Binder Free and Flexible Asymmetric Supercapacitor Exploiting Mn3O4 and MoS2 Nanoflakes on Carbon Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodeposition of Manganese Oxide and Annealing to Spinel Mn3O4
2.2. Synthesis of MoS2 Flakes and Electrodes Fabrication
2.3. Physical-Chemical Characterization
2.4. Electrochemical Characterization and Device Assembling
3. Results and Discussion
3.1. Characterization of the Material at the Cathode
3.2. Characterization of the Material at the Anode
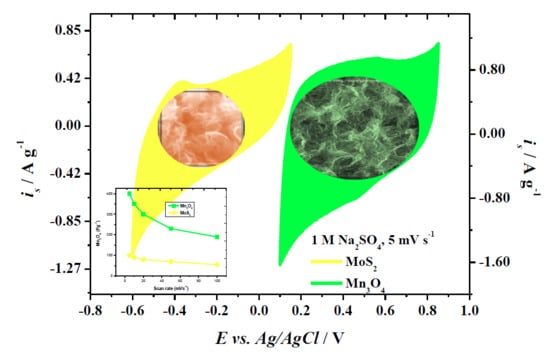
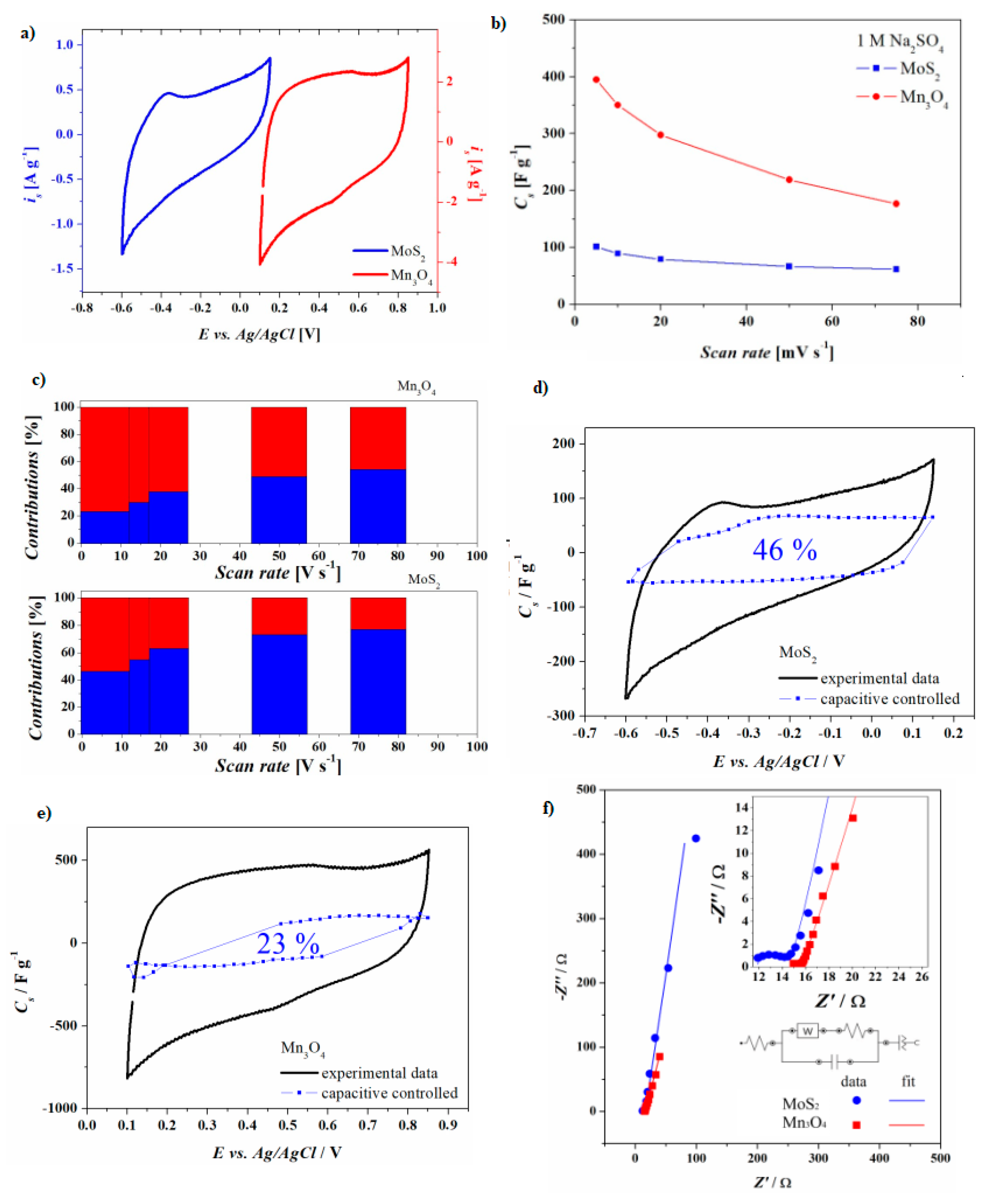
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Z.; Li, L.; Yan, J.; Zhang, X. Materials Design and System Construction for Conventional and New-Concept Supercapacitors. Adv. Sci. News 2017, 4, 1600382. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, M.; Balasubramanian, K. Simple capacitors to supercapacitors-an overview. Int. J. Electrochem. Sci. 2008, 3, 1196–1217. [Google Scholar]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Huang, X.; Ding, R.; Hu, Y.; Jiang, J.; Liao, L. Direct growth of SnO2 nanorod array electrodes for lithium-ion batteries. J. Mater. Chem. 2009, 19, 1859–1864. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Commun. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Zhanga, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Xie, K.; Wei, B. Nanomaterials for stretchable energy storage and conversion devices. Nanomater. Sustain. Energy 2016, 159–191. [Google Scholar] [CrossRef]
- Dai, Z.; Peng, C.; Chae, J.H.; Ng, K.C.; Chen, G.Z. Cell voltage versus electrode potential range in aqueous supercapacitors. Sci. Rep. 2015, 5, 9854. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Chung, H.-S.; Moore, J.; Thomas, J.; Jung, Y. High-Performance One-Body Core/Shell Nanowire Supercapacitor Enabled by Conformal Growth of Capacitive 2D WS2 Layers. ACS Nano 2016, 10, 10726–10735. [Google Scholar] [CrossRef] [PubMed]
- Graeme, C.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menze, R.; Shaffer, M.S.P.; Coleman, J.N. Solvent exfoliation of transition metal dichalcogenides: Dispersibility of exfoliated nanosheets varies only weakly between compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar]
- Lou, W.; Chen, M.; Wang, X.; Liu, W. Novel single-source precursors approach to prepare highly uniform Bi2S3 and Sb2S3 nanorods via a solvothermal treatment. Chem. Mater. 2007, 19, 872–878. [Google Scholar] [CrossRef]
- Fang, L.; Qiu, Y.; Zhai, T.; Wang, F.; Lan, M.; Haung, K.; Jing, Q. Flower-like nanoarchitecture assembled from Bi2S3 nanorod/MoS2 nanosheet heterostructures for high-performance supercapacitor electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 41–48. [Google Scholar] [CrossRef]
