Formation of Nanocrystalline Cobalt Oxide-Decorated Graphene for Secondary Lithium-Air Battery and Its Catalytic Performance in Concentrated Alkaline Solutions
Abstract
:1. Introduction
2. Materials and Methods
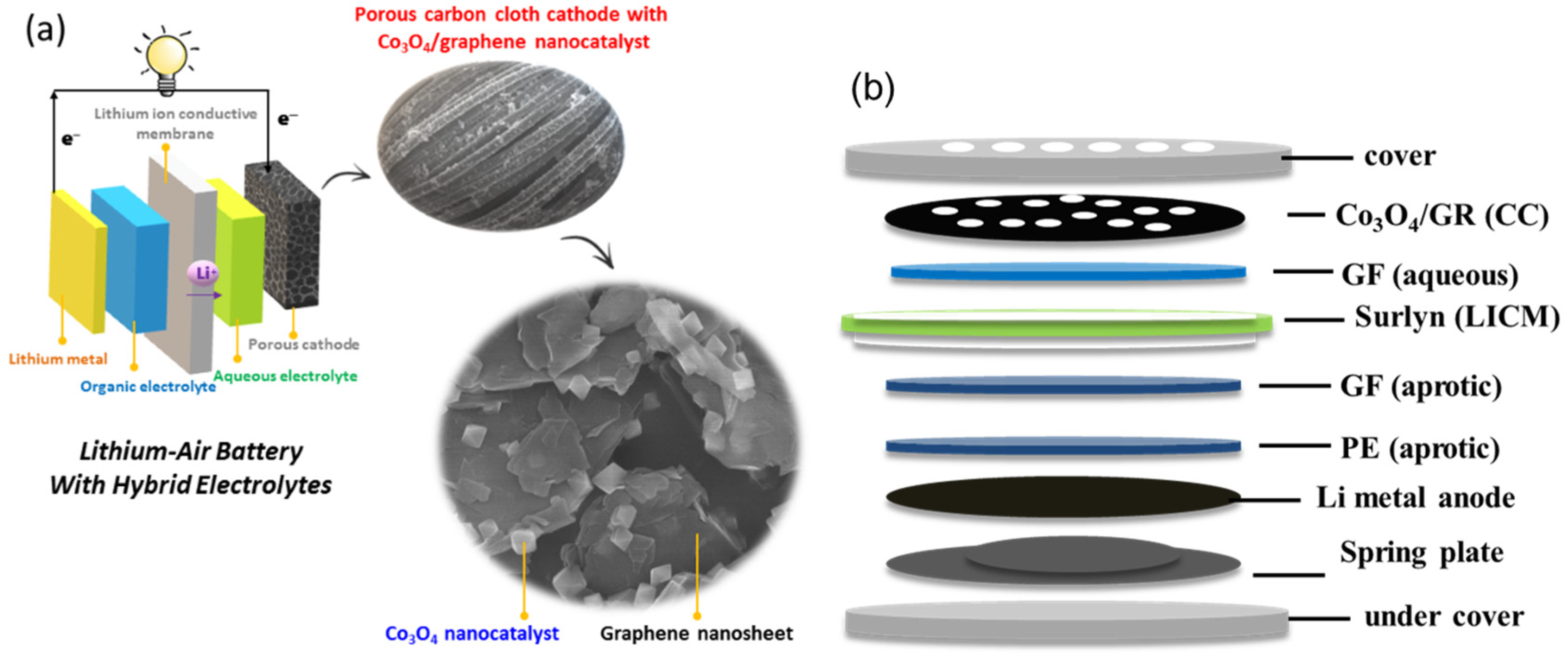
2.1. Synthesis of Co3O4/GR Composites
2.2. Preparation of Lithium Ion Conductive Membrane (LICM)
2.3. Preparation and Characterization of Air Cathode
2.4. Electrochemical and Battery Performance Measurements
3. Results and Discussion
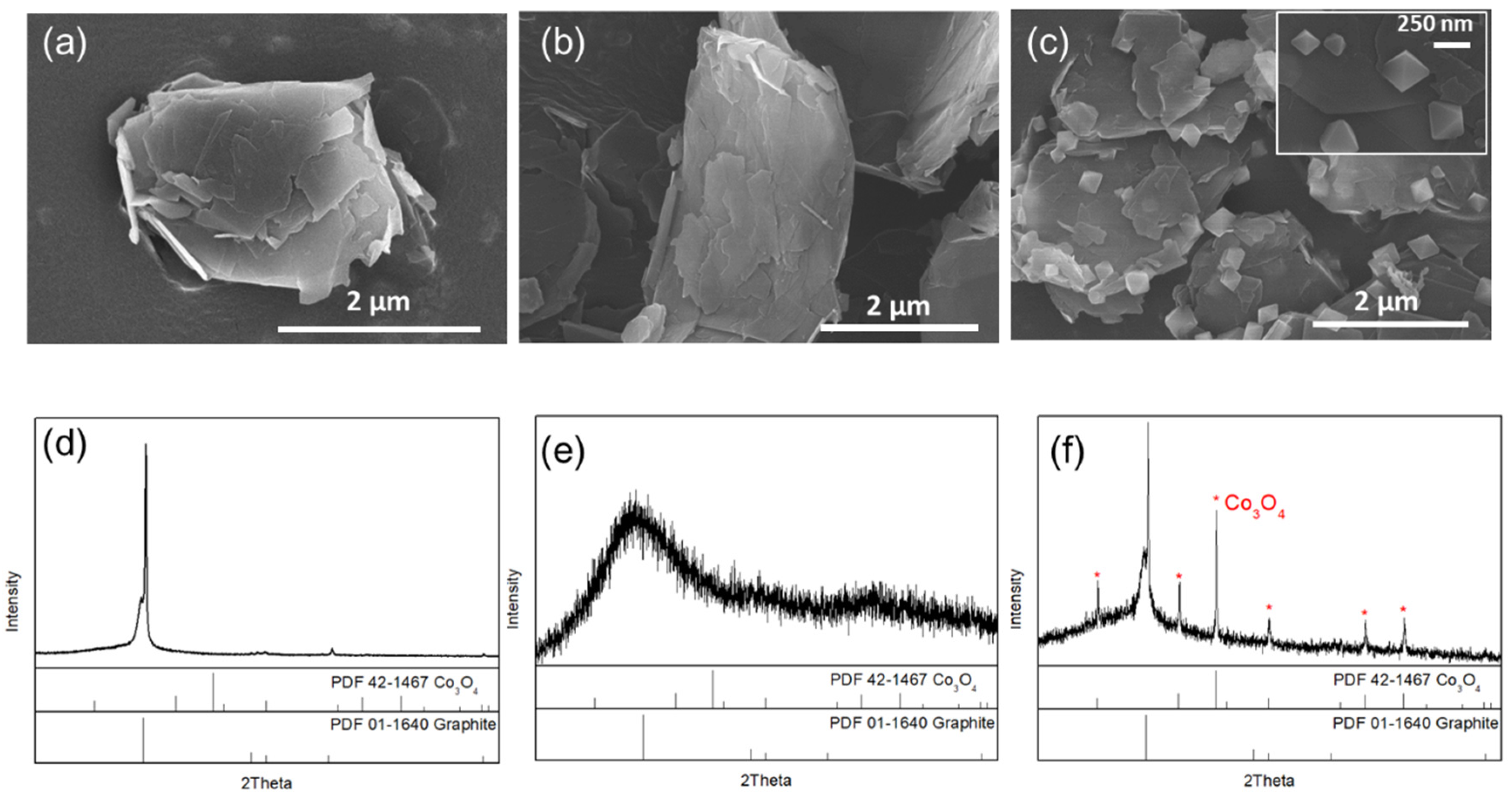
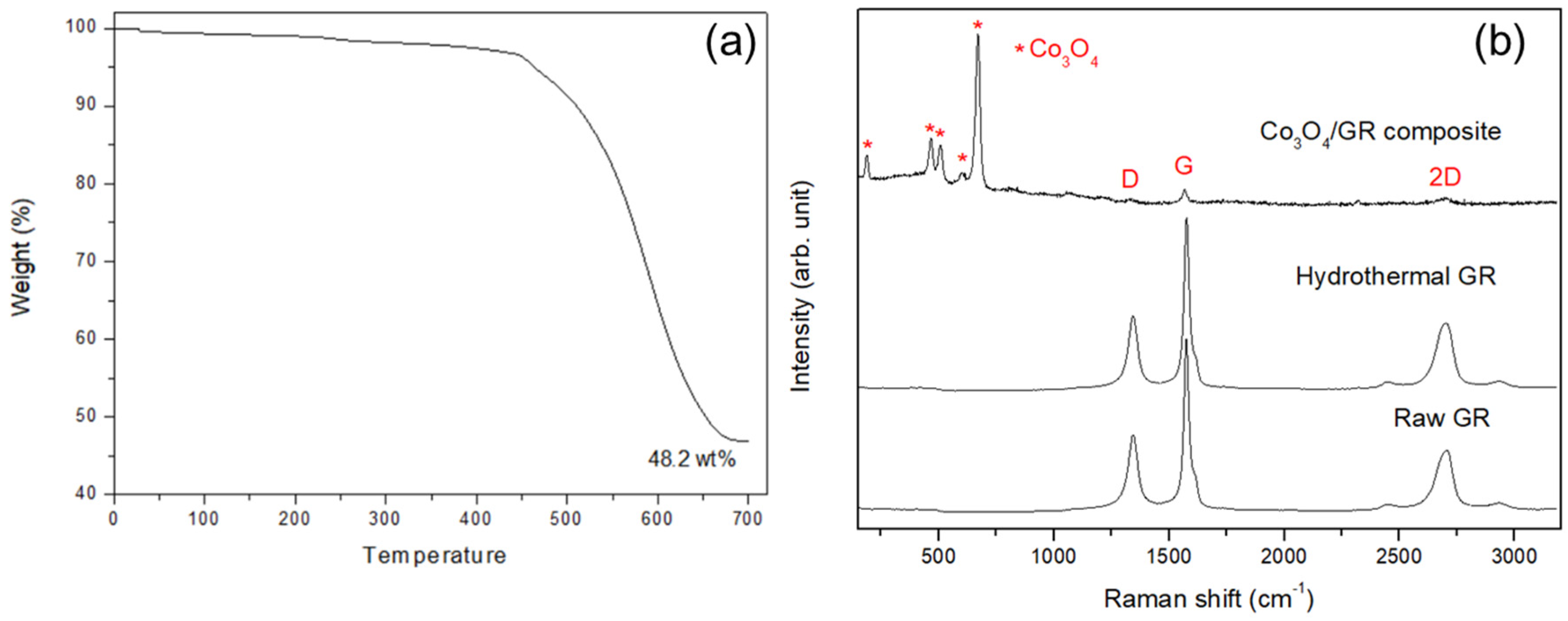
3.1. Characterization of Synthesized Co3O4/GR Composite
3.2. Prepared Air Cathode Characterization
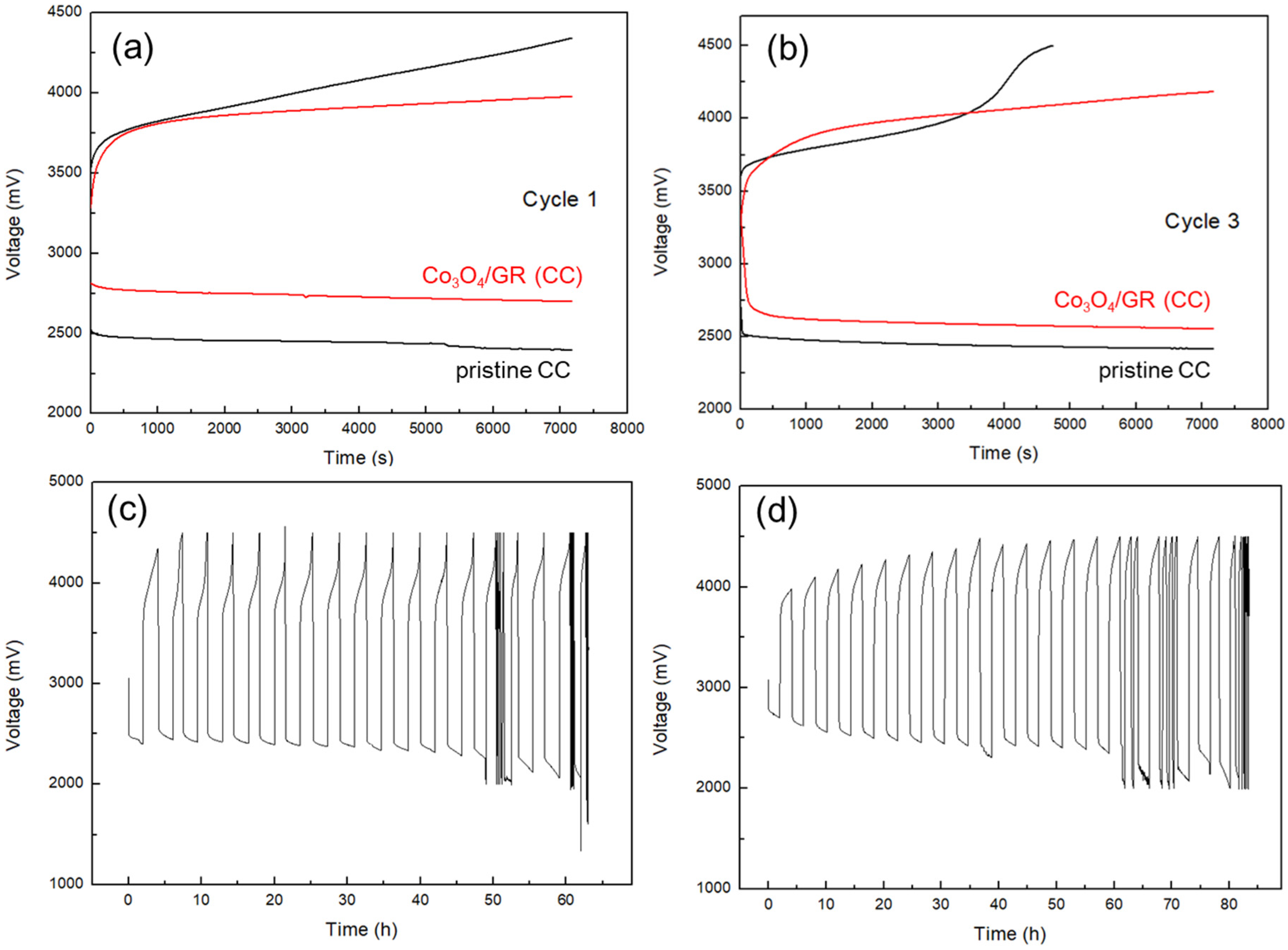
3.3. Cathode Catalytic Performance and HELAB Cycling Performance
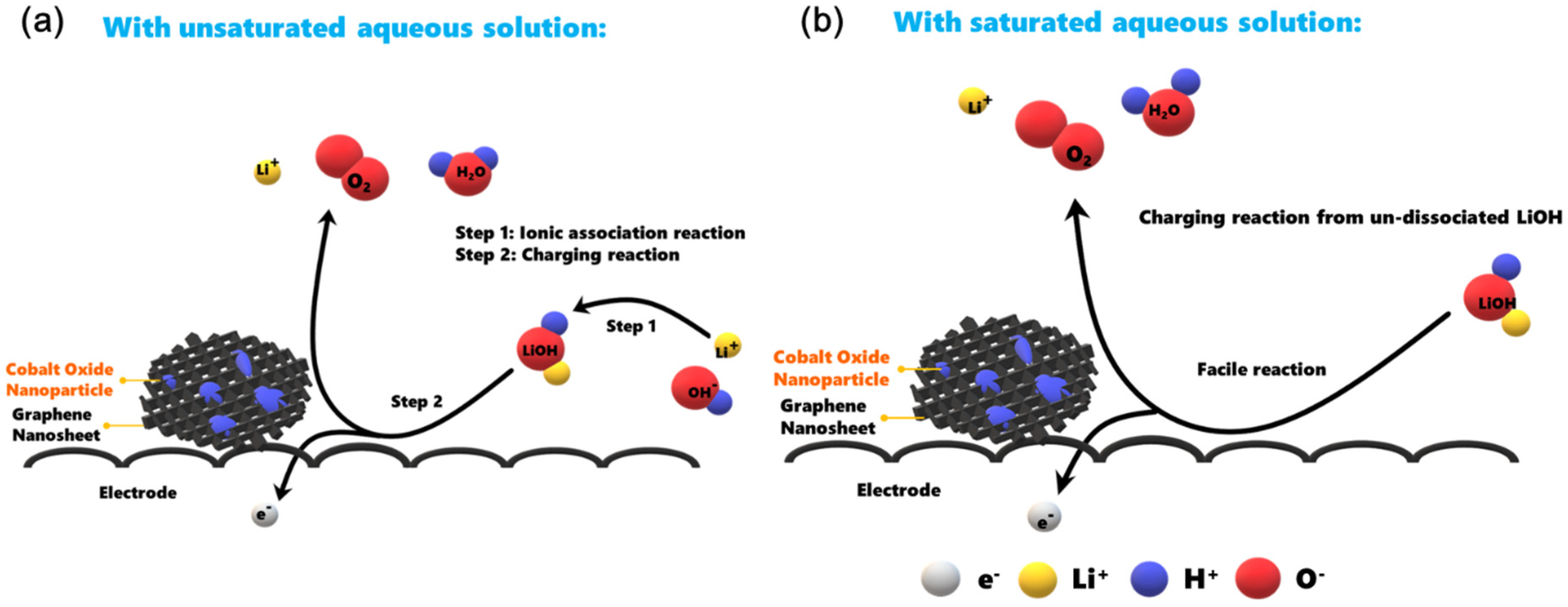
3.4. Optimization of HELAB Using Saturated LiOH in 11.6 M LiCl
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, B.; Kumar, J.; Leese, R.; Fellner, J.P.; Rodrigues, S.J.; Abraham, K.M. A solid-state, rechargeable, long cycle life lithium–air battery. J. Electrochem. Soc. 2010, 157, A50. [Google Scholar] [CrossRef]
- Imanishi, N.; Yamamoto, O. Perspectives and challenges of rechargeable lithium-air batteries. Mater. Today Adv. 2019, 4, 100031. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Liu, J.; Wang, Y.; Xia, Y. A long-life lithium–air battery in ambient air with a polymer electrolyte containing a redox mediator. Angew. Chem. Int. Ed. 2017, 56, 7505–7509. [Google Scholar] [CrossRef] [PubMed]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium-air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Christensen, A.; Albertus, P.; Sanchez-Carrera, R.S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. A