Rapid and Facile Synthesis of High-Performance Silver Nanowires by a Halide-Mediated, Modified Polyol Method for Transparent Conductive Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silver Nanowire
2.3. Preparation of Silver Nanowires Transparent Conductive Films
2.4. Characterization of Silver Nanowires and Transparent Conductive Films
3. Results
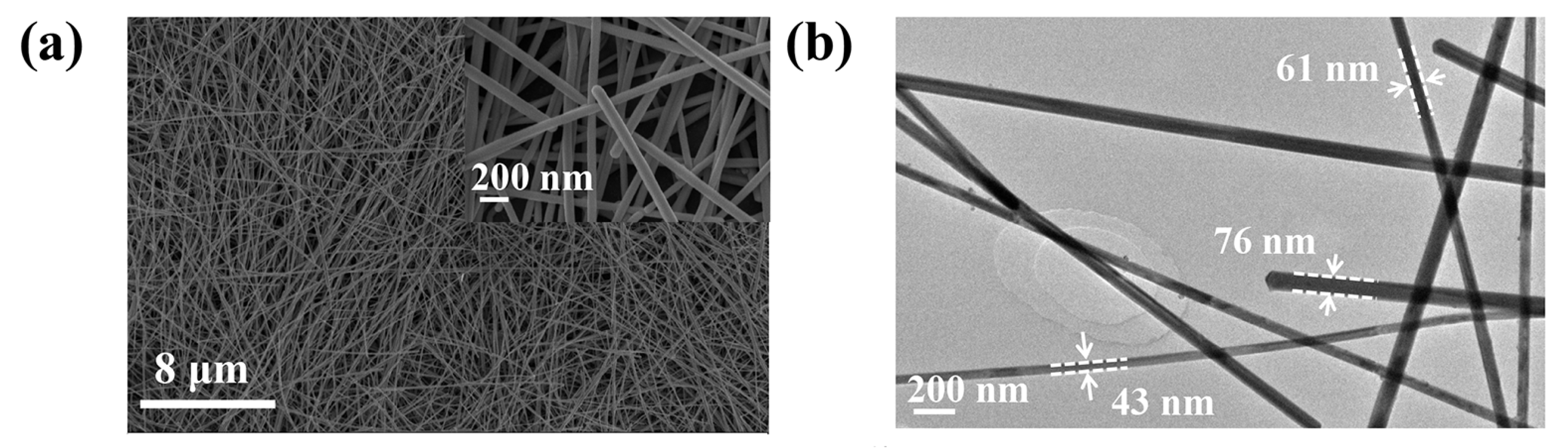
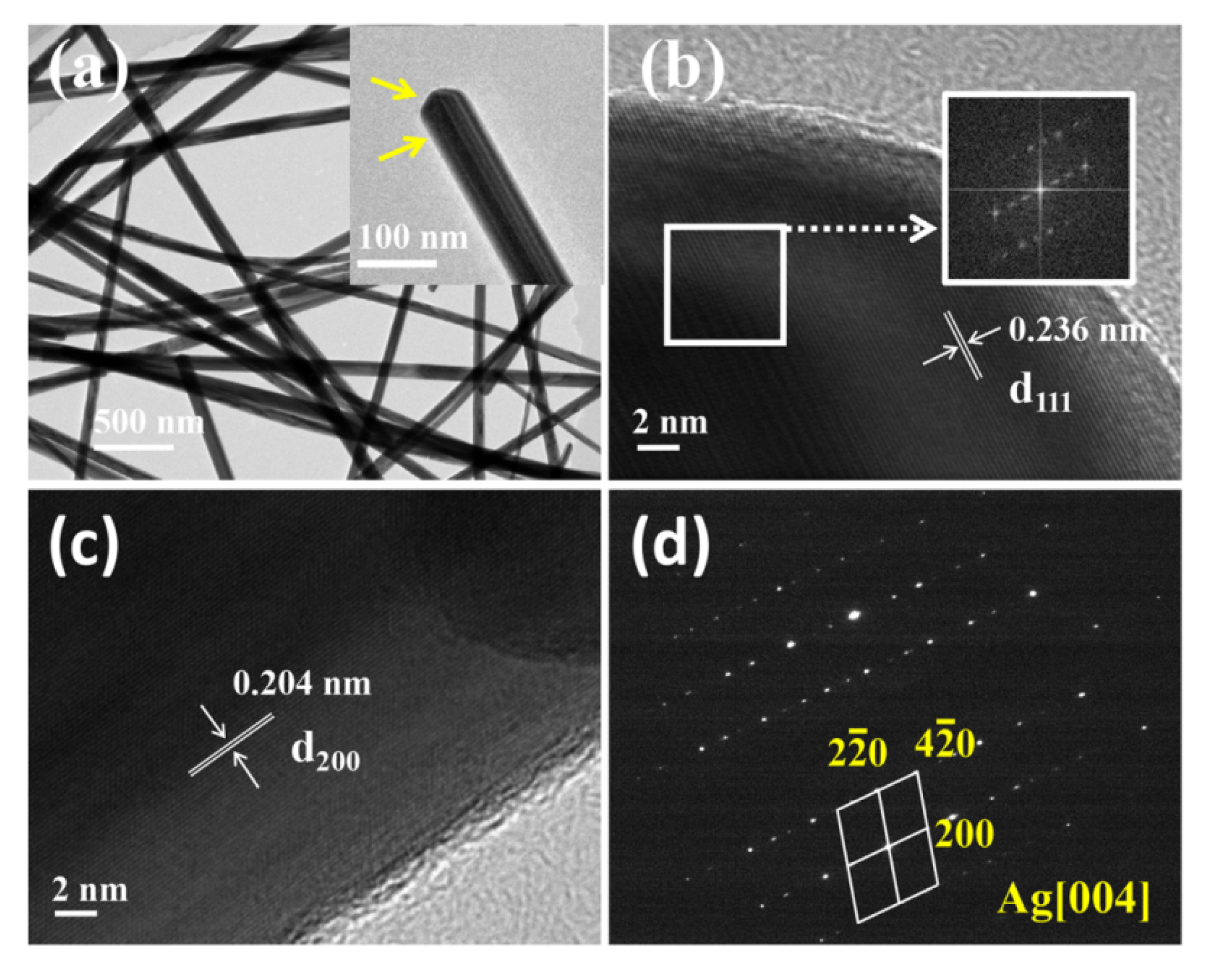
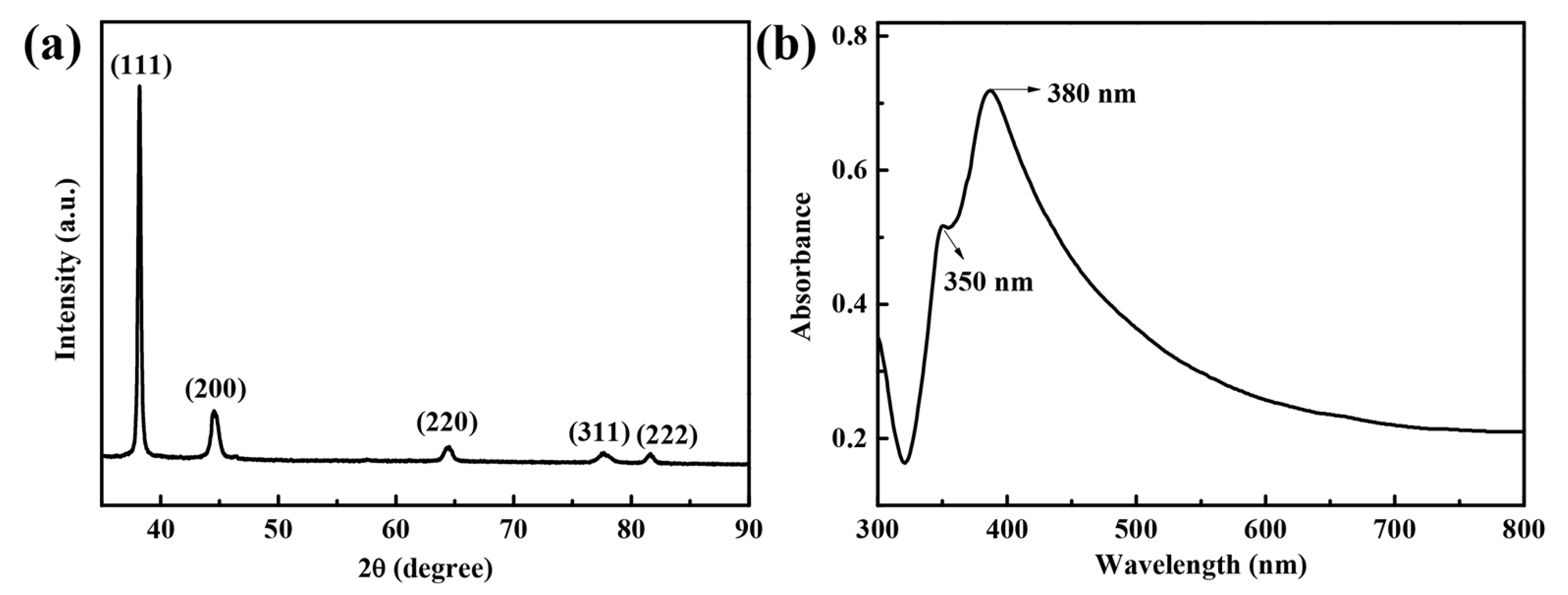
3.1. Characterization of Silver Nanowires
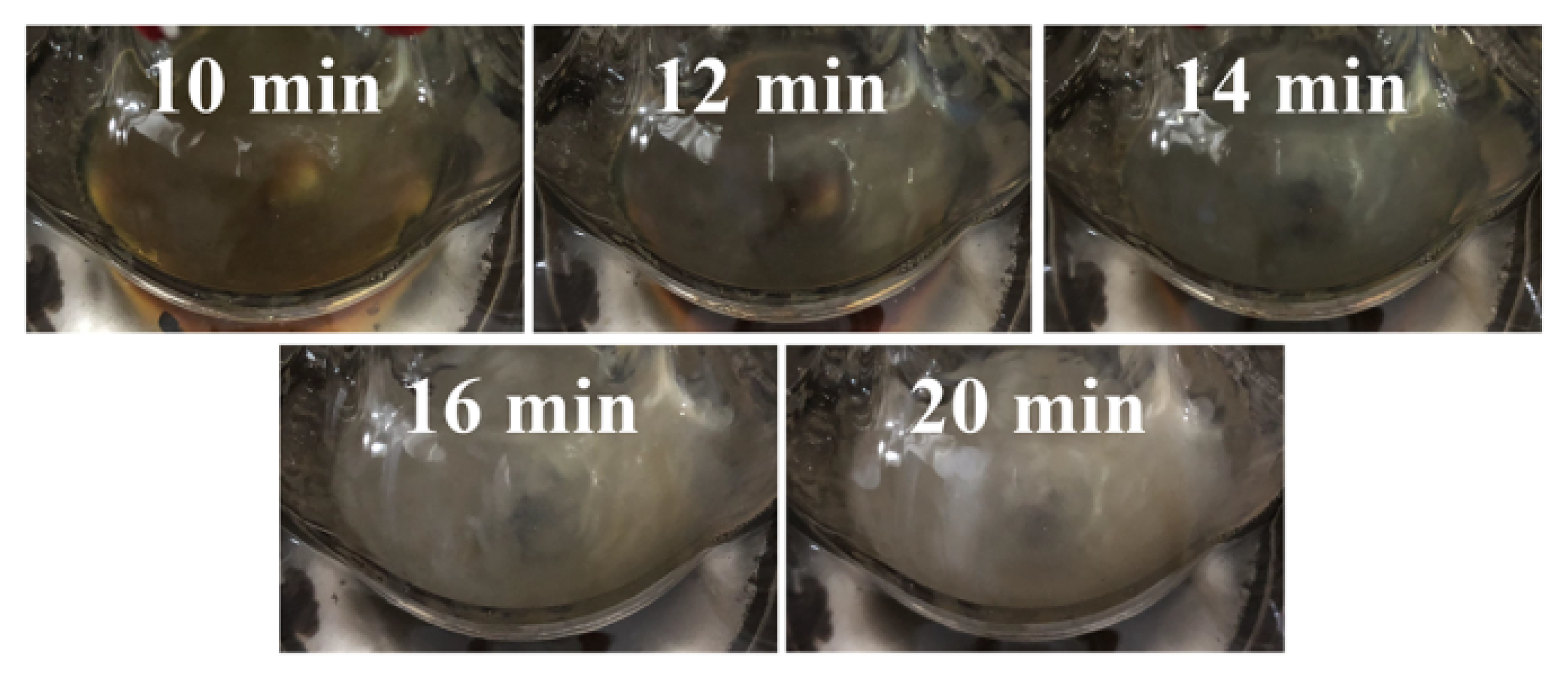
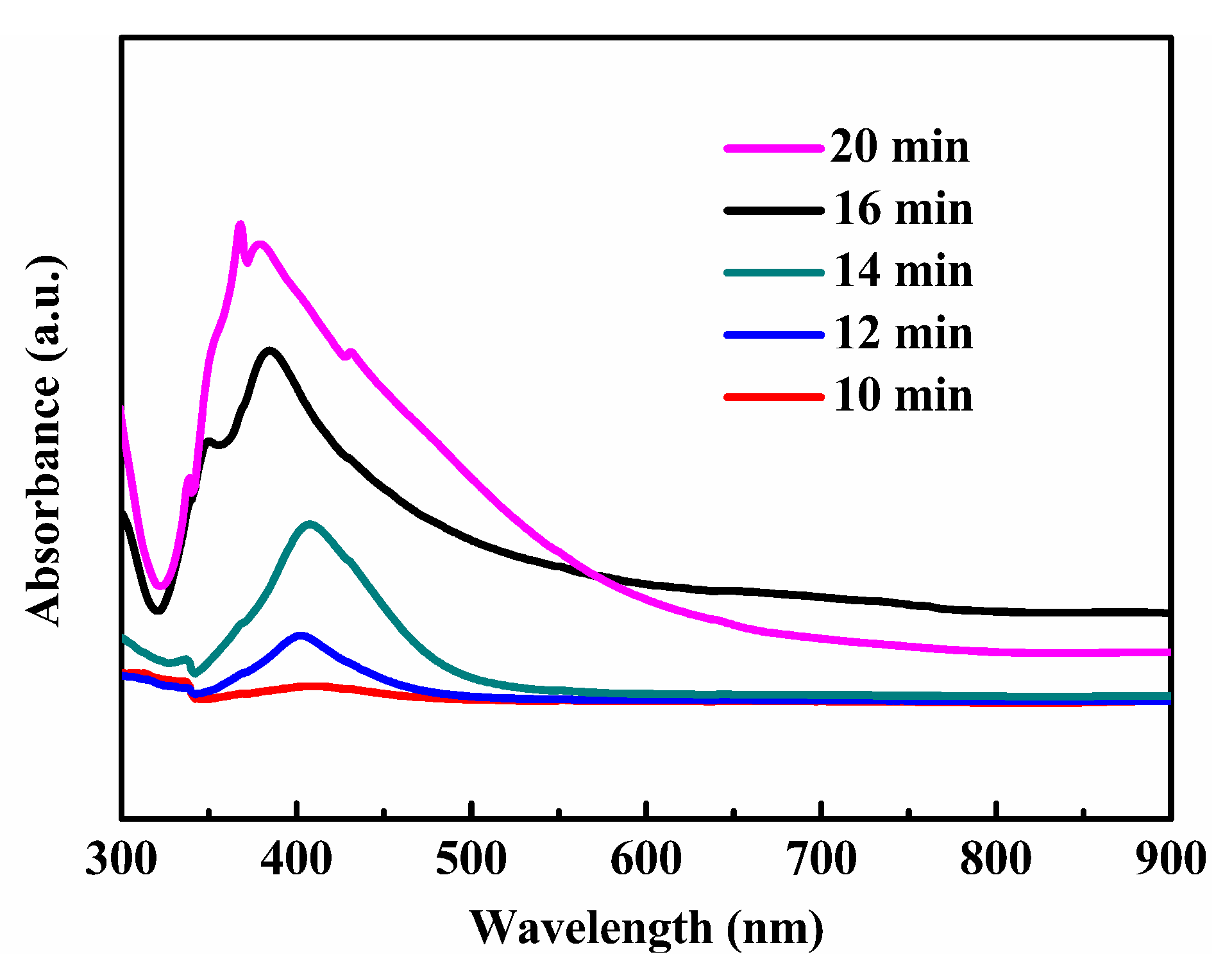
3.2. Growth Mechanism of Silver Nanowires
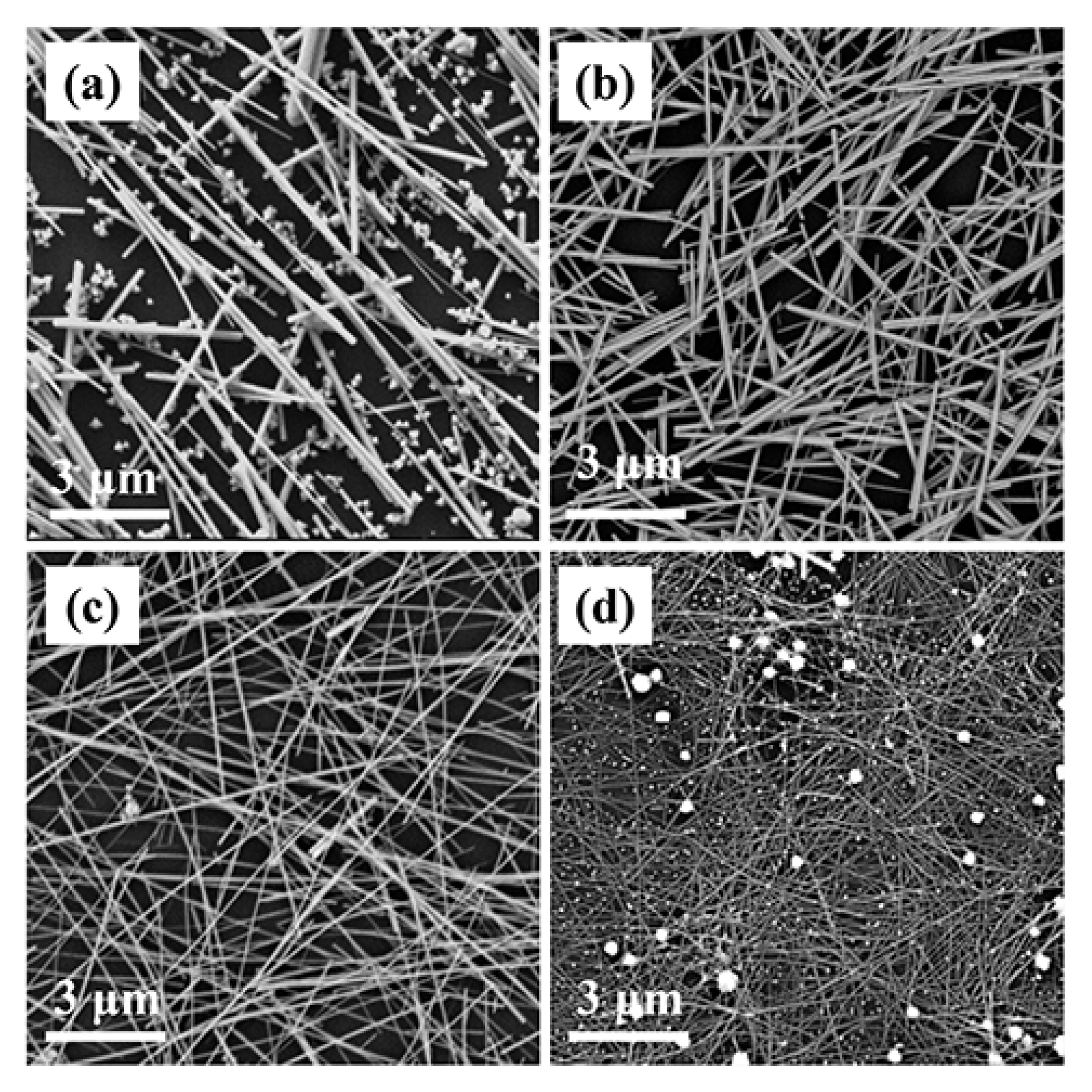
3.3. Influence of the Reaction Parameters
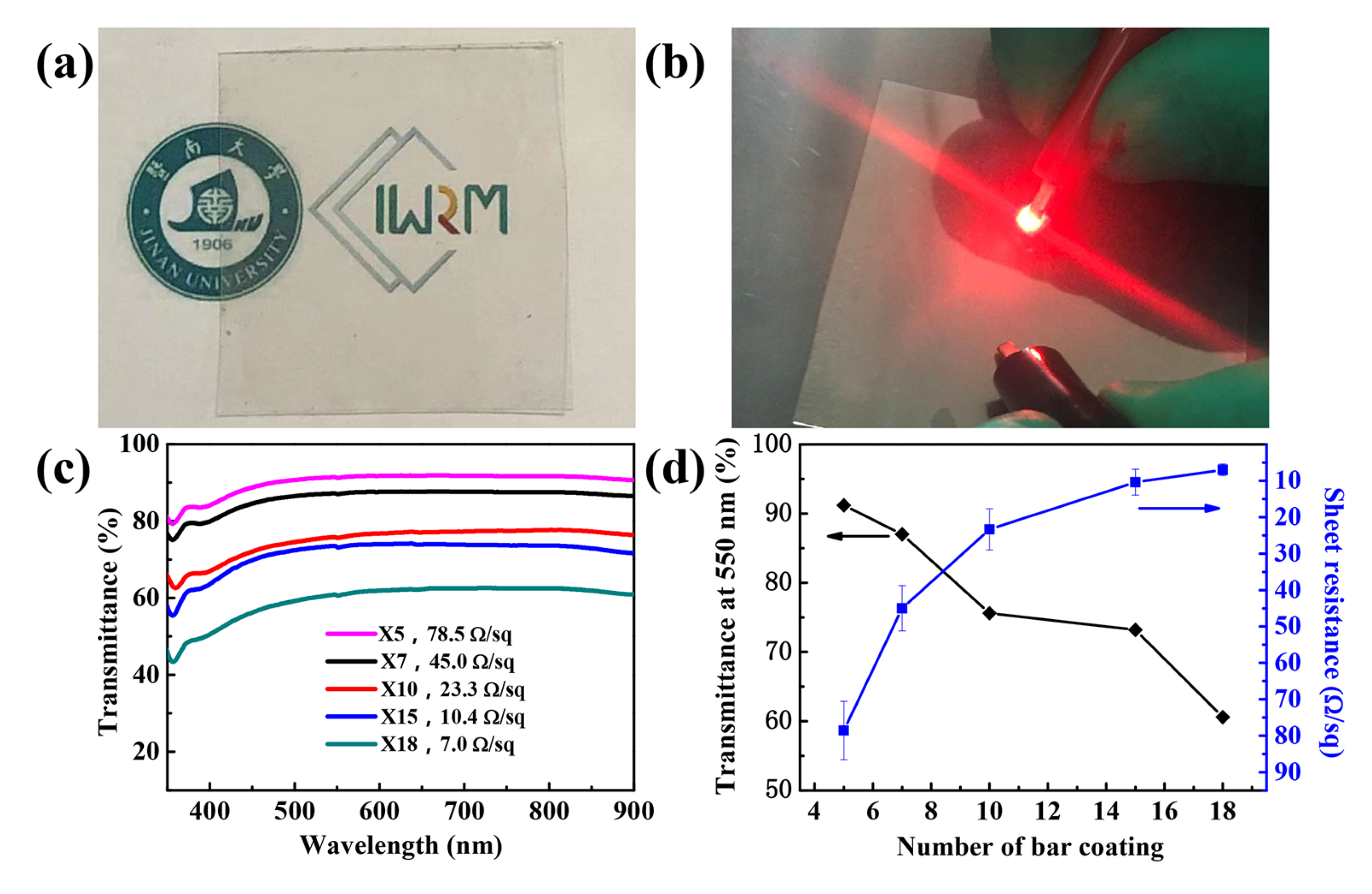
3.4. Performance of Silver Nanowire Transparent Conductive Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.Y.; Lee, W.K.; Chen, Y.J.; Lu, C.Y.; Lin, H.Y.; Wu, C.C. Enhancing optical out-coupling of organic light-emitting devices with nanostructured composite electrodes consisting of indium tin oxide nanomesh and conducting polymer. Adv. Mater. 2015, 27, 4883–4888. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ye, C. Flexible transparent conductive films on the basis of Ag nanowires: Design and applications: A review. J. Mater. Sci. Technol. 2015, 31, 581–588. [Google Scholar] [CrossRef]