- Tsai, H.-W.; Yagoubi, A.; Chan, T.-C.; Wang, C.-C.; Liu, W.-T.; Liao, C.-N.; Lu, S.-Y.; Chen, L.-J.; Chueh, Y.-L. Electrochemical synthesis of ultrafast and gram-scale surfactant-free tellurium nanowires by gas-solid transformation and their applications as supercapacitor electrodes for p-doping of graphene transistors. Nanoscale 2015, 7, 7535–7539. [Google Scholar] [CrossRef]
- Karade, S.S.; Sankapal, B.R. Two dimensional cryptomelane like growth of MoSe2 over MWCNTs: Symmetric all-solid-state supercapacitor. J. Electroanal. Chem. 2017, 802, 131–138. [Google Scholar] [CrossRef]
- Peng, H.; Wei, C.; Wang, K.; Meng, T.; Ma, G.; Lei, Z.; Gong, X. Ni0.85Se@MoSe2 Nanosheet Arrays as the Electrode for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 17067–17075. [Google Scholar] [CrossRef]
- Iu, M.L.; Wang, X.; Huang, Z.; Guo, P.; Wang, Z. In-situ solution synthesis of graphene supported lamellar 1T’-MoTe2 for enhanced pseudocapacitors. Mater. Lett. 2017, 206, 229–232. [Google Scholar]
- Liu, M.; Wang, Z.; Liu, G.; Jiao, D.; Li, Y.; An, C.; Jun, Z. Synthesis of few-layer 1T′-MoTe2 ultrathin nanosheets for high-performance pseudocapacitors. J. Mater. Chem. A 2017, 5, 1035–1042. [Google Scholar] [CrossRef]
- Hou, X.; Peng, T.; Cheng, J.; Yu, Q.; Luo, R.; Lu, Y.; Lu, Y.; Liu, X.; Kim, J.-K.; He, J.; et al. Ultrathin ZnS nanosheet/carbon nanotube hybrid electrode for high-performance flexible all-solid-state supercapacitor. Nano Res. 2017, 10, 2570–2583. [Google Scholar] [CrossRef]
- Li, G.-C.; Liu, M.; Wu, M.-K.; Liu, P.-F.; Zhou, Z.; Zhu, S.-R.; Liu, R.; Han, L. MOF-derived self-sacrificing route to hollow NiS2/ZnS nanospheres for high performance supercapacitors. RSC Adv. 2016, 6, 103517–103522. [Google Scholar] [CrossRef]
- Pei, L.; Yang, Y.; Chu, H.; Shen, J.; Ye, M. elf-assembled flower-like FeS2/graphene aerogel composite with enhanced electrochemical properties. Ceram. Int. 2016, 42, 5053–5061. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Gigot, A.; Fontana, M.; Serrapede, M.; Castellino, M.; Bianco, S.; Armandi, M.; Bonelli, B.; Pirri, C.F.; Tresso, E.; Rivolo, P. Mixed 1T-2H Phase MoS2/Reduced Graphene Oxide as Active Electrode for Enhanced Supercapacitive Performance. ACS Appl. Mater. Interfaces 2016, 8, 32842–32852. [Google Scholar] [CrossRef]
- Yan, W.; Ayvazian, T.; Kim, J.; Liu, Y.; Donavan, K.C.; Xing, W.; Yang, Y.; Hemminger, J.C.; Penner, R.M. Mesoporous manganese oxide nanowires for high-capacity, high-rate, hybrid electrical energy storage. ACS Nano 2011, 5, 8275–8287. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Kou, S.; Wu, N. Investigation of Pseudocapacitive Charge-Storage Reaction of MnO2·nH2O Supercapacitors in Aqueous Electrolytes. J. Electrochem. Soc. 2006, 153, A1317–A1324. [Google Scholar]
- Broughton, J.N.; Brett, M.J. Variations in MnO2 electrodeposition for electrochemical capacitors. Electrochim. Acta 2005, 50, 4814–4819. [Google Scholar] [CrossRef]
