1704740. [Google Scholar]
- Bai, J.; Ma, Y.; Yuan, G.; Chen, X.; Mei, J.; Zhang, L.; Ren, L. Solvent-controlled and solvent-dependent strategies for the synthesis of multicolor carbon dots for pH sensing and cell imaging. J. Mater. Chem. C 2019, 7, 9709–9718. [Google Scholar] [CrossRef]
- Wei, W.; Xu, C.; Wu, L.; Wang, J.; Ren, J.; Qu, X. Non-enzymatic-browning-reaction: A versatile route for production of nitrogen-doped carbon dots with tunable multicolor luminescent display. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef]
- Xiong, Y.; Schneider, J.; Reckmeier, C.J.; Huang, H.; Kasák, P.; Rogach, A.L. Carbonization conditions influence the emission characteristics and the stability against photobleaching of nitrogen doped carbon dots. Nanoscale 2017, 9, 11730–11738. [Google Scholar] [CrossRef]
- Su, W.; Wu, H.; Xu, H.; Zhang, Y.; Li, Y.; Li, X.; Fan, L. Carbon dots: A booming material for biomedical applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Teradal, N.L.; Jelinek, R. Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Bhaisare, M.L.; Talib, A.; Khan, M.S.; Pandey, S.; Wu, H.-F. Synthesis of fluorescent carbon dots via microwave carbonization of citric acid in presence of tetraoctylammonium ion, and their application to cellular bioimaging. Microchim. Acta 2015, 182, 2173–2181. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; Yang, Q.; Wu, Q.; Shi, J.; Gong, A.; Yang, M. Efficient room-temperature phosphorescence from nitrogen-doped carbon dots in composite matrices. Chem. Mater. 2016, 28, 8221–8227. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Activating Room Temperature Long Afterglow of Carbon Dots via Covalent Fixation. Chem. Mater. 2017, 29, 4866–4873. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Zhou, Z.; Zhang, H.; Zhang, X.; Zhuang, J.; Liu, Y.; Lei, B.; Hu, C. A Universal Strategy for Activating the Multicolor Room-Temperature Afterglow of Carbon Dots in a Boric Acid Matrix. Angew. Chem. 2019, 131, 7356–7361. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Y.K.; Sharma, G.; Dong, Y.; Zheng, X.; Li, P.; Johnston, A.; Bappi, G.; Fan, J.Z.; Kung, H.; et al. Bright high-colour-purity deep-blue carbon dot light-emitting diodes via efficient edge amination. Nat. Photonics 2020, 14, 171–176. [Google Scholar] [CrossRef]
- Chen, B.; Feng, J. White-light-emitting polymer composite film based on carbon dots and lanthanide complexes. J. Phys. Chem. C 2015, 119, 7865–7872. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Wang, Y.; Yang, Y.; Liu, X. Efficient resistance against solid-state quenching of carbon dots towards white light emitting diodes by physical embedding into silica. Carbon N. Y. 2018, 126, 426–436. [Google Scholar] [CrossRef]
- Zdražil, L.; Kalytchuk, S.; Hola, K.; Petr, M.; Zmeškal, O.; Kment, S.; Rogach, A.; Zboril, R. Carbon dot–based tandem luminescent solar concentrator. Nanoscale 2020, 12, 6664–6672. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, N.; Marinovic, A.; Yoshizawa, N.; Goode, A.E.; Fay, M.; Khlobystov, A.; Titirici, M.-M.; Sapelkin, A. Structure and solvents effects on the optical properties of sugar-derived carbon nanodots. Sci. Rep. 2018, 8, 6559. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, M.; Xiao, Y.; Dong, H.; Zhang, H.; Zhuang, J.; Hu, H.; Lei, B.; Liu, Y. A Self-Quenching-Resistant Carbon-Dot Powder with Tunable Solid-State Fluorescence and Construction of Dual-Fluorescence Morphologies for White Light-Emission. Adv. Mater. 2016, 28, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bao, L.; Tang, B.; Zhao, J.Y.; Zhang, Z.L.; Xiong, L.H.; Hu, J.; Wu, L.L.; Pang, D.W. Fluorescence-Converging Carbon Nanodots-Hybridized Silica Nanosphere. Small 2016, 12, 4702–4706. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Baltimore, MD, USA, 2006; ISBN 0387312781. [Google Scholar]
