Plant Extracts Activated by Cold Atmospheric Pressure Plasmas as Suitable Tools for Synthesis of Gold Nanostructures with Catalytic Uses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Plant Extract Preparation
2.3. Plant Extracts Activation by Cold Atmospheric Pressure Plasmas
2.4. Synthesis of Gold Nanostructures Using Untreated as Well as CAPP-Treated Aqueous Plant Extracts
2.5. Characterization of the Optical and Granulometric Properties of Gold Nanostructures
2.6. Studies of the Interactions and Processes Occur at the Extract-Cold Atmospheric Pressure Plasma Interface and Leading to the Plant Extract Activation
2.7. Gold Nanoparticles Stabilization by Active Compounds Originating from Plant Extracts
2.8. Homogenous Catalysis
3. Results and Discussion
3.1. Optical Properties of Gold Nanostructures
3.2. Granulometric Properties of Gold Nanostructures
3.3. Nature of Plant Extracts Activated by Proper CAPP Source
3.4. Gold Nanoparticles Stabilization by Active Compounds Originating from Plant Extracts
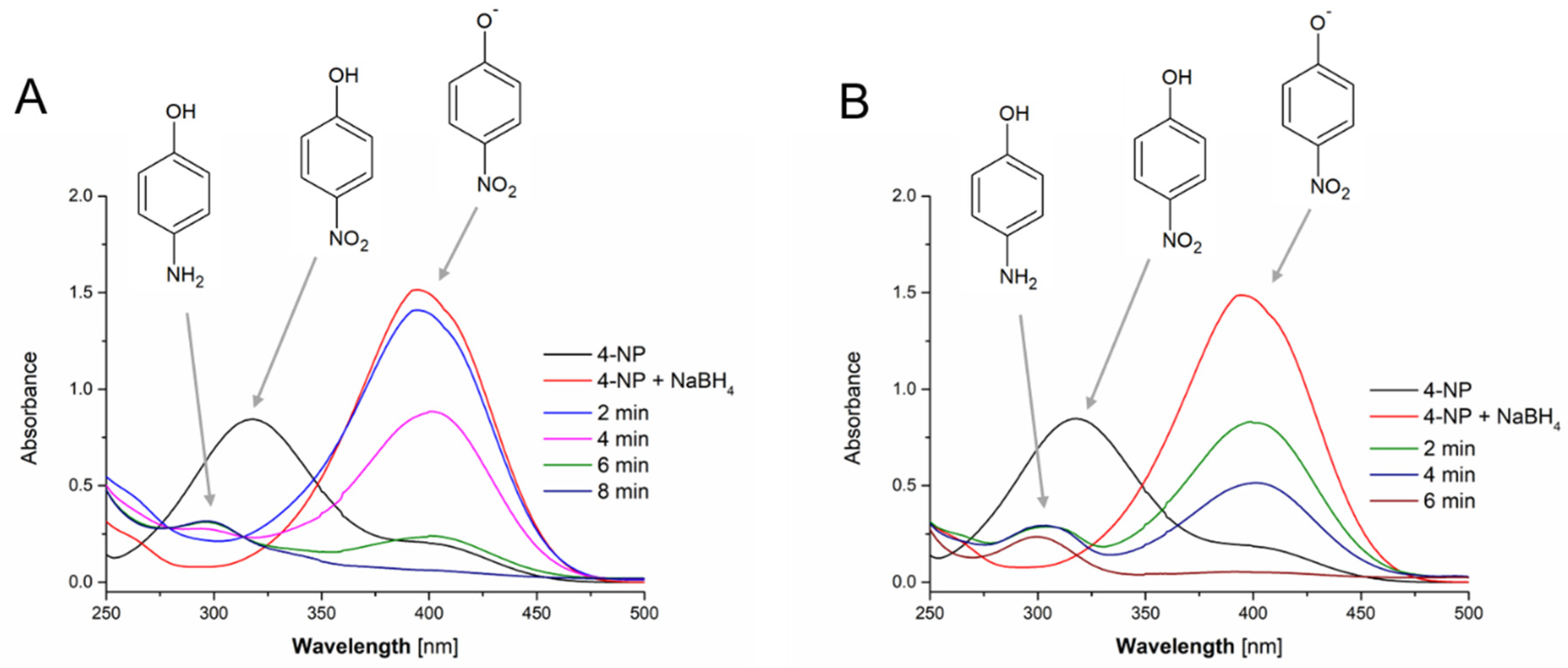
3.5. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Chen, X.; Zheng, Z.; Ke, X.; Jaatinen, E.; Zhao, J.; Guo, C.; Xie, T.; Wang, D. Mechanism of supported gold nanoparticles as photocatalysts under ultraviolet and visible light irradiation. Chem. Commun. 2009, 48, 7524–7526. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface Plasmon Resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt. Commun. 2016, 367, 102–107. [Google Scholar]
- Jiang, Y.; Zhao, H.; Lin, Y.; Zhu, N.; Ma, Y.; Mao, L. Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew. Chem. 2010, 49, 4800. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096. [Google Scholar] [CrossRef]
- Haruta, M. When gold is not noble: Catalysis by nanoparticles. Chem. Rec. 2003, 3, 75. [Google Scholar] [CrossRef]
- Haruta, M.; Date, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A. Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; MubarakAli, D.; Loke, M.F.; Thajuddin, N.; Alharbi, N.S.; Yadavalli, T.; Alagiri, M.; Vadivelu, J. Anti-Helicobacter pylori, cytotoxicity and catalytic activity of biosynthesized gold nanoparticles: Multifaceted application. Arab. J. Chem. 2019, 12, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Kumar, I.; Mondal, M.; Meyappan, V.; Sakthivel, N. Green one-pot synthesis of gold nanoparticles using Sansevieria roxburghiana leaf extract for the catalytic degradation of toxic organic pollutants. Mater. Res. Bull. 2019, 117, 18–27. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Fiore, B.; La Parola, V.; Lazzara, G.; Guernelli, S.; Zaccheroni, N.; Riela, S. Gold nanoparticles stabilized by modified halloysite nanotubes for catalytic applications. Appl. Organomet. Chem. 2019, 33, e4665. [Google Scholar] [CrossRef]