critical review of Li/air batteries. J. Electrochem. Soc. 2012, 159, R1–R30. [Google Scholar] [CrossRef]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.-B.; Lee, Y.-J.; Sun, Y.-K.; Passerini, S.; Scrosati, B. The lithium/air battery: Still an emerging system or a practical reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.; Zhang, Y.; Cheng, X.; Liu, L.; Peng, H. A Li–air battery with ultralong cycle life in ambient air. Adv. Mater. 2018, 30, 1704378. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, J.; Peng, Z. Achilles Heel of lithium–air batteries: Lithium carbonate. Angew. Chem. Int. Ed. 2018, 57, 3874–3886. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A lithium–oxygen battery with a long cycle life in an air-like atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef]
- Huang, Z.; Ren, J.; Zhang, W.; Xie, M.; Li, Y.; Sun, D.; Shen, Y.; Huang, Y. Protecting the Li-metal anode in a Li–O2 battery by using boric acid as an SEI-forming additive. Adv. Mater. 2018, 30, 1803270. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chang, J.C.; Kumar, S.R.; Lue, S.J. Glyme-based electrolyte formulation analysis in aprotic lithium-oxygen battery and its cyclic stability. Energy 2019, 187, 115926. [Google Scholar] [CrossRef]
- Lai, J.; Xing, Y.; Chen, N.; Li, L.; Wu, F.; Chen, R. Electrolytes for rechargeable lithium–air batteries. Angew. Chem. Int. Ed. 2019, 59, 2974–2997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Zhou, H.S. A lithium-air battery with a potential to continuously reduce O2 from air for delivering energy. J. Power Sources 2010, 195, 358–361. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhou, H.S. A lithium–air fuel cell using copper to catalyze oxygen-reduction based on copper-corrosion mechanism. Chem. Commun. 2010, 46, 6305–6307. [Google Scholar] [CrossRef]
- Lu, Y.; Goodenough, J.B.; Kim, Y. Aqueous cathode for next-generation alkali-ion batteries. J. Am. Chem. Soc. 2011, 133, 5756–5759. [Google Scholar] [CrossRef]
- He, H.; Niu, W.; Asl, N.M.; Salim, J.; Chen, R.R.; Kim, Y. Effects of aqueous electrolytes on the voltage behaviors of rechargeable Li-air batteries. Electrochim. Acta 2012, 67, 87–94. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Lu, Y.; Xu, M.; Zhang, D.; Ruoff, R.S.; Stevenson, K.J.; Goodenough, J.B. CoMn2O4 spinel nanoparticles grown on graphene as bifunctional catalyst for lithium-air batteries. J. Electrochem. Soc. 2011, 158, A1379–A1382. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhao, T.S.; Wei, Z.H.; Tan, P.; An, L. A high-rate and long cycle life solid-state lithium–air battery. Energy Environ. Sci. 2015, 8, 3745–3754. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, J.; Li, F.; Wang, Z.L.; Sun, C. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Imanishi, N.; Hasegawa, S.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Lithium anode for lithium-air secondary batteries. J. Power Sources 2008, 185, 1392–1397. [Google Scholar] [CrossRef]
- Pan, J.; Tian, X.L.; Zaman, S.; Dong, Z.; Liu, H.; Park, H.S.; Xia, B.Y. Recent progress on transition metal oxides as bifunctional catalysts for lithium-air and zinc-air batteries. Batter. Supercaps 2019, 2, 336–347. [Google Scholar] [CrossRef]