- Wu, H.; Hu, L.; Carney, T.J.; Ruan, Z.; Kong, D.; Yu, Z.; Yao, Y.; Cha, J.J.; Zhu, J.; Fan, S.; et al. Low reflectivity and high flexibility of tin-doped indium oxide nanofiber transparent electrodes. J. Am. Chem. Soc. 2011, 133, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, Y.W.; Kim, J.E.; Yoon, S.W.; Kim, T.Y.; Noh, J.-S.; Suh, K.S. Synthesis of dimension-controlled silver nanowires for highly conductive and transparent nanowire films. Acta Mater. 2015, 83, 84–90. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Kim, I.; Lee, H. Flexible and stretchable optoelectronic devices using silver nanowires and graphene. Adv. Mater. 2016, 28, 4541–4548. [Google Scholar] [CrossRef]
- Ha, J.; Park, S.; Kim, D.; Ryu, J.; Lee, C.; Hong, B.H.; Hong, Y. High-performance polymer light emitting diodes with interface-engineered graphene anodes. Organ. Electron. 2013, 14, 2324–2330. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.; Kim, K.S.; Lee, S.-K.; Bae, S.; Ahn, J.-H.; Kim, Y.-J.; Choi, J.-B.; Hong, B.H. High-performance graphene-based transparent flexible heaters. Nano Lett. 2011, 11, 5154–5158. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-U.; Nam, S.; Lee, M.-S.; Lieber, C.M. Synthesis of monolithic graphene–graphite integrated electronics. Nat. Mater. 