- Nadaf, N.Y.; Kanase, S.S. Biosynthesis of gold nanoparticles by Bacillus marisflavi and its potential in catalytic dye degradation. Arab. J. Chem. 2019, 12, 4806–4814. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.; Nair, K.M.; Paul, N.; Koshy, E.P.; Mathew, B. Catalytic activities of green synthesized silver and gold nanoparticles. Mater. Today 2019, 9, 97–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Bragg, L.M.; Servos, M.R.; Liu, J. Gold nanoparticles as dehydrogenase mimicking nanozymes for estradiol degradation. Chin. Chem. Lett. 2019, 30, 1655–1658. [Google Scholar] [CrossRef]
- Correia, L.S.; Grenman, H.; Warna, J.; Salmi, T.; Murzin, D.Y. Catalytic oxidation kinetics of arabinose on supported gold nanoparticles. Chem. Eng. J. 2019, 370, 952–961. [Google Scholar] [CrossRef]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D.; Jamroz, P.; Pohl, P.; Dzimitrowicz, A. Hydrogel-based nanocomposite catalyst containing uncoated gold nanoparticles synthesized using cold atmospheric pressure plasma for the catalytic decomposition of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123886. [Google Scholar] [CrossRef]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D.; Lesniewicz, A.; Pohl, P.; Dzimitrowicz, A. Highly efficient and convenient nanocomposite catalysts produced using in-situ approach for decomposition of 4-nitrophenol Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124452. [Google Scholar] [CrossRef]
- Cyganowski, P.; Lesniewicz, A.; Dzimitrowicz, A.; Wolska, J.; Pohl, P.; Jermakowicz-Bartkowiak, D. Molecular reactors for synthesis of polymeric nanocomposites with noble metal nanoparticles for catalytic decomposition of 4-nitrophenol. J. Colloid Interface Sci. 2019, 541, 226–233. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, L.; Yuan, H.; Liu, Y.; Gui, Y.; Zhang, B.; Zhou, L.; Fang, S. Heterogeneous gold catalysts for selective hydrogenation: From nanoparticles to atomically precise nanoclusters. Nanoscale 2019, 11, 11429–11436. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W. Homogeneous Catalysis: Understanding the Art; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Pandey, P.; Shukla, S.; Pandey, Y. 3-Aminopropyltrimethoxysilane and grapheme oxide/reduced graphene oxide-induced generation of gold nanoparticles and their nanocomposites: Electrocatalytic and kinetic activity. RSC Adv. 2016, 6, 80549. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Dzimitrowicz, A.; Berent, S.; Motyka, A.; Jamroz, P.; Kurcbach, K.; Sledz, W.; Pohl, P. Comparison of the characteristics of gold nanoparticles synthesized using aqueous plant extracts and natural plant essential oils of Eucalyptus globulus and Rosmarinus officinalis. Arab. J. Chem. 2019, 12, 4795–4805. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Jamroz, P.; Sergiel, I.; Kozlecki, T.; Pohl, P. Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. Arab. J. Chem. 2019, 12, 4118–4130. [Google Scholar] [CrossRef] [Green Version]
- Zayed, M.F.; Mahfoze, R.A.; El-kousy, S.M.; Al-Ashkar, E.A. In-vitro antioxidant and antimicrobial activities of metal nanoparticles biosynthesized using optimized Pimpinella anisum extract. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124167. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Kala, S.M.J.; Pushparaj, T.L. Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J. Drug Deliv. Sci. Technol. 2020, 57, 101620. [Google Scholar] [CrossRef]
- Haddada, M.B.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef]
- Vo, T.T.; Dang, C.H.; Doan, V.D.; Dang, V.S.; Nguyen, T.D. Biogenic synthesis of silver and gold nanoparticles from Lactuca indica leaf extract and their application in catalytic degradation of toxic compounds. J. Inorg. Organomet. Polym. Mater. 2020, 30, 388–399. [Google Scholar] [CrossRef]
- Vijilvani, C.; Bindhu, M.R.; Frincy, F.C.; AlSalhi, M.S.; Sabitha, S.; Saravanakumar, K.; Devanesan, S.; Umadevi, M.; Aljaafreh, M.J.; Atif, M. Antimicrobial and catalytic activities of biosynthesized gold, silver and palladium nanoparticles from Solanum nigurum leaves. J. Photochem. Photobiol. B 2020, 202, 111713. [Google Scholar] [CrossRef]
- Unal, I.S.; Demirbas, A.; Onal, I.; Ildiz, N.; Ocsoy, I. One step preparation of stable gold nanoparticle using red cabbage extracts under UV light and its catalytic activity. J. Photochem. Photobiol. B 2020, 204, 111800. [Google Scholar] [CrossRef]
- Uzma, M.; Sunayana, N.; Raghavendra, V.B.; Madhu, C.S.; Shanmuganathan, R.; Brindhadevi, K. Biogenic synthesis of gold nanoparticles using Commiphora wightii and their cytotoxic effects on breast cancer cell line (MCF-7). Process. Biochem. 2020, 92, 269–276. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, G.S.; Tang, Y.P.; Qian, Y.F.; Guan, H.L.; Pang, H.; Qin, Y. Simultaneous quantification of flavonol glycosides, terpene lactones, biflavones, proanthocyanidins, and ginkgolic acids in Ginkgo biloba leaves from fruit cultivars by ultrahigh-performance liquid chromatography coupled with triple quadrupole mass spectrometry. BioMed Res. Int. 2013, 2013, 1–11. [Google Scholar]