- Cao, X.; Yang, R. Advances in electrocatalysts for the cathode of Li-air batteries. Chin. Sci. Bull. 2019, 64, 3340–3349. [Google Scholar]
- Abraham, K.M.; Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Lu, Y.C.; Kwabi, D.G.; Yao, K.P.C.; Hardling, J.R.; Zhou, J.G.; Zuin, L.; Shao-Horn, Y. The discharge rate capability of rechargeable Li–O2 batteries. Energy Environ. Sci. 2011, 4, 2999–3007. [Google Scholar] [CrossRef]
- Beattie, S.D.; Manolescu, D.M.; Blair, S.L. High-capacity lithium–air cathodes. J. Electrochem. Soc. 2009, 156, A44–A47. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Gasteiger, H.A.; Shao-Horn, Y. Catalytic activity trends of oxygen reduction reaction for nonaqueous Li-air batteries. J. Am. Chem. Soc. 2011, 133, 19048–19051. [Google Scholar] [CrossRef]
- Roena, L.M.; Paikb, C.H.; Jarvic, T.D. Electrocatalytic corrosion of carbon support in PEMFC cathodes. Electrochem. Solid-State Lett. 2004, 7, A19–A22. [Google Scholar] [CrossRef]
- Kangasniemia, K.H.; Conditb, D.A.; Jarvic, T.D. Characterization of Vulcan electrochemically oxidized under simulated PEM fuel cell conditions. J. Electrochem. Soc. 2004, 151, E125–E132. [Google Scholar] [CrossRef]
- Antolini, E. Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells. J. Mater. Sci. 2003, 38, 2995–3005. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Chen, Z.; Waje, M.; Yan, Y. Durability investigation of carbon nanotube as catalyst support for proton exchange membrane fuel cell. J. Power Sources 2006, 154, 154–159. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y.; Shi, P. Durability study of Pt/C and Pt/CNTs catalysts under simulated PEM fuel cell conditions. J. Electrochem. Soc. 2006, 153, A1093–A1097. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Li, F.; Ma, C.; Wang, Y.; Ren, Y.; Yang, W.; Ma, Z.; Li, J.; Chen, Y.; Kim, Y.; et al. Graphene–Co3O4 nanocomposite as an efficient bifunctional catalyst for lithium–air batteries. J. Mater. Chem. A 2014, 2, 7188. [Google Scholar] [CrossRef]
- Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Bezerra, C.W.B.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wu, D.; Feng, X.; Muellen, K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. 2010, 49, 2565–2569. [Google Scholar] [CrossRef]
- Hang, Y.; Zhang, C.; Luo, X.; Xie, Y.; Xin, S.; Li, Y.; Zhang, D.; Goodenough, J.B. α-MnO2 nanorods supported on porous graphitic carbon nitride as efficient electrocatalysts for lithium-air batteries. J. Power Sources 2018, 392, 15–22. [Google Scholar] [CrossRef]
- Surya, K.; Michael, M.S.; Prabaharan, S.R.S. A review on advancement in non-noble metal based oxides as bifunctional catalysts for rechargeable non-aqueous Li/air battery. Solid State Ion. 2018, 317, 89–96. [Google Scholar] [CrossRef]
- Lim, H.; Gwon, H.; Kim, H.; Kim, S.; Yoon, T.; Choi, J.W.; Oh, S.M.; Kang, K. Mechanism of Co3O4/grapheme catalytic activity in Li–O2 batteries using carbonate based electrolyte. Electrochim. Acta 2013, 90, 63–70. [Google Scholar] [CrossRef]
- Peng, S.H.; Chen, T.H.; Li, C.H.; Lu, H.C.; Lue, S.J. Optimal cobalt oxide (Co3O4):graphene (GR) ratio in Co3O4/GR as air cathode catalyst for air-breathing hybrid electrolyte lithium-air battery. J. Power Sources 2020, in press. [Google Scholar]