2012, 11, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ryu, K.; Liu, X.; Polikarpov, E.; Ly, J.; Tompson, M.E.; Zhou, C. Transparent, conductive, and flexible carbon nanotube films and their application in organic light-emitting diodes. Nano Lett. 2006, 6, 1880–1886. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, conductive carbon nanotube films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Kim, J.-W.; Kang, D.; Lee, D.W.; Ko, S.-J.; Lee, H.J.; Lee, C.-L.; Kim, J.Y.; Shin, H.S.; Song, M.H. Highly efficient polymer light-emitting diodes using graphene oxide as a hole transport layer. ACS Nano 2012, 6, 2984–2991. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Giubileo, F.; Grillo, A.; Luongo, G.; Iemmo, L.; Urban, F.; Lozzi, L.; Capista, D.; Nardone, M.; Passacantando, M. Bias Tunable Photocurrent in Metal-Insulator-Semiconductor Heterostructures with Photoresponse Enhanced by Carbon Nanotubes. Nanomaterials 2019, 9, 1598. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhou, X.; Zhang, J.; Bruck, A.M.; Bond, A.C.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Yu, G. Nanostructured conductive polymer gels as a general framework material to improve electrochemical performance of cathode materials in Li-ion batteries. Nano Lett. 2017, 17, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Elschner, A.; Lövenich, W. Solution-deposited PEDOT for transparent conductive applications. MRS Bull. 2011, 36, 794–798. [Google Scholar] [CrossRef]