- Velmurugan, P.; Shim, J.; Bang, K.K.; Oh, B.T. Gold nanoparticles mediated coloring of fabrics and leather for antibacterial activity. J. Photochem. Photobiol. B 2016, 160, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Dong, C.; Wang, X.; Zhang, X.; Xiao, X.; Yang, X. Green synthesis and characterization of monodisperse gold nanoparticles using Ginkgo biloba leaf extract. Optik 2017, 144, 511–521. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Mahmud, S.; Liu, H. Gold nanoparticles biosynthesized using Ginkgo biloba leaf aqueous extract for the decolorization of azo-dyes and fluorescent detection of Cr (VI). J. Clust. Sci. 2019, 31, 549–560. [Google Scholar] [CrossRef]
- Lu, J.M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Leonard, K.; Ahmmad, B.; Okamura, H.; Kurawaki, J. In situ green synthesis of biocompatible ginseng capped gold nanoparticles with remarkable stability. Colloids Surf. B Biointerfaces 2011, 82, 391–396. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Markus, J.; Kim, Y.J.; Wang, C.; Perez, Z.E.J.; Ahn, S.; Aceituno, V.C.; Mathiyalagan, R.; Yang, D.C. Coalescence of functional gold and monodisperse silver nanoparticles mediated by black Panax ginseng Meyer root extract. Int. J. Nanomed. 2016, 11, 6621–6634. [Google Scholar] [CrossRef] [Green Version]
- Hurh, J.; Markus, J.; Kim, Y.J.; Ahn, S.; Castro-Aceituno, V.; Mathiyalagan, R.; Kim, J.Y.; Yang, D.C. Facile reduction and stabilization of ginsenoside-functionalized gold nanoparticles: Optimization, characterization, and in vitro cytotoxicity studies. J. Nanopart. Res. 2017, 19, 313. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Li, Y. A review of plasma-liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar] [CrossRef] [Green Version]
- Zadi, T.; Assadi, A.A.; Nasrallah, N.; Bouallouche, R.; Tri, P.N.; Bouzaza, A.; Azizi, M.M.; Maachi, R.; Wolbert, D. Treatment of hospital indoor air by a hybrid system of combined plasma with photocatalysis: Case of trichloromethane. Chem. Eng. J. 2018, 349, 276–286. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Comparative study between laboratory and large pilot scales for VOC’s removal from gas streams in continuous flow surface discharge plasma. Chem. Eng. Res. Des. 2016, 106, 308–314. [Google Scholar] [CrossRef]