- Jiang, Z.C.; Lin, T.N.; Lin, H.T.; Talite, M.J.; Tzeng, T.T.; Hsu, C.L.; Chiu, K.P.; Lin, C.A.J.; Shen, J.L.; Yuan, C.T. A Facile and Low-Cost Method to Enhance the Internal Quantum Yield and External Light-Extraction Efficiency for Flexible Light-Emitting Carbon-Dot Films. Sci. Rep. 2016, 6, 19991. [Google Scholar] [CrossRef]
- Tian, Z.; Li, D.; Ushakova, E.V.; Maslov, V.G.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Rogach, A.L. Multilevel Data Encryption Using Thermal-Treatment Controlled Room Temperature Phosphorescence of Carbon Dot/Polyvinylalcohol Composites. Adv. Sci. 2018, 5, 1800795. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Xu, X.; Zhuang, J.; Zhang, H.; Zhang, X.; Hu, C.; Lei, B.; Kaminski, C.F.; Liu, Y. Carbon Dot-Silica Nanoparticle Composites for Ultralong Lifetime Phosphorescence Imaging in Tissue and Cells at Room Temperature. Chem. Mater. 2019, 31, 9887–9894. [Google Scholar] [CrossRef]
- Joseph, J.; Anappara, A.A. Cool white, persistent room-temperature phosphorescence in carbon dots embedded in a silica gel matrix. Phys. Chem. Chem. Phys. 2017, 19, 15137–15144. [Google Scholar] [CrossRef]
- Kuzema, P.; Bolbukh, Y.; Lipke, A.; Majdan, M.; Tertykh, V. Luminescent Sol-Gel Glasses from Silicate–Citrate–(Thio)Ureate Precursors. Colloids Interfaces 2019, 3, 11. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Yu, Y.; Yan, Y.; Zhang, H.; Li, J.; Yu, J. Carbon dots in zeolites: A new class of thermally activated delayed fluorescence materials with ultralong lifetimes. Sci. Adv. 2017, 3, e1603171. [Google Scholar] [CrossRef]
- Dong, X.; Wei, L.; Su, Y.; Li, Z.; Geng, H.; Yang, C.; Zhang, Y. Efficient long lifetime room temperature phosphorescence of carbon dots in a potash alum matrix. J. Mater. Chem. C 2015, 3, 2798–2801. [Google Scholar] [CrossRef]
- Sun, M.; Qu, S.; Hao, Z.; Ji, W.; Jing, P.; Zhang, H.; Zhang, L.; Zhao, J.; Shen, D. Towards efficient solid-state photoluminescence based on carbon-nanodots and starch composites. Nanoscale 2014, 6, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sun, X.; Wang, Y.; Song, R.; Xie, Z.; Zhou, S.; Chen, P. Controllable Photoluminescent and Nonlinear Optical Properties of Polymerizable Carbon Dots and Their Arbitrary Copolymerized Gel Glasses. Adv. Opt. Mater. 2018, 6, 1701273. [Google Scholar] [CrossRef]
- Lin, S.; Lin, C.; He, M.; Yuan, R.; Zhang, Y.; Zhou, Y.; Xiang, W.; Liang, X. Solvatochromism of bright carbon dots with tunable long-wavelength emission from green to red and their application as solid-state materials for warm WLEDs. RSC Adv. 2017, 7, 41552–41560. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward Efficient Orange Emissive Carbon Nanodots through Conjugated sp2-Domain Controlling and Surface Charges Engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- Bagnich, S.A.; Bogomolov, V.N.; Kurdyukov, D.A.; Pershukevich, P.P. Phosphorescence of aromatic compounds in a porous matrix of sodium borosilicate glass and their interaction with the pore walls. Phys. Solid State 1995, 37, 1642–1645. [Google Scholar]
- Vasincu, A.; Paulsen, B.; Diallo, D.; Vasincu, I.; Aprotosoaie, A.; Bild, V.; Charalambous, C.; Constantinou, A.; Miron, A.; Gavrilescu, C. Vernonia kotschyana Roots: Therapeutic Potential via Antioxidant Activity. Molecules 2014, 19, 19114–19136. [Google Scholar] [CrossRef]
- Ehrat, F.; Bhattacharyya, S.; Schneider, J.; Löf, A.; Wyrwich, R.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Tracking the Source of Carbon Dot Photoluminescence: Aromatic Domains versus Molecular Fluorophores. Nano Lett. 2017, 17, 7710–7716. [Google Scholar] [CrossRef]