- Trung, T.N.; Arepalli, V.K.; Gudala, R.; Kim, E.-T. Polyol synthesis of ultrathin and high-aspect-ratio Ag nanowires for transparent conductive films. Mater. Lett. 2017, 194, 66–69. [Google Scholar] [CrossRef]
- Hwang, B.; An, C.-H.; Becker, S. Highly robust Ag nanowire flexible transparent electrode with UV-curable polyurethane-based overcoating layer. Mater. Des. 2017, 129, 180–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Xu, D.; Sun, Y.; Yan, F. One-pot synthesis and purification of ultralong silver nanowires for flexible transparent conductive electrodes. ACS Appl. Mater. Interfaces 2017, 9, 25465–25473. [Google Scholar] [CrossRef]
- Hwang, C.; An, J.; Choi, B.D.; Kim, K.; Jung, S.-W.; Baeg, K.-J.; Kim, M.-G.; Ok, K.M.; Hong, J. Controlled aqueous synthesis of ultra-long copper nanowires for stretchable transparent conducting electrode. J. Mater. Chem. C 2016, 4, 1441–1447. [Google Scholar] [CrossRef]
- Chu, H.-C.; Chang, Y.-C.; Lin, Y.; Chang, S.-H.; Chang, W.-C.; Li, G.-A.; Tuan, H.-Y. Spray-deposited large-area copper nanowire transparent conductive electrodes and their uses for touch screen applications. ACS Appl. Mater. Interfaces 2016, 8, 13009–13017. [Google Scholar] [CrossRef]