- Assadi, A.A.; Loganathan, S.; Tri, P.N.; Ghaida, S.G.A.; Bouzaza, A.; Tuan, A.N.; Wolbert, D. Pilot scale degradation of mono and multi volatile organic compounds by surface discharge plasma/TiO2 reactor: Investigation of competition and synergism. J. Hazard. Mater. 2018, 357, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Dzimitrowicz, A.; Greda, K.; Lesniewicz, T.; Jamroz, P.; Nyk, M.; Pohl, P. Size-controlled synthesis of gold nanoparticles by a novel atmospheric pressure glow discharge system with a metallic pin electrode and a flowing liquid electrode. RSC Adv. 2016, 6, 80773–80783. [Google Scholar] [CrossRef] [Green Version]
- Mie, G. A contribution to the optics of turbid media, especially colloidal metallic suspensions. Ann. Phys. 1908, 25, 377–445. [Google Scholar] [CrossRef]
- Shirai, N.; Uchida, S.; Tochikubo, F. Synthesis of metal nanoparticles by dual plasma electrolysis using atmospheric dc glow discharge in contact with liquid. Jpn. J. Appl. Phys. 2014, 53, 046202. [Google Scholar] [CrossRef]
- Tochikubo, F.; Shimokawa, Y.; Shirai, N.; Uchida, S. Chemical reactions in liquid induced by atmospheric-pressure dc glow discharge in contact with liquid. Jpn. J. Appl. Phys. 2014, 53, 126201. [Google Scholar] [CrossRef]
- Badi’ah, H.I.; Seedeh, F.; Supriyanto, G.; Zaidan, A.H. Synthesis of Silver Nanoparticles and the Development in Analysis Method. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jawa Timur, Indonesia, 11–12 October 2018; Volume 217, p. 012005. [Google Scholar]
- Koczka, N.; Stefanovits-Banyai, E. Element composition of Ginkgo Biloba, L. leaves. In Ginkgo Biloba: Biology, Uses and Health Benefits; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 29–35. [Google Scholar]
- Dzimitrowicz, A.; Bielawska-Pohl, A.; Pohl, P.; Jermakowicz-Bartkowiak, D.; Jamroz, P.; Malik-Gajewska, M.; Klimczak, A.; Cyganowski, P. Application of Oil-in-Water Nanoemulsion Carrying Size-Defined Gold Nanoparticles Synthesized by Non-Thermal Plasma for the Human Breast Cancer Cell Lines Migration and Apoptosis. Plasma Chem. Plasma Process. 2020. [Google Scholar] [CrossRef] [Green Version]
- Goswami, A.M.; Ghosh, S. Biological synthesis of colloidal gold nanoprisms using Penicillium citrinum MTCC9999. J. Biomater. Nanobiotechnol. 2013, 4, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Jamroz, P.; Greda, K.; Pohl, P.; Zyrnicki, W. Atmospheric Pressure Glow Discharges Generated in Contact with Flowing Liquid Cathode: Production of Active Species and Application in Wastewater Purification Processes. Plasma Chem. Plasma Process. 2014, 34, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Perala, S.R.K.; Kumar, S. On the two-step mechanism for synthesis of transition-metal nanoparticles. Langmuir 2014, 30, 12703–12711. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Nemcova, L.; Maguire, P.; Graham, W.G.; Mariotti, D. Synthesis of surfactant-free electrostatically stabilized gold nanoparticles by plasma-induced liquid chemistry. Nanotechnology 2013, 24, 245604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, S.; Ghosh, S.K.; Nath, S.; Pande, S.; Praharaj, S.; Panigrahi, S.; Basu, S.; Endo, T.; Pal, T. Synthesis of silver nanoshell-coated cationic polystyrene beads: A solid phase catalyst for the reduction of 4-nitrophenol. Appl. Catal. A Gen. 2006, 313, 41–48. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Panicker, S.; Chehimi, M.M.; Monge, M.; Lopez-de-Luzuriaga, J.M.; Mohamed, A.A.; Bruce, A.E.; Bruce, M.R. Synthesis of water-soluble gold–aryl nanoparticles with distinct catalytic performance in the reduction of the environmental pollutant 4-nitrophenol. Catal. Sci. Technol. 2019, 9, 6059–6071. [Google Scholar] [CrossRef]
- Larm, N.E.; Thon, J.A.; Vazmitsel, Y.; Atwood, J.L.; Baker, G.A. Borohydride stabilized gold–silver bimetallic nanocatalysts for highly efficient 4-nitrophenol reduction. Nanoscale Adv. 2019, 1, 4665–4668. [Google Scholar] [CrossRef] [Green Version]
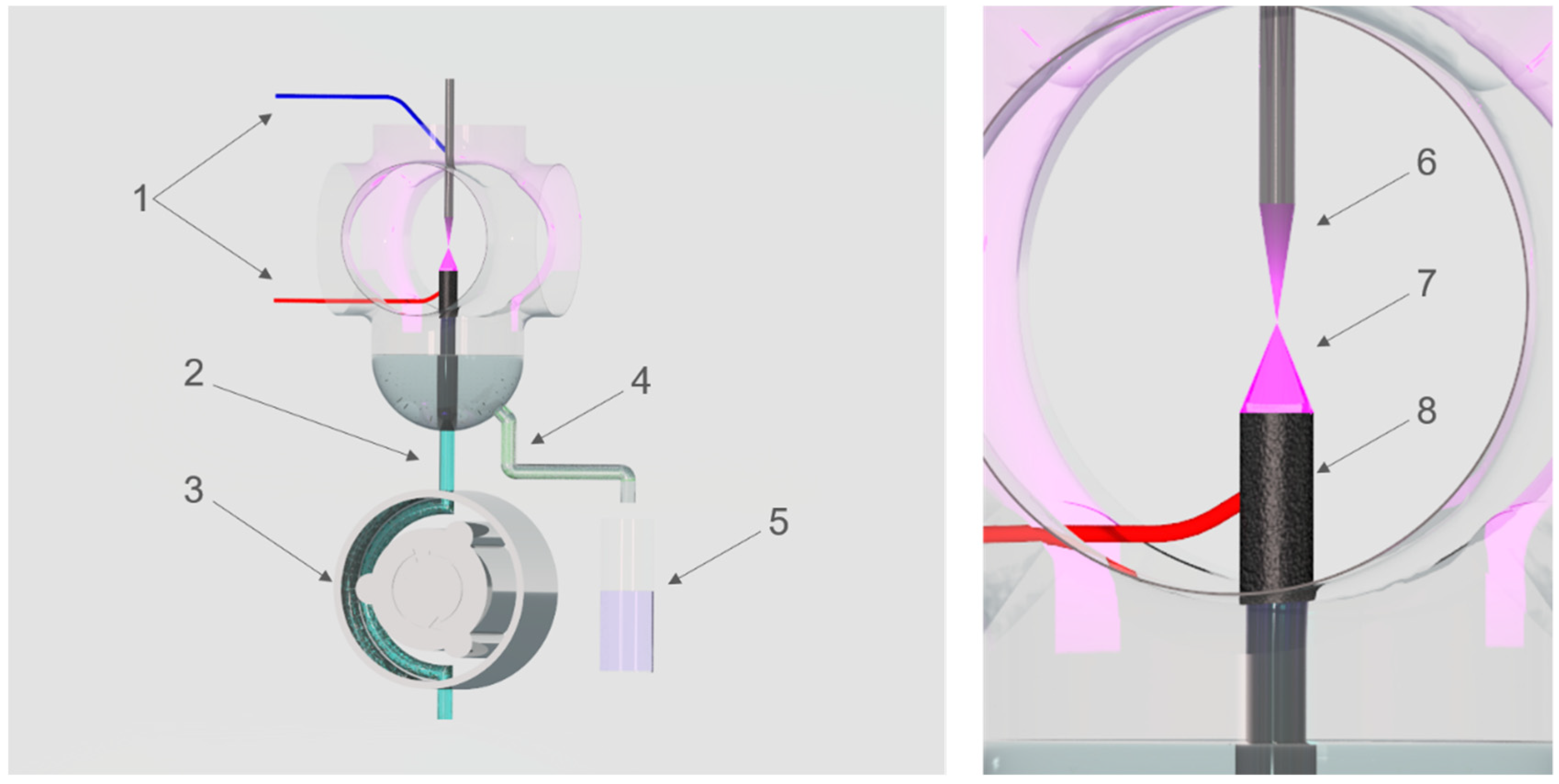





| Type of Plant Extract | Sample | Au(III) Concentration (mg L−1) | λmax (nm) | A |
|---|---|---|---|---|
| G. biloba | untreated | 125 | 501.2 | 0.1444 |
| G. biloba | FLA-dc-APGD | 125 | 529.6 | 0.1897 |
| G. biloba | FLC-dc-APGD | 125 | 524.8 | 0.2010 |
| G. biloba | untreated | 250 | 531.6 | 0.2087 |
| G. biloba | FLA-dc-APGD | 250 | 533.6 | 0.2437 |
| G. biloba | FLC-dc-APGD | 250 | 533.6 | 0.2297 |
| G. biloba | untreated | 500 | 536.0 | 0.3028 |
| G. biloba | FLA-dc-APGD | 500 | 535.4 | 0.4736 |
| G. biloba | FLC-dc-APGD | 500 | 544.2 | 0.4503 |
| P. ginseng | untreated | 125 | 546.6 | 0.0570 |
| P. ginseng | FLA-dc-APGD | 125 | 547.8 | 0.1333 |
| P. ginseng | FLC-dc-APGD | 125 | 542.0 | 0.1465 |
| P. ginseng | untreated | 250 | 547.2 | 0.0519 |
| P. ginseng | FLA-dc-APGD | 250 | 555.2 | 0.0835 |
| P. ginseng | FLC-dc-APGD | 250 | 551.4 | 0.0802 |
| P. ginseng | untreated | 500 | 545.2 | 0.0470 |
| P. ginseng | FLA-dc-APGD | 500 | 554.2 | 0.0766 |
| P. ginseng | FLC-dc-APGD | 500 | 546.4 | 0.0669 |
| Shape Distribution (%) | Diameter (nm) | ||||||
|---|---|---|---|---|---|---|---|
| Plant Extract | Sample | Spherical | Rod | Triangular | Pentagonal | Hexagonal | |
| G. biloba | untreated | 94.6 | 1.2 | 3.9 | 0.7 | 0.8 | 15.6 ± 7.0 |
| G. biloba | FLA-dc-APGD | 87.0 | 1.8 | 5.3 | 2.6 | 3.3 | 24.4 ± 4.4 |
