Functionalized Graphene Derivatives and TiO2 for High Visible Light Photodegradation of Azo Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide (GO)
2.2. Synthesis of Heteroatom Reduced Graphene Oxide (rGO)
2.3. Synthesis of Graphene Derivative-TiO2 Composites
2.4. Characterization Techniques
2.5. Photocatalytic Experiments
3. Results and Discussion
3.1. Materials Characterization
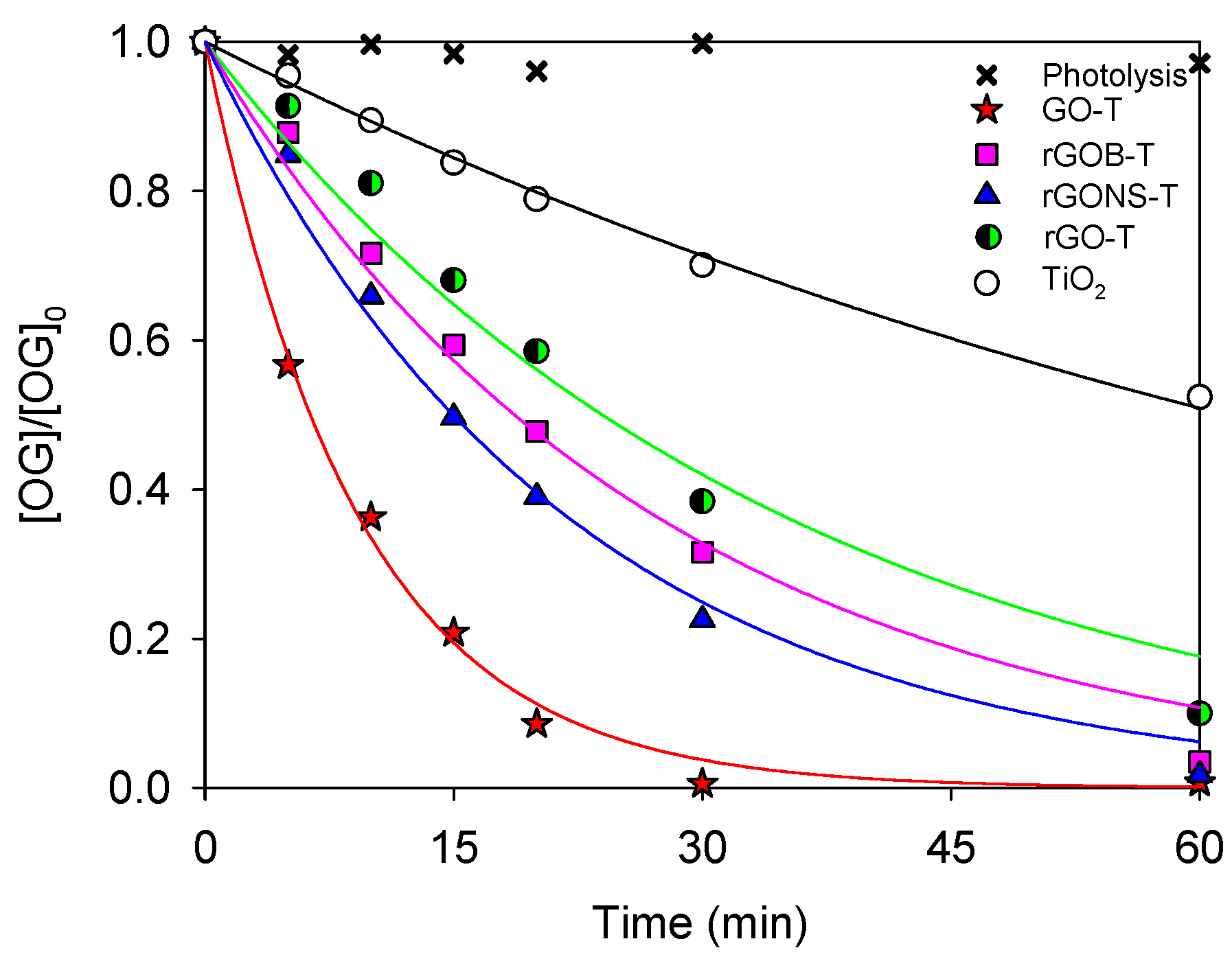
3.2. Photocatalytic Degradation of OG
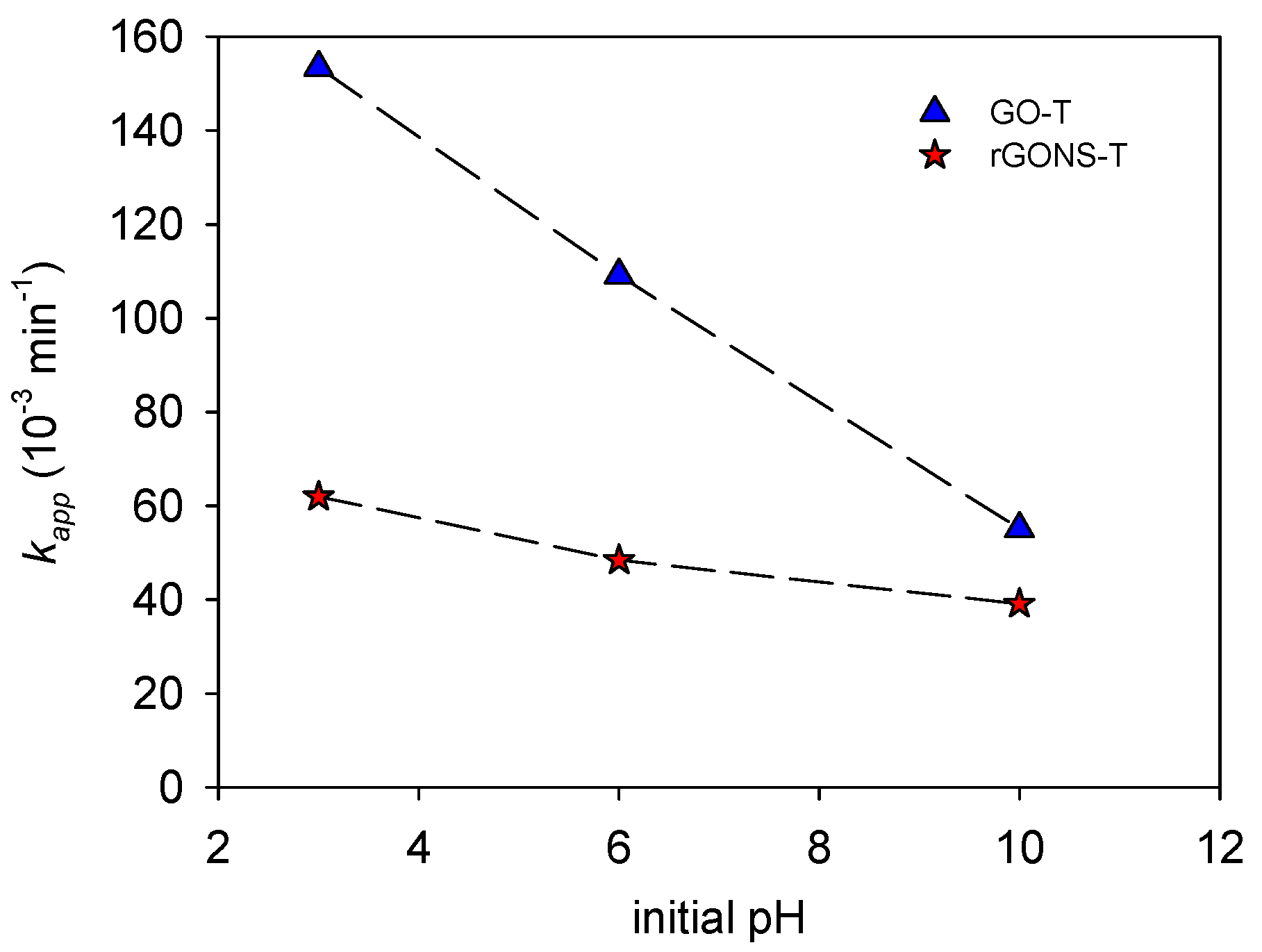
3.2.1. Influence of pH on OG Degradation
3.2.2. Photocatalytic Degradation Pathway
3.2.3. Reutilization Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Rodríguez, S.M.; Galvez, J.B.; Gasca, C.A.E. Photocatalysis. Sol. Energy 2004, 77, 443–444. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123, 241–256. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Konstantinou, I.; A Albanis, T. TiO2-Assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Maeda, K.; Higashi, M.; Lu, D.; Abe, R.; Domen, K. Efficient Nonsacrificial Water Splitting through Two-Step Photoexcitation by Visible Light using a Modified Oxynitride as a Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2010, 132, 5858–5868. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.-G.; Lu, G.M.; Cheng, H.-M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.S.; Grady, A.M.; Koodali, R.T. Lanthanide modified semiconductor photocatalysts. Catal. Sci. Technol. 2012, 2, 683. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Majima, T.; Choi, W. Photocatalysis of Dye-Sensitized TiO2 Nanoparticles with Thin Overcoat of Al2O3: Enhanced Activity for H2 Production and Dechlorination of CCl4. J. Phys. Chem. C 2009, 113, 10603–10609. [Google Scholar] [CrossRef]
- Police, A.K.R.; Vattikuti, S.P.; Baik, Y.-J.; Byon, C.; Vatikuti, S.P. Eco-Friendly, hydrogen fluoride-Free, morphology-Oriented synthesis of TiO2 with exposed (001) facets. Ceram. Int. 2019, 45, 2178–2184. [Google Scholar] [CrossRef]