- Zhong, Z.; Lee, H.; Kang, D.; Kwon, S.; Choi, Y.-M.; Kim, I.; Kim, K.-Y.; Lee, Y.; Woo, K.; Moon, J. Continuous patterning of copper nanowire-based transparent conducting electrodes for use in flexible electronic applications. ACS Nano 2016, 10, 7847–7854. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, P.; Lee, H.B.; Hong, S.; Lee, I.; Yeo, J.; Lee, S.S.; Kim, T.S.; Lee, D.; Ko, S.H. Room-temperature nanosoldering of a very long metal nanowire network by conducting-polymer-assisted joining for a flexible touch-panel application. Adv. Funct. Mater. 2013, 23, 4171–4176. [Google Scholar] [CrossRef]
- Yuksel, R.; Coskun, S.; Unalan, H.E. Coaxial silver nanowire network core molybdenum oxide shell supercapacitor electrodes. Electrochim. Acta 2016, 193, 39–44. [Google Scholar] [CrossRef]
- Bergin, S.M.; Chen, Y.-H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.-Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef] [Green Version]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating simulations and experiments to predict sheet resistance and optical transmittance in nanowire films for transparent conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef]
- Jiu, J.; Araki, T.; Wang, J.; Nogi, M.; Sugahara, T.; Nagao, S.; Koga, H.; Suganuma, K.; Nakazawa, E.; Hara, M. Facile synthesis of very-long silver nanowires for transparent electrodes. J. Mater. Chem. A 2014, 2, 6326–6330. [Google Scholar] [CrossRef]