- Shinomiya, T.; Vinay, G.; Norio, M. Effects of electrochemical-deposition method and microstructure on the capacitive characteristics of nano-sized manganese oxide. Electrochim. Acta 2006, 51, 4412–4419. [Google Scholar] [CrossRef]
- Wu, M.-S. Electrochemical capacitance from manganese oxide nanowire structure synthesized by cyclic voltammetric electrodeposition. Appl. Phys. Lett. 2005, 87, 153102. [Google Scholar] [CrossRef]
- Chou, S.; Cheng, F.; Chen, J. Electrodeposition synthesis and electrochemical properties of nanostructured γ-MnO2 films. J. Power Source 2006, 162, 727–734. [Google Scholar] [CrossRef]
- Hu, C.-C.; Tsou, T.-W. Ideal capacitive behavior of hydrous manganese oxide prepared by anodic deposition. Electrochem. Commun. 2002, 4, 105–109. [Google Scholar] [CrossRef]
- Zhai, T.; Xie, S.; Yu, M.; Fang, P.; Liang, C.; Lu, X.; Tong, Y. Oxygen vacancies enhancing capacitive properties of MnO2 nanorods for wearable asymmetric supercapacitors. Nano Energy 2014, 8, 255–293. [Google Scholar] [CrossRef]
- Serrapede, M.; Rafique, A.; Fontana, M.; Zine, A.; Rivolo, P.; Bianco, S.; Chetibi, L.; Tresso, E.; Lamberti, A. Fiber-shaped asymmetric supercapacitor exploiting rGO/Fe2O3 aerogel and electrodeposited MnOx nanosheets on carbon fibers. Carbon 2019, 144, 91–100. [Google Scholar] [CrossRef]
- Rafique, A.; Massa, A.; Fontana, M.; Bianco, S.; Chiodoni, A.; Pirri, C.F.; Hernández, S.; Lamberti, A. Highly Uniform Anodically Deposited Film of MnO2 Nanoflakes on Carbon Fibers for Flexible and Wearable Fiber-Shaped Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 28386–28393. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 Electrodeposited Films for the Oxygen Evolution Reaction of Water. J. Phys. Chem. C 2014, 118, 14073–14083. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Ardizzione, S.; Bianchi, C.L.; Tirelli, D. Mn3O4 and γ-MnOOH powders, preparation, phase composition and XPS characterisation. Collied Surf. A Physicochem. Eng. Asp. 1998, 134, 305–312. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Mineral. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Chiodoni, A.; Salvador, G.P.; Massaglia, G.; Delmondo, L.; Munoz-Tabares, J.A.; Sacco, A.; Garino, N.; Castellino, M.; Ahmed, M.D.; Pirri, C.F.; et al. MnxOy- based cathodes for oxygen reduction reaction catalysis in microbial fuel cells. Int. J. Hydrog. Energy 2019, 44, 4432–4441. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Ambrosi, A.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Transition metal dichalcogenides (MoS2, MoSe2, WS2 and WSe2) exfoliation technique has strong influence upon their capacitance. Electrochem. Commun. 2015, 56, 24–28. [Google Scholar] [CrossRef]
- Karade, S.S.; Dubal, D.P.; Sankapal, B.R. MoS2 ultrathin nanoflakes for high performance supercapacitors: Room temperature chemical bath deposition (CBD). RSC Adv. 2016, 6, 39159–39165. [Google Scholar] [CrossRef]