- Ehrhardt, C.; Gjikaj, M.; Brockner, W. Thermal decomposition of cobalt nitrato compounds: Preparation of anhydrous cobalt(II)nitrate and its characterisation by Infrared and Raman spectra. Thermochim. Acta 2005, 432, 36–40. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, Y.; Fang, P.; Sha, H.; Cha, L.; Ming, Z. Co2O3-NH2-MCM-41 decorated graphite as an effective electrode:synthesis, characterization and its application for electro-catalytic oxidation of acid red 1. Electroanalysis 2017, 29, 794–805. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Esmaeili, A.; Entezari, M.H. Facile and fast synthesis of graphene oxide nanosheets via bath ultrasonic irradiation. J. Colloid Interface Sci. 2014, 432, 19–25. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.H. Effect of oxygen content on structures of graphite oxides. Ind. Eng. Chem. Res. 2011, 50, 6132–6137. [Google Scholar]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaría, R.; Menéndez, R.; Corma, A.; García, H. Surface area measurement of graphene oxide in aqueous solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 nanoparticles: Synthesis, characterizations, and raman spectroscopy. J. Nanomater. 2013, 2013, 714853. [Google Scholar] [CrossRef] [Green Version]
- Samal, R.; Dash, B.; Sarangi, C.K.; Sanjay, K.; Subbaiah, T.; Senanayake, G.; Minakshi, M. Influence of Synthesis Temperature on the Growthand Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications. Nanomaterials 2017, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lu, C.; Qin, J.; Zhang, R.; Tang, H.; Song, H. A facile one-step hydrothermal method to produce graphene–MoO3 nanorod bundle composites. Mater. Lett. 2011, 65, 2341–2344. [Google Scholar] [CrossRef]