- Jeong, C.K.; Lee, J.; Han, S.; Ryu, J.; Hwang, G.T.; Park, D.Y.; Park, J.H.; Lee, S.S.; Byun, M.; Ko, S.H.; et al. A hyper-stretchable elastic-composite energy harvester. Adv. Mater. 2015, 27, 2866–2875. [Google Scholar] [CrossRef]
- Schuette, W.M.; Buhro, W.E. Polyol synthesis of silver nanowires by heterogeneous nucleation; mechanistic aspects influencing nanowire diameter and length. Chem. Mater. 2014, 26, 6410–6417. [Google Scholar] [CrossRef]
- Li, B.; Ye, S.; Stewart, I.E.; Alvarez, S.; Wiley, B.J. Synthesis and purification of silver nanowires to make conducting films with a transmittance of 99%. Nano Lett. 2015, 15, 6722–6726. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Wu, X.; Lian, L.; Yang, S.; Dong, D.; Feng, D.; He, G. A highly conductive and smooth AgNW/PEDOT: PSS film treated by hot-pressing as electrode for organic light emitting diode. Organ. Electron. 2017, 43, 182–188. [Google Scholar] [CrossRef]
- Nekahi, A.; Marashi, S.; Fatmesari, D.H. High yield polyol synthesis of round-and sharp-end silver nanowires with high aspect ratio. Mater. Chem. Phys. 2016, 184, 130–137. [Google Scholar]
- Tang, C.; Sun, W.; Lu, J.; Yan, W. Role of the anions in the hydrothermally formed silver nanowires and their antibacterial property. J. Colloid Interface Sci. 2014, 416, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef]
