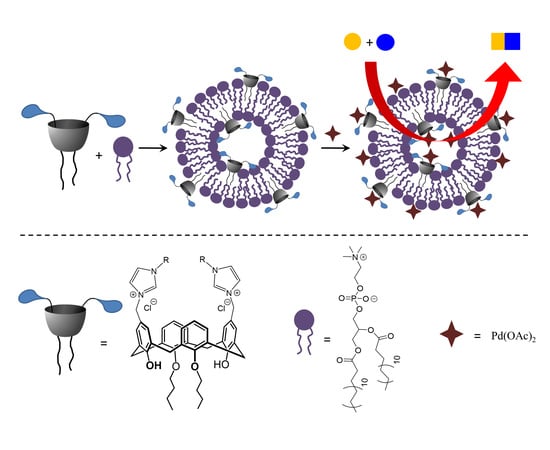
New Amphiphilic Imidazolium/Benzimidazolium Calix[4]arene Derivatives: Synthesis, Aggregation Behavior and Decoration of DPPC Vesicles for Suzuki Coupling in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterisation Methods
2.2. Reagents
2.3. Dynamic Light Scattering and Zeta-Potential
2.4. Critical Aggregation Concentration Determination
2.5. Vesicles Preparation
2.6. Turbidity Measurements
2.7. Gas Chromatography Mass Spectrometry
2.8. Suzuki–Miyaura Coupling
3. Results and Discussion
3.1. Synthesis of Imidazolium/Benzimidazolium Calix[4]arene Derivatives
3.2. Aggregation Behavior of 5–9 in Aqueous Solutions
3.3. Complexes of 5–9 with Pd(II) Obtained In Situ in Model Suzuki–Miyaura Coupling
3.4. Embedding of 5 into DPPC Vesicles and Their Catalytic Activity in Suzuki–Miyaura Coupling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beletskaya, I.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 73874–73922. [Google Scholar] [CrossRef] [PubMed]
- Corbet, J.-P.; Mignani, G.R. Selected patented cross-coupling reaction technologies. Chem. Rev. 2006, 106, 2651–2710. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P.; Scott, N.M. N-Heterocyclic Carbenes in Synthesis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef] [PubMed]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Udvardy, A.; Czegeni, C.E.; Joo, F. Poly-N-heterocyclic carbene complexes with applications in aqueous media. Coord. Chem. Rev. 2019, 400, 213038. [Google Scholar] [CrossRef]
- Levin, E.; Ivry, E.; Diesendruck, C.E.; Lemcoff, N.G. Water in N-Heterocyclic Carbene-Assisted Catalysis. Chem. Rev. 2015, 115, 4607–4692. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Sorella, G.L.; Strukul, G.; Scarso, A. Recent advances in catalysis in micellar media. Green Chem. 2015, 17, 644–683. [Google Scholar] [CrossRef]
- Donner, A.; Hagedorn, K.; Mattes, L.; Drechsler, M.; Polarz, S. Hybrid Surfactants with N-Heterocyclic Carbene Heads as a Multifunctional Platform for Interfacial Catalysis. Chem. Eur. J. 2017, 23, 18129–18133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Liu, J.; Wang, Y.; Wang, J. Synthesis, structure and catalysis/applications of N-heterocyclic carbene based on macrocycles. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 15–37. [Google Scholar] [CrossRef]
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; van Leeuwen, P.W.N.M. Supramolecular catalysis. Part 2: Artificial enzyme mimics. Chem. Soc. Rev. 2014, 43, 1734–1787. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, S.E.; Burilov, V.A.; Antipin, I.S. Thiacalix[4]arene’s Lower Rim Derivatives: Synthesis and Supramolecular Properties. Macroheterocycles 2017, 10, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Helttunen, K.; Shahgaldian, P. Self-assembly of amphiphilic calixarenes and resorcinarenes in water. New J. Chem. 2010, 34, 2704–2714. [Google Scholar] [CrossRef]