- Shia, P.; Sua, R.; Zhua, S.; Zhua, M.; Li, D.; Xua, S. Supported cobalt oxide on graphene oxide: Highly efficient catalysts for the removal of Orange II from water. J. Hazard. Mater. 2012, 229–230, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yang, Z.; Wanga, H.; Cheng, W.; Chen, M.; Shangguan, W.; Ding, G. TiO2-graphene nanocomposites for photocatalytic hydrogen production from splitting water. Int. J. Hydrogen Energy 2012, 37, 2224–2230. [Google Scholar] [CrossRef]
- Safanama, D.; Adams, S. High efficiency aqueous and hybrid lithium-air batteries enabled by Li1.5Al0.5Ge1.5(PO4)3 ceramic anode-protecting membranes. J. Power Sources 2017, 340, 294–301. [Google Scholar] [CrossRef]
- Shimonishi, Y.; Zhang, T.; Imanishi, N.; Im, D.; Joon Lee, D.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A study on lithium/air secondary batteries—Stability of the NASICON-type lithium ion conducting solid electrolyte in alkaline aqueous solutions. J. Power Sources 2011, 196, 5128–5132. [Google Scholar] [CrossRef]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathambyd, K. Synthesis and Characterization of Li(Co0.5Ni0.5)PO4 Cathode for Li-Ion Aqueous Battery Applications. Electrochem Solid-State Lett. 2011, 14, A86–A89. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; De Marco, R. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery Part III. Electrochemical behavior of-MnO2 in aqueous lithium hydroxide electrolyte. J. Power Sources 2006, 153, 165. [Google Scholar] [CrossRef]



| Property | Crystalline Size (nm) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (Å) |