- Toybou, D.; Celle, C.; Aude-Garcia, C.; Rabilloud, T.; Simonato, J.-P. A toxicology-informed, safer by design approach for the fabrication of transparent electrodes based on silver nanowires. Environ. Sci. Nano 2019, 6, 684–694. [Google Scholar] [CrossRef]
- Lei, B.; Wang, J.; Du, Y.; Zhang, K. Controlling the size of silver nanowires through one-pot polyol method with trace halide and its effect on kinetic process. Mater. Res. Express 2017, 4, 075052. [Google Scholar] [CrossRef]
- Rui, Y.; Zhao, W.; Zhu, D.; Wang, H.; Song, G.; Swihart, M.T.; Wan, N.; Gu, D.; Tang, X.; Yang, Y.; et al. Understanding the effects of NaCl, NaBr and their mixtures on silver nanowire nucleation and growth in terms of the distribution of electron traps in silver halide crystals. Nanomaterials 2018, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Tetsumoto, T.; Gotoh, Y.; Ishiwatari, T. Mechanistic studies on the formation of silver nanowires by a hydrothermal method. J. Colloid Interface Sci. 2011, 362, 267–273. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Luu, Q.N.; Doorn, J.M.; Berry, M.T.; Jiang, C.; Lin, C.; May, P.S. Preparation and optical properties of silver nanowires and silver-nanowire thin films. J. Colloid Interface Sci. 2011, 356, 151–158. [Google Scholar] [CrossRef]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- Zhang, K.; Du, Y.; Chen, S. Sub 30 nm silver nanowire synthesized using KBr as co-nucleant through one-pot polyol method for optoelectronic applications. Organ. Electron. 