- Police, A.K.R.; Vattikuti, S.V.P.; Mandari, K.K.; Chennaiahgari, M.; Mangalampalli, V.P.S.; Valluri, K.; Byon, C. Bismuth oxide cocatalyst and copper oxide sensitizer in Cu2O/TiO2/Bi2O3 ternary photocatalyst for efficient hydrogen production under solar light irradiation. Ceram. Int. 2018, 44, 11783–11791. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Reddy, P.A.K.; Nagajyothi, P.; Shim, J.; Byon, C. Hydrothermally synthesized Na2Ti3O7 nanotube–V2O5 heterostructures with improved visible photocatalytic degradation and hydrogen evolution Its photocorrosion suppression. J. Alloys Compd. 2018, 740, 574–586. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M. Design of graphene-Based TiO2 photocatalysts—A review. Environ. Sci. Pollut. Res. 2012, 19, 3676–3687. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Silva, T.L.S.; Pastrana-Martínez, L.M.; Brandão, A.T.; Figueiredo, J.L.; Silva, A.M. Modification of the surface chemistry of single and multi-Walled carbon nanotubes by HNO3 and H2SO4 hydrothermal oxidation for application in direct contact membrane distillation. Phys. Chem. Chem. Phys. 2014, 16, 12237–12250. [Google Scholar] [CrossRef]
- Liang, D.; Cui, C.; Hub, H.; Wang, Y.; Xu, S.; Ying, B.; Li, P.; Lu, B.; Shen, H. One-Step hydrothermal synthesis of anatase TiO2/reduced graphene oxide nanocomposites with enhanced photocatalytic activity. J. Alloys Compd. 2014, 582, 236–240. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Chenab, C.; Wang, X. Enhanced photocatalytic degradation of methylene blue under visible irradiation on graphene@TiO2 dyade structure. Appl. Catal. B Environ. 2012, 111, 303–308. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 photocatalysts with enhanced photogenerated charges separation and reactant adsorption for high selective photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Fattahi, A.; Liang, R.; Kaur, A.; Schneider, O.; Arlos, M.J.; Peng, P.; Servos, M.; Zhou, N. Photocatalytic degradation using TiO2-Graphene nanocomposite under UV-LED illumination: Optimization using response surface methodology. J. Environ. Chem. Eng. 2019, 7, 103366. [Google Scholar] [CrossRef]
