Tuning the Solubility Parameters of Carbon Nanotubes by Means of Their Adducts with Janus Pyrrole Compounds
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Multi Walled Carbon Nanotubes (CNTs)
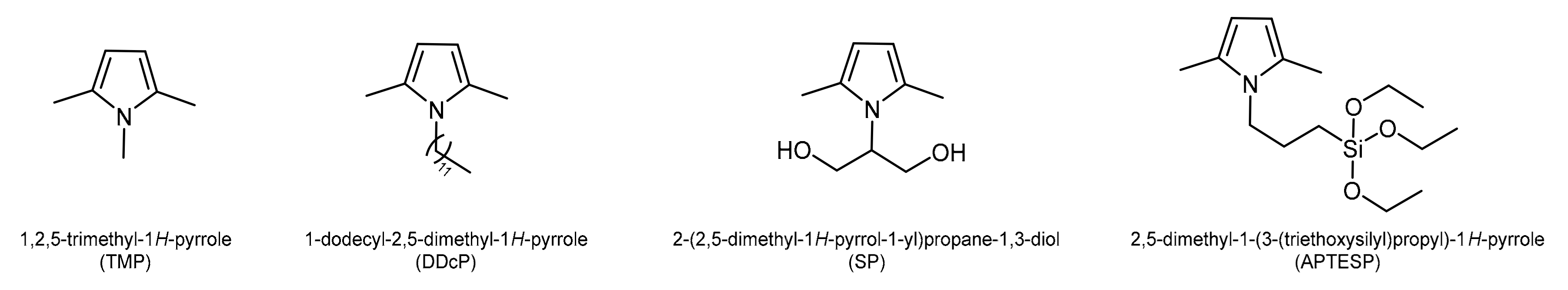
2.1.2. Reagents for PyCs Synthesis
2.1.3. Ingredients for the Preparation of Coating Layers
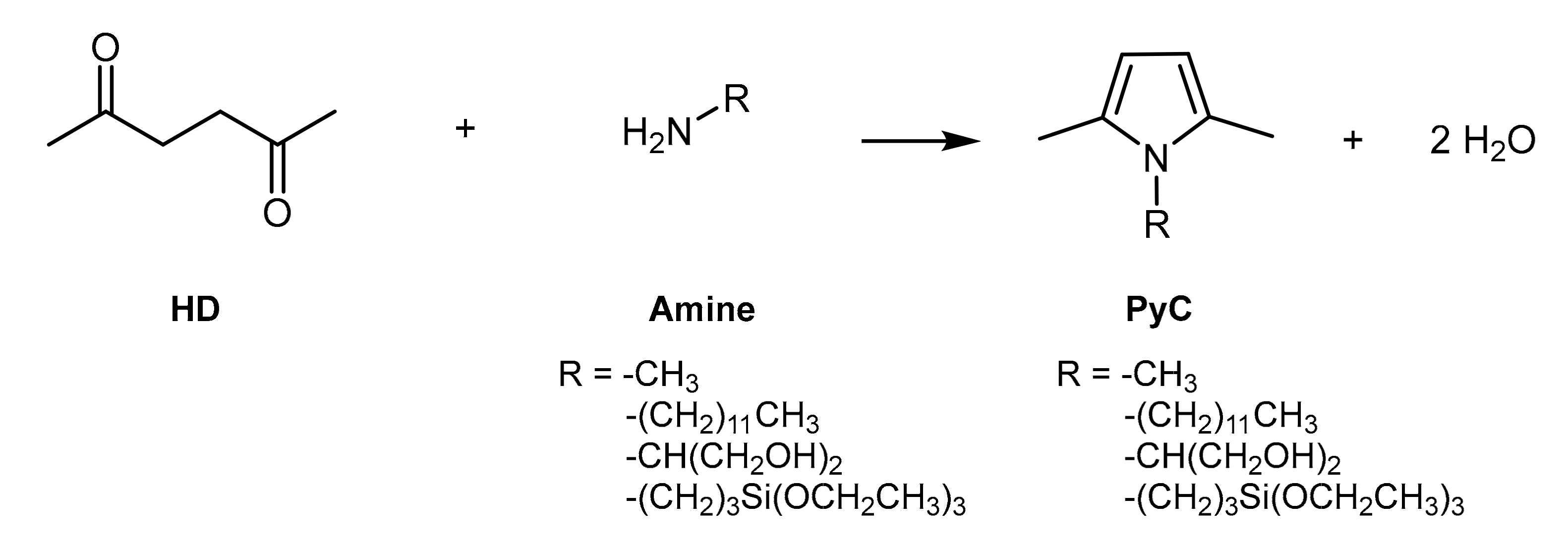
2.2. Synthesis of PyCs
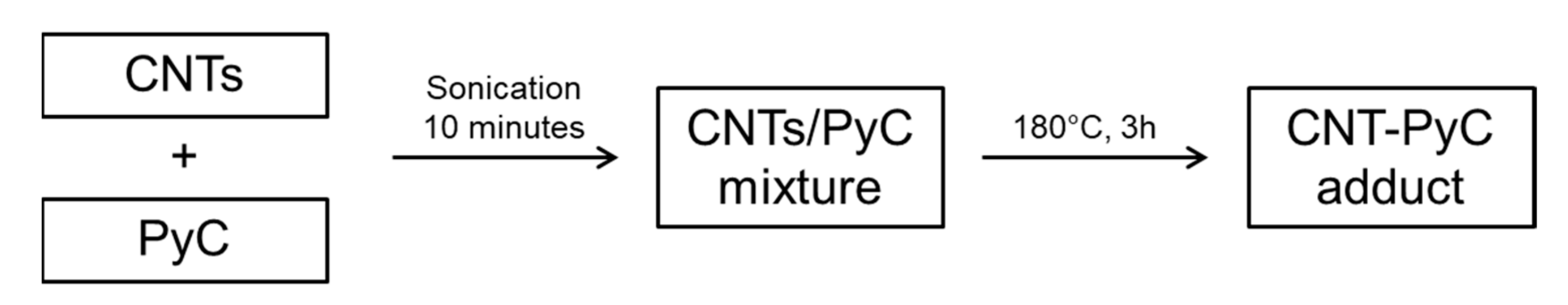
2.3. Preparation of CNT-PyC Adducts