- Burilov, V.A.; Valiyakhmetova, A.M.; Mironova, D.A.; Sultanova, E.D.; Evtugyn, V.G.; Osin, Y.N.; Katsyuba, S.A.; Burganov, T.I.; Solovieva, S.E.; Antipin, I.S. Novel amphiphilic conjugates of: P-tert -butylthiacalix[4]arene with 10,12-pentacosadiynoic acid in 1,3-alternate stereoisomeric form synthesis and chromatic properties in the presence of metal ions. New J. Chem. 2018, 42, 2942–2951. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Padnya, P.L.; Kunafina, A.F.; Nugmanova, A.R.; Stoikov, I.I. Sulfobetaine derivatives of thiacalix[4]arene: Synthesis and supramolecular self-assembly of submicron aggregates with AgI cations. Mendeleev Commun. 2019, 29, 86–88. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 6th ed.; Elsevier: New York, NY, USA, 2009. [Google Scholar]
- Kumar, N.; Jain, R. Convenient syntheses of bulky group containing imidazolium ionic liquids. J. Heterocycl. Chem. 2012, 49, 370–374. [Google Scholar] [CrossRef]
- Jingping, L.; Jingbo, C.; Jingfeng, Z.; Yuanhong, Z.; Liang, L.; Hongbin, Z. A modified procedure for the synthesis of 1-arylimidazoles. Synthesis 2003, 17, 2661–2666. [Google Scholar]
- Gutsche, D.C.; Dhawan, B.; No, K.H.; Muthukrishnan, R. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J. Am. Chem. Soc. 1981, 103, 3782–3792. [Google Scholar] [CrossRef]
- Gutsche, D.C.; Levine, J.A. Calixarenes. 6. Synthesis of a functionalizable calix[4]arene in a conformationally rigid cone conformation. J. Am. Chem. Soc. 1982, 104, 2653–2655. [Google Scholar] [CrossRef]
- Frank, M.; Maas, G.; Schatz, J. Calix[4]arene-Supported N-Heterocyclic Carbene Ligands as Catalysts for Suzuki Cross-Coupling Reactions of Chlorotoluene. Eur. J. Org. Chem. 2004, 3, 607–613. [Google Scholar] [CrossRef]
- Eker, F.; Durmus, H.O.; Akinoglu, B.G.; Severcan, F. Application of turbidity technique on peptide-lipid and drug-lipid interactions. J. Mol. Struct. 1999, 482–483, 693–697. [Google Scholar] [CrossRef]
- Fahlbusch, T.; Frank, M.; Maas, G.; Schatz, J. N-Heterocyclic Carbene Complexes of Mercury, Silver, Iridium, Platinum, Ruthenium, and Palladium Based on the Calix[4]arene Skeleton. Organometallics 2009, 28, 6183–6193. [Google Scholar] [CrossRef]
- Fahlbusch, T.; Frank, M.; Schatz, J. The Suzuki Coupling of Aryl Chlorides in Aqueous Media Catalyzed by in situ Generated Calix[4]arene-Based N-Heterocyclic Carbene Ligands. Eur. J. Org. Chem. 2006, 10, 2378–2383. [Google Scholar]
- Larsen, M.; Jørgensen, M. Selective Halogen-Lithium Exchange Reaction of Bromine-Substituted 25,26,27,28-Tetrapropoxycalix[4]arene. J. Org. Chem. 1996, 61, 6651–6655. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-T.; Wang, G.-Q.; Yang, L.-M.; Lou, Y.-X. The Selective Chloromethylation of 25, 27-Dihydroxy-26, 28-Dimethoxycalix{4}arene and Nucleophilic Substitution Therefrom. Synth. Commun. 1995, 25, 1109–1118. [Google Scholar] [CrossRef]
- Rehm, M.; Frank, M.; Schatz, J. Water-soluble calixarenes—Self-aggregation and complexation of noncharged aromatic guests in buffered aqueous solution. Tetrahedron Lett. 2009, 50, 93–96. [Google Scholar] [CrossRef]