- Police, A.K.R.; Chennaiahgari, M.; Boddula, R.; Vattikuti, S.P.; Mandari, K.K.; Chan, B. Single-Step hydrothermal synthesis of wrinkled graphene wrapped TiO2 nanotubes for photocatalytic hydrogen production and supercapacitor applications. Mater. Res. Bull. 2018, 98, 314–321. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Peng, W.-X.; Jermsittiparsert, K.; Hosseinzadeh, G.; Hosseinzadeh, R. Combination of sonochemical and freeze-Drying methods for synthesis of graphene/Ag-Doped TiO2 nanocomposite: A strategy to boost the photocatalytic performance via well distribution of nanoparticles between graphene sheets. Ceram. Int. 2020, 46, 7446–7452. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, Y.-J.; Huang, W.; Chen, P. Heteroatom-Doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-Doping of Graphene Through Electrothermal Reactions with Ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Liang, X.-Q.; Song, W.; Wu, Z.-Y. Identification of the nitrogen species on N-Doped graphene layers and Pt/NG composite catalyst for direct methanol fuel cell. Phys. Chem. Chem. Phys. 2010, 12, 12055. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.; Xia, X.-H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Wu, D.; Liao, L.; Zhao, S.; Peng, H.; Liu, Z. Synthesis of Boron-Doped Graphene Monolayers Using the Sole Solid Feedstock by Chemical Vapor Deposition. Small 2013, 9, 1316–1320. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.; Gou, X.; Xu, W. Phosphorus-Doped graphene nanosheets as efficient metal-Free oxygen reduction electrocatalysts. RSC Adv. 2013, 3, 9978. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2011, 6, 205–211. [Google Scholar] [CrossRef]
- Poh, H.L.; Šimek, P.; Sofer, Z.; Pumera, M. Sulfur-Doped Graphene via Thermal Exfoliation of Graphite Oxide in H2S, SO2, or CS2 Gas. ACS Nano 2013, 7, 5262–5272. [Google Scholar] [CrossRef]
- Putri, L.K.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Heteroatom doped graphene in photocatalysis: A review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Pedrosa, M.; Da Silva, E.S.; Pastrana-Martínez, L.M.; Drazic, G.; Falaras, P.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M. Hummers’ and Brodie’s graphene oxides as photocatalysts for phenol degradation. J. Colloid Interface Sci. 2020, 567, 243–255. [Google Scholar] [CrossRef]
- Mou, Z.; Wu, Y.; Sun, J.; Yang, P.; Du, Y.; Lu, C. TiO2 Nanoparticles-Functionalized N-Doped Graphene with Superior Interfacial Contact and Enhanced Charge Separation for Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2014, 6, 13798–13806. [Google Scholar] [CrossRef]