2015, 26, 380–385. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol synthesis of silver nanowires: An extensive parametric study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- Da Silva, R.R.; Yang, M.; Choi, S.-I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.-Y.; Camargo, P.H.; Ribeiro, S.J.L.; Xia, Y. Facile synthesis of sub-20 nm silver nanowires through a bromide-mediated polyol method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Huang, Q.; Cui, J.; Lin, H.; Li, W.; Lin, Z.; Zhang, P. Rapid and Facile Synthesis of High-Performance Silver Nanowires by a Halide-Mediated, Modified Polyol Method for Transparent Conductive Films. Nanomaterials 2020, 10, 1139. https://doi.org/10.3390/nano10061139
Cao L, Huang Q, Cui J, Lin H, Li W, Lin Z, Zhang P. Rapid and Facile Synthesis of High-Performance Silver Nanowires by a Halide-Mediated, Modified Polyol Method for Transparent Conductive Films. Nanomaterials. 2020; 10(6):1139. https://doi.org/10.3390/nano10061139
Chicago/Turabian StyleCao, Lin, Qin Huang, Jie Cui, Huaijun Lin, Wei Li, Zhidan Lin, and Peng Zhang. 2020. "Rapid and Facile Synthesis of High-Performance Silver Nanowires by a Halide-Mediated, Modified Polyol Method for Transparent Conductive Films" Nanomaterials 10, no. 6: 1139. https://doi.org/10.3390/nano10061139