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T.; et al. Vacancy-Induced Ferromagnetism of MoS2 Nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS₂ nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Liu, T.-C.; Pell, W.G.; Conway, B.E.; Roberson, S.L. Behavior of Molybdenum Nitrides as Materials for Electrochemical Capacitors. J. Electrochem. Soc. 1998, 145, 6. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Belanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 2004, 15, 3184–3190. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Murali, S.; Quarles, N.; Zhang, L.L.; Potts, J.R.; Tan, Z.; Lu, Y.; Zhu, Y.; Ruoff, R.S. Volumetric capacitance of compressed activated microwave-expanded graphite oxide (a-MEGO) electrodes. Nano Energy 2013, 2, 764–768. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Nanoscale 2015, 7, 1502–1505. [Google Scholar] [CrossRef]
- Brusse, T.; Toupin, M.; Dugas, R.; Athouël, L.; Crosnier, O.; Bélanger, D. Crystalline MnO2 as Possible Alternatives to Amorphous Compounds in Electrochemical Supercapacitors. J. Electrochem. Soc. 2006, 153, A2171–A2180. [Google Scholar] [CrossRef]
- Ghodbane, O.; Pascal, J.-L.; Favier, F. Microstructural effects on charge-storage properties in MnO 2-based electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2009, 1, 1130–1139. [Google Scholar] [CrossRef]
- Camacho, R.A.P.; Wu, A.M.; Gao, S.; Jin, X.-Z.; Cao, G.-Z.; Huang, H. Mn3O4 nanoparticles encapsulated in carbon cages as the electrode of dual-mechanism supercapacitors. Mater. Chem. 2019, 12, 361–372. [Google Scholar]
- Toupin, M.; Brousse, T.; Belanger, D. Influence of Microstucture on the Charge Storage Properties of Chemically Synthesized Manganese Dioxide. Chem. Mater. 2002, 14, 3946–3952. [Google Scholar] [CrossRef]
- Brezesinski, K.; Wang, J.; Haetge, J.; Reitz, C.; Steinmueller, S.O.; Tolbert, S.H.; Smarsly, B.M.; Dunn, B.; Brezesinski, T. Pseudocapacitive contributions to charge storage in highly ordered mesoporous group v transition metal oxides with iso-oriented layered nanocrystalline domains. J. Am. Chem. Soc. 2010, 132, 6982–6990. [Google Scholar] [CrossRef] [PubMed]
- Soon, J.M.; Loh, K.P. Electrochemical Double-Layer Capacitance of MoS2 Nanowall Films. Electrochem. Solid State Lett. 2007, 10, A250–A254. [Google Scholar] [CrossRef]
- Rowly-Neal, S.J.; Brownson, D.A.; Smith, G.C.; Sawtell, D.A.; Kelly, P.J.; Banks, C.E. 2D nanosheet molybdenum disulphide (MoS2) modified electrodes explored towards the hydrogen evolution reaction. Nanoscale 2015, 7, 18152–18168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lyu, F.; Du, M.; Zhang, M.; Wang, Q.; Yao, J.; Guo, B. Design of two-dimensional, ultrathin MoS2 nanoplates fabricated within one-dimensional carbon nanofibers with thermosensitive morphology: High-performance electrocatalysts for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2014, 6, 22126–22137. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Huang, S.; Yang, W.; Cao, L. Activating MoS2 for pH-Universal Hydrogen Evolution Catalysis. J. Am. Chem. Soc. 2017, 139, 16194–16200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ji, X.; Xu, K.; Chen, C.; Xiong, X.; Xiong, J.; Yao, Y.; Miao, L.; Jiang, J. Unraveling the different charge storage mechanism in T and H phases of MoS2. Electrochem. Acta 2016, 217, 1–8. [Google Scholar] [CrossRef]
- Dupont, M.; Donne, S.W. Separating Faradaic and Non-Faradaic Charge Storage Contributions in Activated Carbon Electrochemical Capacitors Using Electrochemical Methods: I. Step Potential Electrochemical Spectroscopy. J. Electrochem. Soc. 2015, 162, A1246–A1254. [Google Scholar] [CrossRef]
- Yang, X.; Niu, H.; Jiang, H.; Wang, Q.; Qu, F. A high energy density all-solid-state asymmetric supercapacitor based on MoS2/graphene nanosheets and MnO2/graphene hybrid electrodes. J. Mater. Chem. A 2016, 4, 11264. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, D.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Source 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Ge, Z.; Zhao, C.; Qian, X. Facile construction of MoS2/RCF electrode for high-performance supercapacitor. Carbon 2018, 127, 699–706. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Yan, X.; Yuan, X.; Wang, D.; Zhu, Y.; Cheng, X. Vertical MoS2 nanosheets arrays on carbon cloth as binder-free and flexible electrode for high-performance all-solid-state symmetric supercapacitor. Ceram. Int. 2019, 45, 21534–21543. [Google Scholar] [CrossRef]
- Sun, P.; Wang, R.; Qiang, W.; Wang, H.; Wang, X. Uniform MoS2 nanolayer with sulfur vacancy on carbon nanotube networks as binder-free electrodes for asymmetrical supercapacitor. Appl. Surf. Sci. 2019, 475, 793–802. [Google Scholar] [CrossRef]
- Xue, T.; Yang, Y.; Yan, X.-H.; Zou, Z.-L.; Han, F.; Yang, Z. Free standingand binder free Molybdenumbisulfide nanospheres/reduced graphene oxide composite paper as flexible electrodefor symmetric supercapacitor. Mater. Res. Express 2019, 6, 095029. [Google Scholar] [CrossRef]
- Wang, X.; Ding, W.; Li, H.; Li, H.; Zhu, S.; Zhu, X.; Sun, Y.; Dou, S.X. Unveiling highly ambient-stable multilayered 1T- MoS2 towards all-solid-state flexible supercapacitors. J. Mater. Chem. A 2019, 7, 19152. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Zhang, H.; Dong, H.; Zhang, M.; Li, R.; et al. Flexible all-solid-state asymmetric supercapacitor based on three-dimensional MoS2/Ketjen black nanoflower arrays. Int. J. Hydrog. Energy 2019, 44, 13690–13699. [Google Scholar] [CrossRef]
- He, J.; Yang, D.; Li, H.; Cao, X.; Kang, L.; He, X.; Jiang, R.; Sun, J.; Lei, Z.; Liu, Z.-H. Mn3O4/RGO/SWCNT hybrid film for all-solid-state flexible supercapacitor with high energy density. Electrochim. Acta 2018, 283, 174–182. [Google Scholar] [CrossRef]