- Pedrosa, M.; Pastrana-Martínez, L.M.; Pereira, M.F.R.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M. N/S-Doped graphene derivatives and TiO2 for catalytic ozonation and photocatalysis of water pollutants. Chem. Eng. J. 2018, 348, 888–897. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Joshi, H.M.; Kumar, P.; Panchakarla, L.; Rao, C. Selectivity in the photocatalytic properties of the composites of TiO2 nanoparticles with B- and N-doped graphenes. Chem. Phys. Lett. 2011, 511, 304–308. [Google Scholar] [CrossRef]
- Albero, J.; Garcia, H. Doped graphenes in catalysis. J. Mol. Catal. A Chem. 2015, 408, 296–309. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jiang, Y.; Muthukaruppan, S.; Balasubramanian, R. Effect of boron doping level on the photocatalytic activity of graphene aerogels. Carbon 2018, 128, 237–248. [Google Scholar] [CrossRef]
- Cai, M.Q.; Su, J.; Zhu, Y.; Wei, X.; Dionysiou, D.D.; Zhang, H.; Dong, C.; Wei, Z. Decolorization of azo dyes Orange G using hydrodynamic cavitation coupled with heterogeneous Fenton process. Ultrason. SonoChem. 2016, 28, 302–310. [Google Scholar] [CrossRef]
- Verma, P.; Samanta, S.K. Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ. Chem. Lett. 2018, 16, 969–1007. [Google Scholar] [CrossRef]
- Tarkwa, J.-B.; Oturan, M.A.; Acayanka, E.; Laminsi, S.; Oturan, M.A. Photo-Fenton oxidation of Orange G azo dye: Process optimization and mineralization mechanism. Environ. Chem. Lett. 2018, 17, 473–479. [Google Scholar] [CrossRef]
- Tekin, D.; Kiziltas, H.; Ungan, H. Kinetic evaluation of ZnO/TiO2 thin film photocatalyst in photocatalytic degradation of Orange G. J. Mol. Liq. 2020, 306, 112905. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Fernández-Sáez, N.; Villela-Martinez, D.E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Pastrana-Martínez, L.M. Heteroatom-doped graphene aerogels and carbon-magnetite catalysts for the heterogeneous electro-Fenton degradation of acetaminophen in aqueous solution. J. Catal. 2019, 378, 68–79. [Google Scholar] [CrossRef]