|---|---|---|---|---|
| Raw GR | − a | 18.8 | 0.0475 | 101 |
| Co3O4/GR | 73.1 | 14.2 | 0.0311 | 87.5 |
| Sample | D Band (cm−1) | G Band (cm−1) | 2D Band (cm−1) | ID/IG | I2D/IG |
|---|---|---|---|---|---|
| Raw GR | 1343 | 1574 | 2708 | 0.44 ± 0.02 | 0.35 ± 0.04 |
| Hydrothermal GR | 1343 | 1576 | 2701 | 0.42 | 0.38 |
| Co3O4/GR composite | 1338 | 1566 | 2694 | 0.47 ± 0.04 | 0.51 ± 0.04 |
| Air Cathode | Thickness (μm) | Sheet Resistance (ohm/□) |
|---|---|---|
| Raw carbon cloth | 373 | 0.541 |
| Carbon cloth (PVDF) dried at 80 °C | 397 | 0.906 |
| Carbon cloth (PVDF) dried at 100 °C | 397 | 0.918 |
| Carbon cloth (PVDF) dried at 120 °C | 397 | 0.821 |
| Carbon cloth (PVDF + Co3O4/GR) dried at 120 °C | 419 | 0.325–0.386 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.-H.; Lu, H.-C.; Jessie Lue, S. Formation of Nanocrystalline Cobalt Oxide-Decorated Graphene for Secondary Lithium-Air Battery and Its Catalytic Performance in Concentrated Alkaline Solutions. Nanomaterials 2020, 10, 1122. https://doi.org/10.3390/nano10061122
Peng S-H, Lu H-C, Jessie Lue S. Formation of Nanocrystalline Cobalt Oxide-Decorated Graphene for Secondary Lithium-Air Battery and Its Catalytic Performance in Concentrated Alkaline Solutions. Nanomaterials. 2020; 10(6):1122. https://doi.org/10.3390/nano10061122
Chicago/Turabian StylePeng, Si-Han, Hsin-Chun Lu, and Shingjiang Jessie Lue. 2020. "Formation of Nanocrystalline Cobalt Oxide-Decorated Graphene for Secondary Lithium-Air Battery and Its Catalytic Performance in Concentrated Alkaline Solutions" Nanomaterials 10, no. 6: 1122. https://doi.org/10.3390/nano10061122