- Regnouf-de-Vains, J.-B.; Berthalon, S.; Lamartine, R. Electrospray mass spectrometric evidence of calixarene p-quinone methide formation. J. Mass Spectrom. 1998, 33, 968–970. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes; Royal Society of Chemistry: Cambridge, UK, 1989. [Google Scholar]
- Rodik, R.V.; Anthony, A.-S.; Kalchenko, V.I.; Mely, Y.; Klymchenko, A.S. Cationic amphiphilic calixarenes to compact DNA into small nanoparticles for gene delivery. New J. Chem. 2015, 39, 1654–1664. [Google Scholar] [CrossRef]
- Burilov, V.A.; Mironova, D.A.; Ibragimova, R.R.; Evtugyn, V.G.; Osin, Y.N.; Solovieva, S.E.; Antipin, I.S. Imidazolium p-tert-Butylthiacalix[4]arene Amphiphiles—Aggregation in Water Solutions and Binding with Adenosine 5-Triphosphate Dipotassium Salt. BioNanoScience 2018, 8, 337–343. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolıvar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Rozengart, E.; Basova, N. Ammonium Compounds with Localized and Delocalized Charge as Reversible Inhibitors of Cholinesterases of Different Origin. J. Evol. Biochem. Physiol. 2001, 37, 604–610. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes−Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Akkoç, S.; Gök, Y.; Özer lhan, l.; Kayser, V. In situ Generation of Efficient Palladium N-heterocyclic Carbene Catalysts Using Benzimidazolium Salts for the Suzuki-Miyaura Cross-coupling Reaction. Curr. Org. Synth. 2016, 13, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd–N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Szilvási, T.; Veszprémi, T. Internal Catalytic Effect of Bulky NHC Ligands in Suzuki–Miyaura Cross-Coupling Reaction. ACS Catal. 2013, 3, 1984–1991. [Google Scholar] [CrossRef]
- Burilov, V.; Gafiatullin, B.; Mironova, D.; Sultanova, E.; Evtugyn, V.; Osin, Y.; Islamov, D.; Usachev, K.; Solovieva, S.; Antipin, I. Amphiphilic Pd (II)-NHC complexes on 1,3-alternate p-tertbutylthiacalix[4]arene platform: Synthesis and catalytic activities in coupling and hydrogenation reactions. Eur. J. Org. Chem. 2020. [Google Scholar] [CrossRef]
- Kostyukovich, A.Y.; Tsedilin, A.M.; Sushchenko, E.D.; Eremin, D.B.; Kashin, A.S.; Topchiy, M.A.; Asachenko, A.F.; Nechaev, M.S.; Ananikov, V.P. In situ transformations of Pd/NHC complexes with N-heterocyclic carbene ligands of different nature into colloidal Pd nanoparticles. Inorg. Chem. Front. 2018, 6, 482–492. [Google Scholar] [CrossRef]
- Lasic, D.D.; Barenholz, Y. Handbook of Nonmedical Applications of Liposomes, 1st ed.; CRC Press Inc.: Boca Raton, FL, USA, 1996. [Google Scholar]