- Ren, X.; Guo, H.; Feng, J.; Si, P.; Zhang, L.; Ci, L. Synergic mechanism of adsorption and metal-free catalysis for phenol degradation by N-doped graphene aerogel. Chemosphere 2018, 191, 389–399. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M. Graphene oxide-P25 photocatalysts for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Surf. Sci. 2013, 275, 361–368. [Google Scholar] [CrossRef]
- García, M.; Ángeles, F.; Rivera-Utrilla, J.; Bautista-Toledo, I.; Moreno-Castilla, C. Adsorption of Humic Substances on Activated Carbon from Aqueous Solutions and Their Effect on the Removal of Cr(III) Ions. Langmuir 1998, 14, 1880–1886. [Google Scholar] [CrossRef]
- Newcombe, G.; Hayes, R.; Drikas, M. Granular activated carbon: Importance of surface properties in the adsorption of naturally occurring organics. Colloids Surf. A Physicochem. Eng. Asp. 1993, 78, 65–71. [Google Scholar] [CrossRef]
- Silva, C.G.; Faria, J.L. Anatase vs. rutile efficiency on the photocatalytic degradation of clofibric acid under near UV to visible irradiation. Photochem. Photobiol. Sci. 2009, 8, 705. [Google Scholar] [CrossRef]
- Mali, S.S.; Desai, S.K.; Dalavi, D.S.; Betty, C.A.; Bhosale, P.N.; Patil, P.S. CdS-Sensitized TiO2 nanocorals: Hydrothermal synthesis, characterization, application. Photochem. Photobiol. Sci. 2011, 10, 1652–1658. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.-L.; Sun, X.-D.; Li, H.-D. Synthesis and characterization of amorphous TiO2 with wormhole-like framework mesostructure. J. Non-Cryst. Solids 2003, 319, 109–116. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Falaras, P.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M. Role of oxygen functionalities on the synthesis of photocatalytically active graphene–TiO2 composites. Appl. Catal. B Environ. 2014, 158, 329–340. [Google Scholar] [CrossRef]
- Van Khai, T.; Gil Na, H.; Kwak, D.S.; Kwon, Y.J.; Ham, H.; Shim, K.B.; Kim, H.W. Significant enhancement of blue emission and electrical conductivity of N-doped graphene. J. Mater. Chem. 2012, 22, 17992. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Wang, Y.; Wang, M.; Qiu, R.; Shu, Z.; Zhang, L.; Hua, Z.; Cui, F.; Wei, C.; et al. One-Step synthesis of sulfur doped graphene foam for oxygen reduction reactions. Dalton Trans. 2014, 43, 3420–3423. [Google Scholar] [CrossRef]