| G. biloba | FLC-dc-APGD | 92.7 | 2.8 | 3.9 | 0.6 | 0.0 | 29.0 ± 3.5 |
| P. ginseng | untreated | 82.6 | 2.7 | 10.4 | 2.3 | 2.0 | 16.0 ± 5.5 |
| P. ginseng | FLA-dc-APGD | 73.2 | 6.8 | 14.1 | 2.0 | 3.9 | 21.3 ± 12.3 |
| P. ginseng | FLC-dc-APGD | 86.8 | 2.2 | 8.4 | 1.3 | 1.3 | 14.2 ± 3.8 |
| Functionality | Band Locations (cm−1) | ||||
|---|---|---|---|---|---|
| Untreated Extract | Extract + AuNPs | Extract Treated by FLA-dc-APGD + AuNPs | Extract Treated by FLC-dc-APGD + AuNPs | ||
| G. biloba | N–H; O–H (Amide A) | 3243 | 3245 | 3246 | 3245 |
| C=O (Amide I) | 1635 | 1634 | 1635 | 1635 | |
| P. ginseng | N–H; O–H (Amide A) | 3254 | 3254 | 3254 | 3254 |
| C=O (Amide I) | 1635 | 1635 | 1635 | 1635 | |
| Nano Catalyst | Synthetic Route | Average Diameter of AuNPs (nm) | km (s−1 mg−1) | Ref. |
|---|---|---|---|---|
| Au | Bio-based using S. roxburghian | 17.48 | 0.43 | [10] |
| Immobilization on thiol-functionalized halloysite nanotubes | 4.20 | 0.16 | [11] | |
| Diazonium-Au (III) reduced in water | 68.20 | 0.24 | [57] | |
| Au-Ag | Borohydride-stabilized | 4.60 | 57.9 (at high Ag concentration) | [58] |
| Au | G. biloba FLC-dc-APGD-activated extract | 29.0 | 2.04 | This work |
| P. ginseng FLC-dc-APGD-activated extract | 14.2 | 3.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzimitrowicz, A.; Cyganowski, P.; Pohl, P.; Milkowska, W.; Jermakowicz-Bartkowiak, D.; Jamroz, P. Plant Extracts Activated by Cold Atmospheric Pressure Plasmas as Suitable Tools for Synthesis of Gold Nanostructures with Catalytic Uses. Nanomaterials 2020, 10, 1088. https://doi.org/10.3390/nano10061088
Dzimitrowicz A, Cyganowski P, Pohl P, Milkowska W, Jermakowicz-Bartkowiak D, Jamroz P. Plant Extracts Activated by Cold Atmospheric Pressure Plasmas as Suitable Tools for Synthesis of Gold Nanostructures with Catalytic Uses. Nanomaterials. 2020; 10(6):1088. https://doi.org/10.3390/nano10061088
Chicago/Turabian StyleDzimitrowicz, Anna, Piotr Cyganowski, Pawel Pohl, Weronika Milkowska, Dorota Jermakowicz-Bartkowiak, and Piotr Jamroz. 2020. "Plant Extracts Activated by Cold Atmospheric Pressure Plasmas as Suitable Tools for Synthesis of Gold Nanostructures with Catalytic Uses" Nanomaterials 10, no. 6: 1088. https://doi.org/10.3390/nano10061088
APA StyleDzimitrowicz, A., Cyganowski, P., Pohl, P., Milkowska, W., Jermakowicz-Bartkowiak, D., & Jamroz, P. (2020). Plant Extracts Activated by Cold Atmospheric Pressure Plasmas as Suitable Tools for Synthesis of Gold Nanostructures with Catalytic Uses. Nanomaterials, 10(6), 1088. https://doi.org/10.3390/nano10061088