- Gruber, B.; König, B. Self-Assembled Vesicles with Functionalized Membranes. Chem. Eur. J. 2012, 19, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Duss, M.; Vallooran, J.J.; Salvati Manni, L.; Kieliger, N.; Handschin, S.; Mezzenga, R.; Jessen, H.J.; Landau, E.M. Lipidic Mesophase-embedded Palladium Nanoparticles: Synthesis and Tunable Catalysts in Suzuki-Miyaura Cross Coupling Reactions. Langmuir 2019, 35, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Muraoka, Y.; Fukushima, K.; Shimozawa, R. Interaction of surfactants with vesicle membrane of dipalmitoylphosphatidylcholine: Fluorescence depolarization study. Chem. Phys. Lipids 1988, 46, 107–115. [Google Scholar] [CrossRef]
- Batna, A.; Spiteller, G. Oxidation of furan fatty acids by soybean lipoxygenase-1 in the presence of linoleic acid. Chem. Phys. Lipids 1994, 70, 179–185. [Google Scholar] [CrossRef]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Khamatgalimov, A.R.; Kovalenko, V.; Zakharova, L.Y. Cationic amphiphiles bearing imidazole fragment: From aggregation properties to potential in biotechnologies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 990–997. [Google Scholar] [CrossRef]






| Calixarene * | CAC, µM | d, nm | PDI | ζ. mV |
|---|---|---|---|---|
| 5 | 65 | 340 ± 13 | 0.426 ± 0.081 | +57 ± 2 |
| 6 | 45 | 351 ± 51 | 0.477 ± 0.042 | +45 ± 1 |
| 7 | 40 | 420 ± 5 | 0.473 ± 0.055 | +52 ± 1 |
| 8 | 53 | 390 ± 2 | 0.513 ± 0.030 | +44 ± 1 |
| 9 | 120 | 410 ± 18 | 0.416 ± 0.070 | +54 ± 0.5 |
| Catalyst * | TON | TOF 10−2 s−1 |
|---|---|---|
| Pd(OAc)2 | 22 | 1.8 |
| 5+Pd(OAc)2 | 89 | 7.4 |
| 6+Pd(OAc)2 | 67 | 5.6 |
| 7+Pd(OAc)2 | 56 | 4.6 |
| 8+Pd(OAc)2 | 62 | 5.2 |
| 9+Pd(OAc)2 | 53 | 4.4 |
| System | Calixarene/DPPC Molar Ratio | d, nm | PDI | ζ. mV | ||
|---|---|---|---|---|---|---|
| before Extrusion | after Extrusion | before Extrusion | after Extrusion | |||
| DPPC | 0 | 600 ± 63 | 106 ± 2 | 0.780 ± 0.140 | 0.078 ± 0.006 | - |
| DPPC+ 5 | 0.04 | 130 ± 5 | 64 ± 5 | 0.703 ± 0.049 | 0.269 ± 0.074 | +22 ± 1 |
| DPPC+ 5 | 0.07 | 60 ± 1 | 51 ± 2 | 0.331 ± 0.015 | 0.239 ± 0.015 | +36 ± 3 |
| DPPC+ 5 | 0.1 | 79 ± 1 | 63 ± 1 | 0.365 ± 0.011 | 0.285 ± 0.025 | +35 ± 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burilov, V.; Garipova, R.; Sultanova, E.; Mironova, D.; Grigoryev, I.; Solovieva, S.; Antipin, I. New Amphiphilic Imidazolium/Benzimidazolium Calix[4]arene Derivatives: Synthesis, Aggregation Behavior and Decoration of DPPC Vesicles for Suzuki Coupling in Aqueous Media. Nanomaterials 2020, 10, 1143. https://doi.org/10.3390/nano10061143
Burilov V, Garipova R, Sultanova E, Mironova D, Grigoryev I, Solovieva S, Antipin I. New Amphiphilic Imidazolium/Benzimidazolium Calix[4]arene Derivatives: Synthesis, Aggregation Behavior and Decoration of DPPC Vesicles for Suzuki Coupling in Aqueous Media. Nanomaterials. 2020; 10(6):1143. https://doi.org/10.3390/nano10061143
Chicago/Turabian StyleBurilov, Vladimir, Ramilya Garipova, Elsa Sultanova, Diana Mironova, Ilya Grigoryev, Svetlana Solovieva, and Igor Antipin. 2020. "New Amphiphilic Imidazolium/Benzimidazolium Calix[4]arene Derivatives: Synthesis, Aggregation Behavior and Decoration of DPPC Vesicles for Suzuki Coupling in Aqueous Media" Nanomaterials 10, no. 6: 1143. https://doi.org/10.3390/nano10061143