- Van Khai, T.; Gil Na, H.; Kwak, D.S.; Kwon, Y.J.; Ham, H.; Shim, K.B.; Kim, H.W. Comparison study of structural and optical properties of boron-doped and undoped graphene oxide films. Chem. Eng. J. 2012, 211, 369–377. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.; Qiao, S.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.-M.; Lu, G. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Papageorgiou, S.K.; Katsaros, F.; Romanos, G.E.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M. Photocatalytic behaviour of nanocarbon–TiO2 composites and immobilization into hollow fibres. Appl. Catal. B Environ. 2013, 142, 101–111. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and Reduced Graphene Oxide as Efficient Photocatalysts for Hydrogen Evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Sun, J.; Qiao, L.; Sun, S.-P.; Wang, G. Photocatalytic degradation of Orange G on nitrogen-Doped TiO2 catalysts under visible light and sunlight irradiation. J. Hazard. Mater. 2008, 155, 312–319. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [Green Version]
- Rengifo-Herrera, J.; Pierzchała, K.; Sienkiewicz, A.; Forro, L.; Kiwi, J.; Pulgarin, C. Abatement of organics and Escherichia coli by N, S co-doped TiO2 under UV and visible light. Implications of the formation of singlet oxygen (1O2) under visible light. Appl. Catal. B Environ. 2009, 88, 398–406. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Zhang, Q.; Li, Y.-H.; Li, L. Three-Dimensional network graphene aerogel for enhancing adsorption and visible light photocatalysis of nitrogen-doped TiO2. Mater. Lett. 2019, 234, 298–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.M.; Park, S.-J. Synthesis and characterization of nitrogen-doped TiO2 coatings on reduced graphene oxide for enhancing the visible light photocatalytic activity. Curr. Appl. Phys. 2018, 18, 163–169. [Google Scholar] [CrossRef]
- Munikrishnappa, C.; Kumar, S.; Shivakumara, S.; Rao, G.M.; Munichandraiah, N. The TiO2-Graphene oxide-Hemin ternary hybrid composite material as an efficient heterogeneous catalyst for the degradation of organic contaminants. J. Sci. Adv. Mater. Devices 2019, 4, 80–88. [Google Scholar] [CrossRef]