- Benetti, D.; Jokar, E.; Yu, C.H.; Fathi, A.; Zhao, H.; Vomiero, A.; Wei-Guang Diau, E.; Rosei, F. Hole-extraction and photostability enhancement in highly efficient inverted perovskite solar cells through carbon dot-based hybrid material. Nano Energy 2019, 62, 781–790. [Google Scholar] [CrossRef]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y.; et al. Near-Infrared Excitation/Emission and Multiphoton-Induced Fluorescence of Carbon Dots. Adv. Mater. 2018, 30, 1705913. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Sarkar, S.; Sudolská, M.; Dubecký, M.; Reckmeier, C.J.; Rogach, A.L.; Zbořil, R.; Otyepka, M. Graphitic Nitrogen Doping in Carbon Dots Causes Red-Shifted Absorption. J. Phys. Chem. C 2016, 120, 1303–1308. [Google Scholar] [CrossRef]
- Kundelev, E.V.; Tepliakov, N.V.; Leonov, M.Y.; Maslov, V.G.; Baranov, A.V.; Fedorov, A.V.; Rukhlenko, I.D.; Rogach, A.L. Amino Functionalization of Carbon Dots Leads to Red Emission Enhancement. J. Phys. Chem. Lett. 2019, 10, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783540938095. [Google Scholar]
- Chai, S.Q.; He, J.H.; Zhan, L.; Li, Y.F.; Li, C.M.; Huang, C.Z. Dy(III)-induced aggregation emission quenching effect of single-layered graphene quantum dots for selective detection of phosphate in the artificial wetlands. Talanta 2019, 196, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, J.S.; Zhang, P.; Niu, X.Q.; Zhao, W.; Zhu, Z.Y.; Ding, H.; Xiong, H.M. Red-Emissive Carbon Dots for Fingerprints Detection by Spray Method: Coffee Ring Effect and Unquenched Fluorescence in Drying Process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Gierschner, J.; Lüer, L.; Milián-Medina, B.; Oelkrug, D.; Egelhaaf, H.J. Highly emissive H-aggregates or aggregation-induced emission quenching? the photophysics of all-trans para-distyrylbenzene. J. Phys. Chem. Lett. 2013, 4, 2686–2697. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002; ISBN 9783527299195. [Google Scholar]
- Ermakova, L.; Sidorova, M.; Jura, N. Electrochemistry of porous glass membranes in electrolyte solutions. J. Memb. Sci. 1996, 115, 11–19. [Google Scholar] [CrossRef]
- Lai, C.W.; Hsiao, Y.H.; Peng, Y.K.; Chou, P.T. Facile synthesis of highly emissive carbon dots from pyrolysis of glycerol; Gram scale production of carbon dots/mSiO2 for cell imaging and drug release. J. Mater. Chem. 2012, 22, 14403–14409. [Google Scholar] [CrossRef]

| Sample | Abs Peak Position, nm | PL Peak Position, nm | PL Lifetime, ns | PL QY, % | ||||
|---|---|---|---|---|---|---|---|---|
| solution | @NSG | solution | @NSG | solution | @NSG | Solution ex. at 350 nm | @NSG ex. at 405 nm | |
| CD-1 | 350 ± 2 | 360 ± 5 | 450 ± 2 | 510 ± 5 | 12.3 ± 0.5 | 7.5 ± 0.5 | 34 ± 1 | 35 ± 5 |
| CD-2 | 430 ± 2 | 430 ± 5 | 500 ± 2 | 510 ± 5 | 8.4 ± 0.5 | 8.7 ± 0.5 | 17 ± 1 | 40 ± 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanidenko, E.A.; Khavlyuk, P.D.; Arefina, I.A.; Cherevkov, S.A.; Xiong, Y.; Döring, A.; Varygin, G.V.; Kurdyukov, D.A.; Eurov, D.A.; Golubev, V.G.; et al. Strongly Luminescent Composites Based on Carbon Dots Embedded in a Nanoporous Silicate Glass. Nanomaterials 2020, 10, 1063. https://doi.org/10.3390/nano10061063
Stepanidenko EA, Khavlyuk PD, Arefina IA, Cherevkov SA, Xiong Y, Döring A, Varygin GV, Kurdyukov DA, Eurov DA, Golubev VG, et al. Strongly Luminescent Composites Based on Carbon Dots Embedded in a Nanoporous Silicate Glass. Nanomaterials. 2020; 10(6):1063. https://doi.org/10.3390/nano10061063
Chicago/Turabian StyleStepanidenko, Evgeniia A., Pavel D. Khavlyuk, Irina A. Arefina, Sergei A. Cherevkov, Yuan Xiong, Aaron Döring, Georgii V. Varygin, Dmitry A. Kurdyukov, Daniil A. Eurov, Valery G. Golubev, and et al. 2020. "Strongly Luminescent Composites Based on Carbon Dots Embedded in a Nanoporous Silicate Glass" Nanomaterials 10, no. 6: 1063. https://doi.org/10.3390/nano10061063
APA StyleStepanidenko, E. A., Khavlyuk, P. D., Arefina, I. A., Cherevkov, S. A., Xiong, Y., Döring, A., Varygin, G. V., Kurdyukov, D. A., Eurov, D. A., Golubev, V. G., Masharin, M. A., Baranov, A. V., Fedorov, A. V., Ushakova, E. V., & Rogach, A. L. (2020). Strongly Luminescent Composites Based on Carbon Dots Embedded in a Nanoporous Silicate Glass. Nanomaterials, 10(6), 1063. https://doi.org/10.3390/nano10061063