- Arul, N.S.; Han, J.; Chen, P.C. Solid State Supercapacitor Based on Manganese Oxide@Reduced Graphene Oxide and Polypyrrole Electrodes. CheElectroChem 2018, 5, 2747–2757. [Google Scholar] [CrossRef]
- Aswathy, R.; Ulaganathan, M.; Rugupathy, P. Mn3O4 nanoparticles grown on surface activated graphite paper for aqueous asymmetric supercapacitors. J. Alloy Compd. 2018, 767, 141–150. [Google Scholar] [CrossRef]
- Beyazay, T.; Oztuna, F.E.S.; Unal, U. Self-Standing Reduced Graphene Oxide Papers Electrodeposited with Manganese Oxide Nanostructures as Electrodes for Electrochemical Capacitors. Electrochim. Acta 2019, 296, 916–924. [Google Scholar] [CrossRef]
- Jian, H.; Zhang, Y.; Wang, C.; Wang, Q.; Meng, C.; Wang, J. Rice husk-derived Mn3O4/manganese silicate/C nanostructured composites for high-performance hybrid supercapacitors. Inorg. Chem. Front. 2019, 6, 2788. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Guo, Z.; Sun, B.; Tian, D.; Feng, Y.; Zhang, N.; Sun, K. Hierarchical Mn3O4 Anchored on 3D Graphene Aerogels via C-O-Mn Linkage with Superior Electrochemical Performance for Flexible Asymmetric Supercapacitor. Chem. Eur. J. 2019, 25, 1–6. [Google Scholar]
- Yu, N.; Yin, H.; Zhang, W.; Liu, Y.; Tang, Z.; Zhu, M.Q. High-Performance Fiber-Shaped All-Solid-State Asymmetric Supercapacitors Based on Ultrathin MnO2 Nanosheet/Carbon Fiber Cathodes for Wearable Electronics. Adv. Energy Mater. 2016, 6, 1501458. [Google Scholar] [CrossRef]
- Zhao, C.; Ge, Z.; Zhou, Y.; Huang, Y.; Wang, G.; Qian, X. Solar-assisting pyrolytically reclaimed carbon fiber and their hybrids of MnO2/RCF for supercapacitor electrodes. Carbon 2017, 114, 230–241. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafique, A.; Zubair, U.; Serrapede, M.; Fontana, M.; Bianco, S.; Rivolo, P.; Pirri, C.F.; Lamberti, A. Binder Free and Flexible Asymmetric Supercapacitor Exploiting Mn3O4 and MoS2 Nanoflakes on Carbon Fibers. Nanomaterials 2020, 10, 1084. https://doi.org/10.3390/nano10061084
Rafique A, Zubair U, Serrapede M, Fontana M, Bianco S, Rivolo P, Pirri CF, Lamberti A. Binder Free and Flexible Asymmetric Supercapacitor Exploiting Mn3O4 and MoS2 Nanoflakes on Carbon Fibers. Nanomaterials. 2020; 10(6):1084. https://doi.org/10.3390/nano10061084
Chicago/Turabian StyleRafique, Amjid, Usman Zubair, Mara Serrapede, Marco Fontana, Stefano Bianco, Paola Rivolo, Candido F. Pirri, and Andrea Lamberti. 2020. "Binder Free and Flexible Asymmetric Supercapacitor Exploiting Mn3O4 and MoS2 Nanoflakes on Carbon Fibers" Nanomaterials 10, no. 6: 1084. https://doi.org/10.3390/nano10061084
APA StyleRafique, A., Zubair, U., Serrapede, M., Fontana, M., Bianco, S., Rivolo, P., Pirri, C. F., & Lamberti, A. (2020). Binder Free and Flexible Asymmetric Supercapacitor Exploiting Mn3O4 and MoS2 Nanoflakes on Carbon Fibers. Nanomaterials, 10(6), 1084. https://doi.org/10.3390/nano10061084