- Prabhu, S.; Cindrella, L.; Kwon, O.J.; Mohanraju, K. Photoelectrochemical, photocatalytic and photochromic performance of rGO-TiO2WO3 composites. Mater. Chem. Phys. 2019, 224, 217–228. [Google Scholar] [CrossRef]
- Kim, S.-R.; Ali, I.; Kim, J.-O. Effect of synthesis parameters on visible light photocatalytic activity of graphene-TiO2 nanocomposites for industrial wastewater treatment. J. Ind. Eng. Chem. 2018, 66, 370–380. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Fluder, M.; Tylus, W.; Jesionowski, T. Investigation of amino-Grafted TiO2/reduced graphene oxide hybrids as a novel photocatalyst used for decomposition of selected organic dyes. J. Environ. Manag. 2018, 212, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Fang, S.-S.; Mei, J.-Y.; Zheng, G.-P.; Zheng, X.-C.; Guan, X.-X. High-Efficiency removal of rhodamine B dye in water using g-C3N4 and TiO2 co-hybridized 3D graphene aerogel composites. Sep. Purif. Technol. 2018, 194, 96–103. [Google Scholar] [CrossRef]

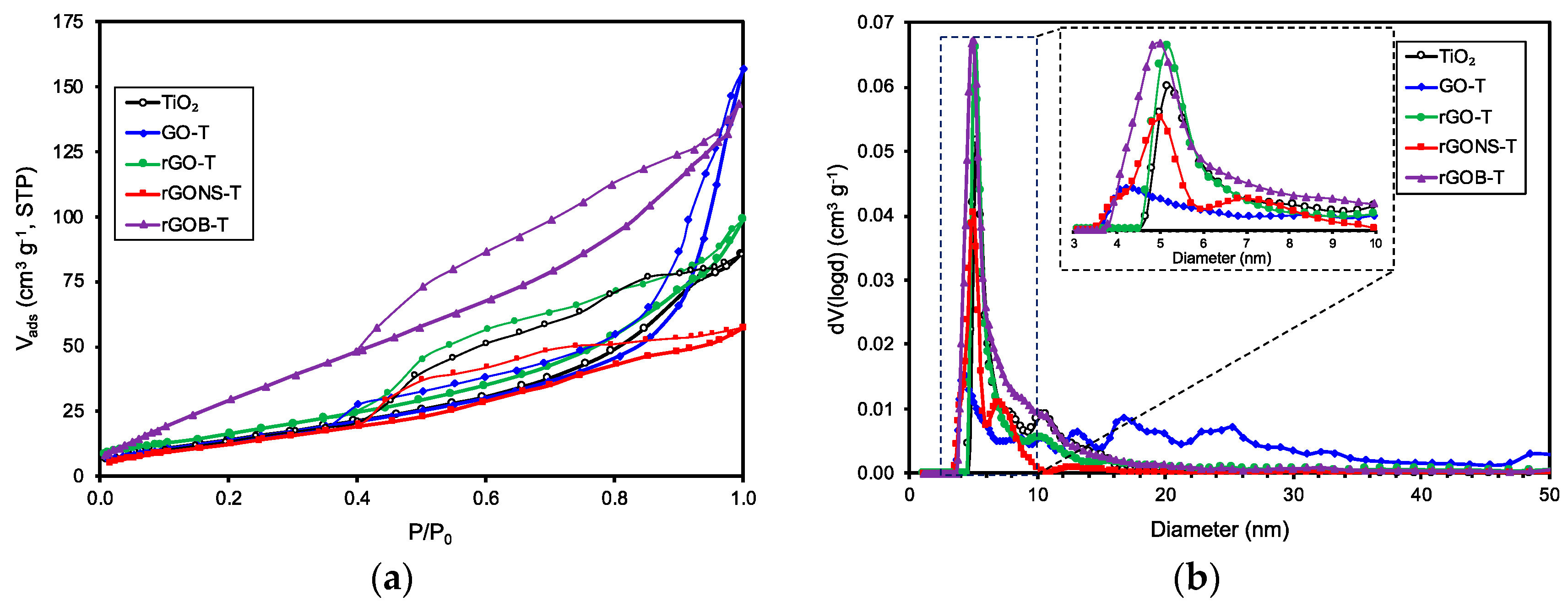

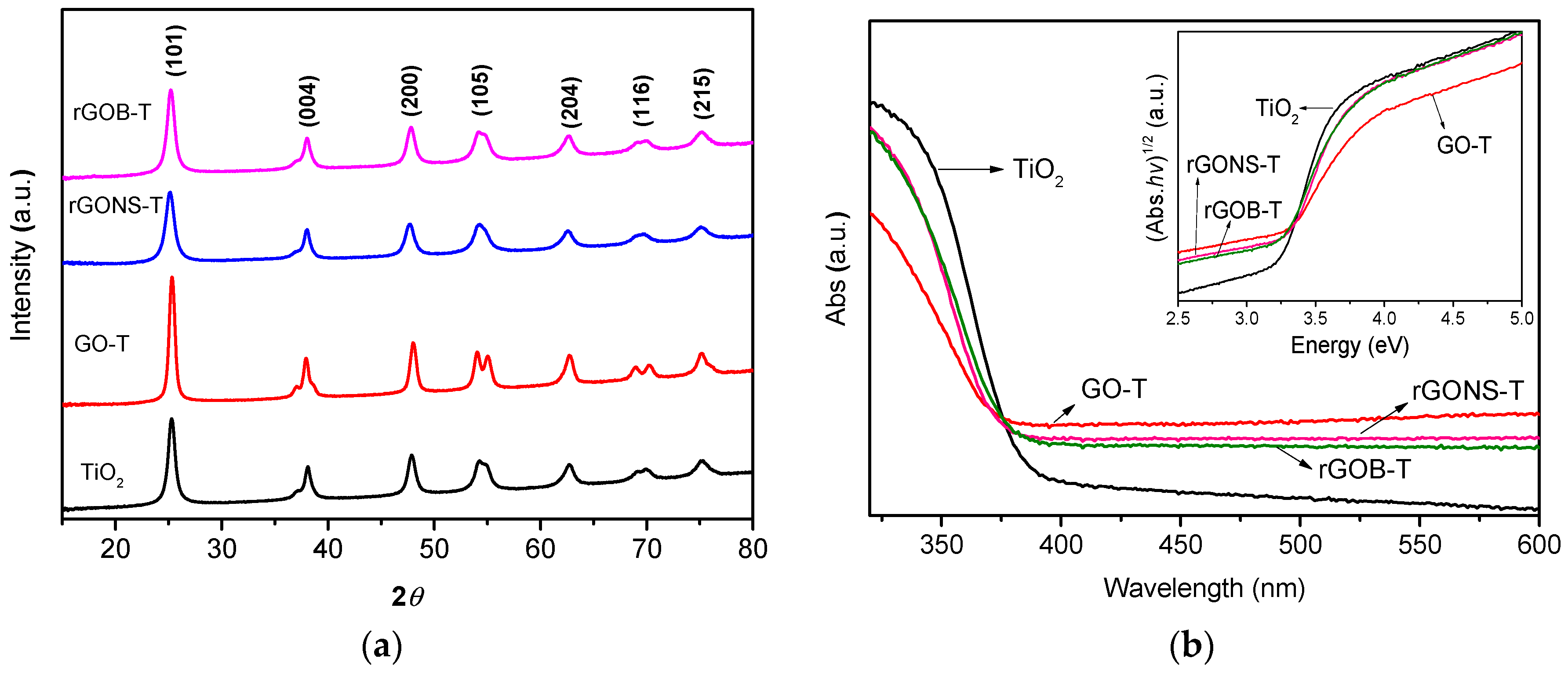

| Samples | SBET (m2 g−1) | Vpore (cm3 g−1) | dpore (nm) | pHPZC | Eg (eV) | Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| TiO2 | 43 | 0.12 | 5.2, 10.3 | 3.5 | 3.20 | 9.7 ± 1.3 |
| GO-T | 55 | 0.17 | 4.2, 16.7, 25.2 | 3.1 | 2.98 | 12.1 ± 1.2 |
| rGO-T | 50 | 0.13 | 5.2, 10.3 | 3.2 | 3.15 | -- |
| rGONS-T | 40 | 0.08 | 5.0, 7.1 | 3.3 | 3.12 | 7.6 ± 1.3 |
| rGOB-T | 80 | 0.20 | 5.0 | 3.2 | 3.12 | 8.9 ± 1.2 |
| Sample | pH | XOG (%) | kapp (10−3 min−1) | r2 |
|---|---|---|---|---|
| Photolysis | 6.0 | 2.8 | −− | −− |
| TiO2 | 6.0 | 47.6 | 11.2 ± 0.3 | 0.996 |
| GO-T | 6.0 | 99.8 | 109.2 ± 4 | 0.996 |
| rGO-T | 6.0 | 90.0 | 29.0 ± 3 | 0.97 |
| rGONS-T | 6.0 | 98.2 | 48.4 ± 2 | 0.993 |
| rGOB-T | 6.0 | 96.5 | 39.1 ± 2 | 0.99 |
| GO-T | 3.0 | 100.0 | 153.4 ± 8 | 0.992 |
| GO-T | 10.0 | 99.6 | 55.1 ± 3 | 0.98 |
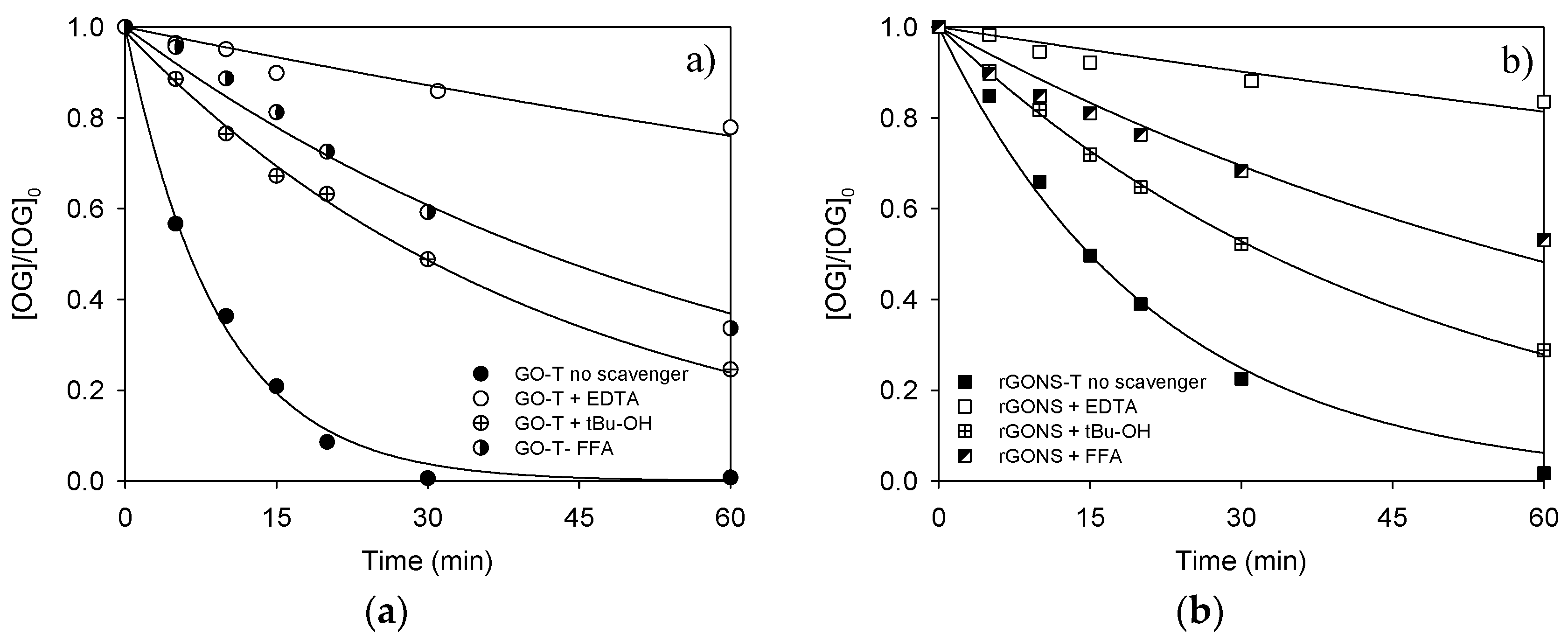
| GO-T + EDTA | 6.0 | 22.1 | 4.5 ± 0.4 | 0.95 |
| GO-T + t-BuOH | 6.0 | 75.4 | 24.1 ± 1 | 0.996 |
| GO-T + FFA | 6.0 | 66.3 | 16.6 ± 1 | 0.98 |
| rGONS-T | 3.0 | 99.5 | 62.0 ± 3 | 0.992 |
| rGONS-T | 10.0 | 87.1 | 39.1 ± 1 | 0.994 |
| rGONS-T + EDTA | 6.0 | 16.4 | 3.4 ± 0.3 | 0.91 |
| rGONS-T + t-BuOH | 6.0 | 71.1 | 21.3 ± 0.3 | 0.999 |
| rGONS-T + FFA | 6.0 | 46.9 | 12.1 ± 0.8 | 0.95 |
| Photocatalyst | Application | Main Results | Ref. |
|---|---|---|---|
| N-TiO2/rGO aerogel | 20 mg L−1, 100 mL MB | Excellent adsorption Removal rates > 90% in 60 min | [68] |
| N-TiO2/rGO | 10 mg L−1, 50 mL RhB | Degradation of 90% in 120 min | [69] |
| GO/TiO2/Hermin | 10 mg L−1, 250 mL RhB under UV/Vis/H2O2. | Degradation of 100% in 40 min | [70] |
| rGO/TiO2/WO3 | MB | Degradation of 83% in 60 min | [71] |
| rGO/TiO2 | 5 mg L−1, 35 mL MB | Degradation of 82% in 140 min Conversion (TOC) 49% in 100 min | [72] |
| rGO/amino-grafted TiO2 | 5 mg L−1, MB 5 mg L−1, Rh6G | Degradation of 91.2% in 40 min (MB) Degradation of 88.3% in 240 min (Rh6G) | [73] |
| Graphene aerogel/TiO2/g-C3N4 | 20 mg L−1, 25 mL RhB | Adsorption of 96.5% in 60 min Degradation of 98.4% in 60 min | [74] |
| GO-TiO2 | 20 mg L−1, 50 mL OG | Degradation of 99.8% in 60 min Conversion (TOC) 40% in 60 min | This work |
| rGONS-TiO2 | 20 mg L−1, 50 mL OG | Degradation of 98.2% in 60 min Conversion (TOC) 22% in 60 min | This work |
| rGOB-TiO2 | 20 mg L−1, 50 mL OG | Degradation of 96.5% in 60 min Conversion (TOC) 17% in 60 min | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Molina, Á.; Morales-Torres, S.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Functionalized Graphene Derivatives and TiO2 for High Visible Light Photodegradation of Azo Dyes. Nanomaterials 2020, 10, 1106. https://doi.org/10.3390/nano10061106
Pérez-Molina Á, Morales-Torres S, Maldonado-Hódar FJ, Pastrana-Martínez LM. Functionalized Graphene Derivatives and TiO2 for High Visible Light Photodegradation of Azo Dyes. Nanomaterials. 2020; 10(6):1106. https://doi.org/10.3390/nano10061106
Chicago/Turabian StylePérez-Molina, Álvaro, Sergio Morales-Torres, Francisco J. Maldonado-Hódar, and Luisa M. Pastrana-Martínez. 2020. "Functionalized Graphene Derivatives and TiO2 for High Visible Light Photodegradation of Azo Dyes" Nanomaterials 10, no. 6: 1106. https://doi.org/10.3390/nano10061106
APA StylePérez-Molina, Á., Morales-Torres, S., Maldonado-Hódar, F. J., & Pastrana-Martínez, L. M. (2020). Functionalized Graphene Derivatives and TiO2 for High Visible Light Photodegradation of Azo Dyes. Nanomaterials, 10(6), 1106. https://doi.org/10.3390/nano10061106