General Procedure
2.4. Characterization of CNT-PyC Adducts
2.4.1. Thermogravimetric Analysis (TGA)
2.4.2. Fourier Transform-Infra Red Spectroscopy (FT-IR)
2.4.3. Raman Spectroscopy
2.4.4. Wide Angle X-ray Diffraction (WAXD)
2.4.5. High-Resolution Transmission Electron Microscopy (HRTEM)
2.5. Preparation, Stability Evaluation and Characterization of Dispersions of CNT-PyC Adducts in Different Solvents
2.5.1. Preparation and Stability Evaluation
2.5.2. Calculation of the Hansen Solubility Parameters (HSP) and Hansen Solubility Sphere
2.6. Preparation of CNT-Based Coating Layers from Water Dispersions, Either Based on CNT-SP or Commercially Available
3. Results and Discussion
3.1. Preparation of CNT-PyC Adducts
3.2. Characterization of CNT-PyC Adducts
Solvent Extraction and Yield of Functionalization
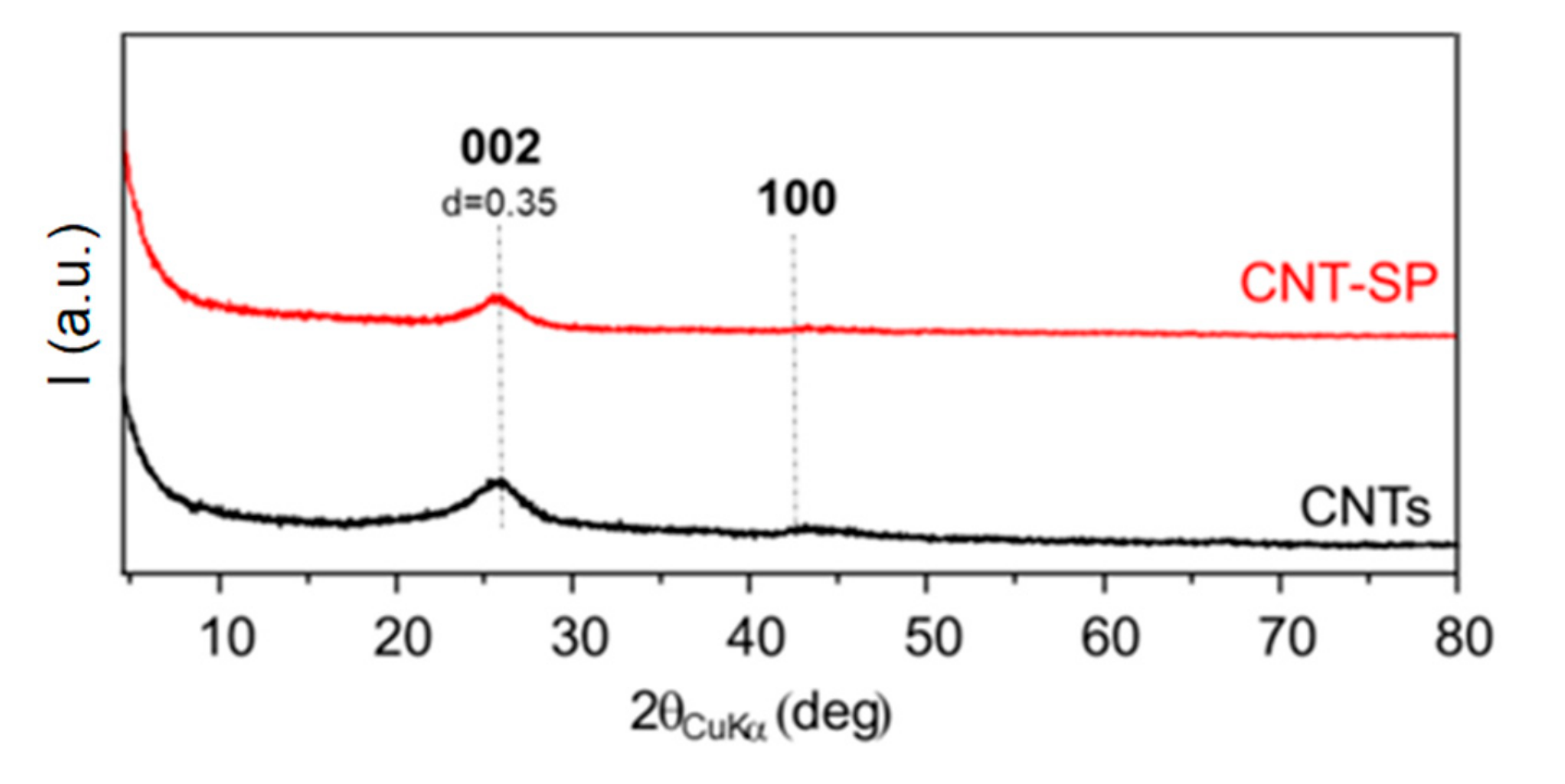
3.3. WAXD and Raman Analysis
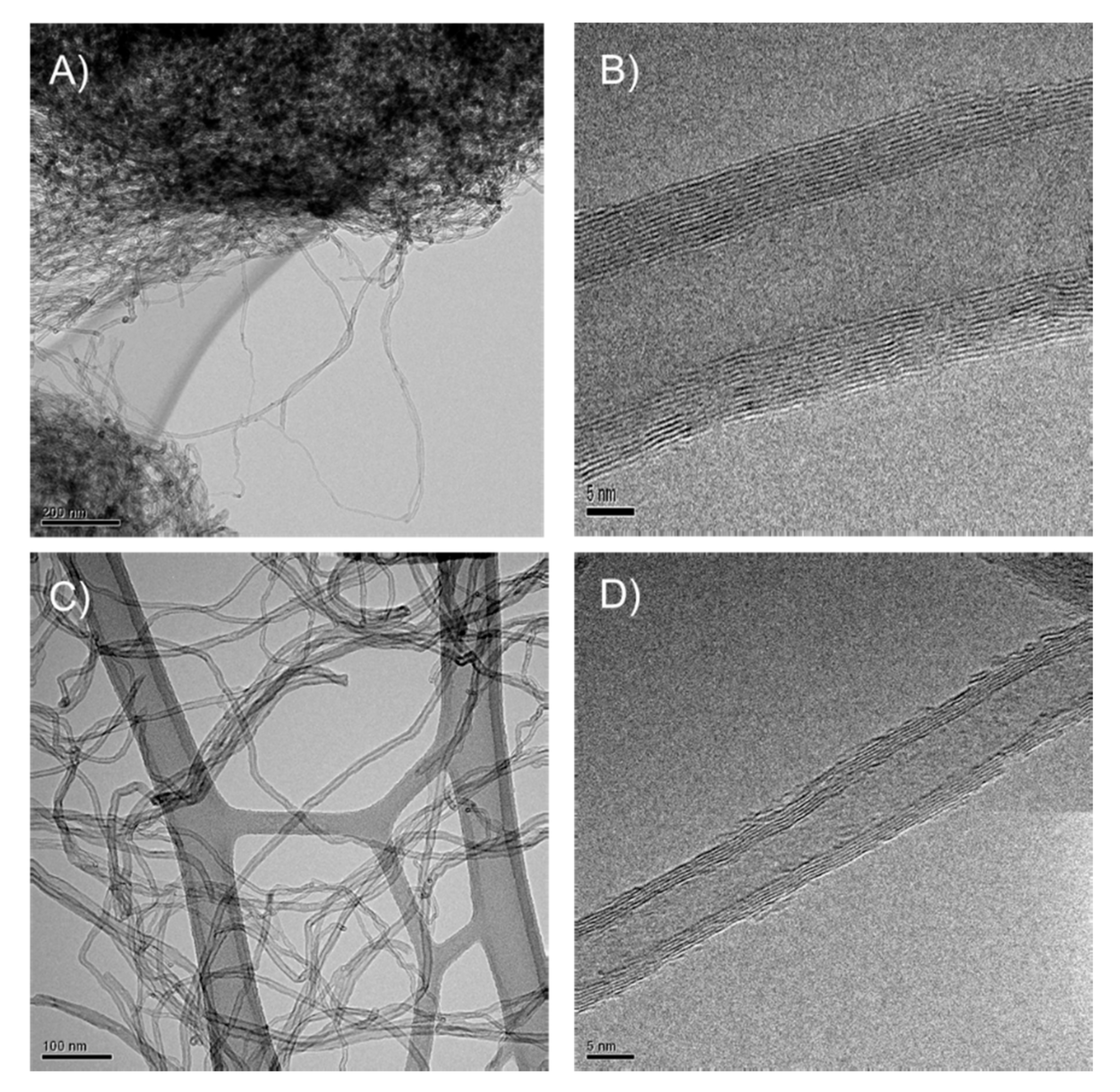
3.4. HRTEM Analysis
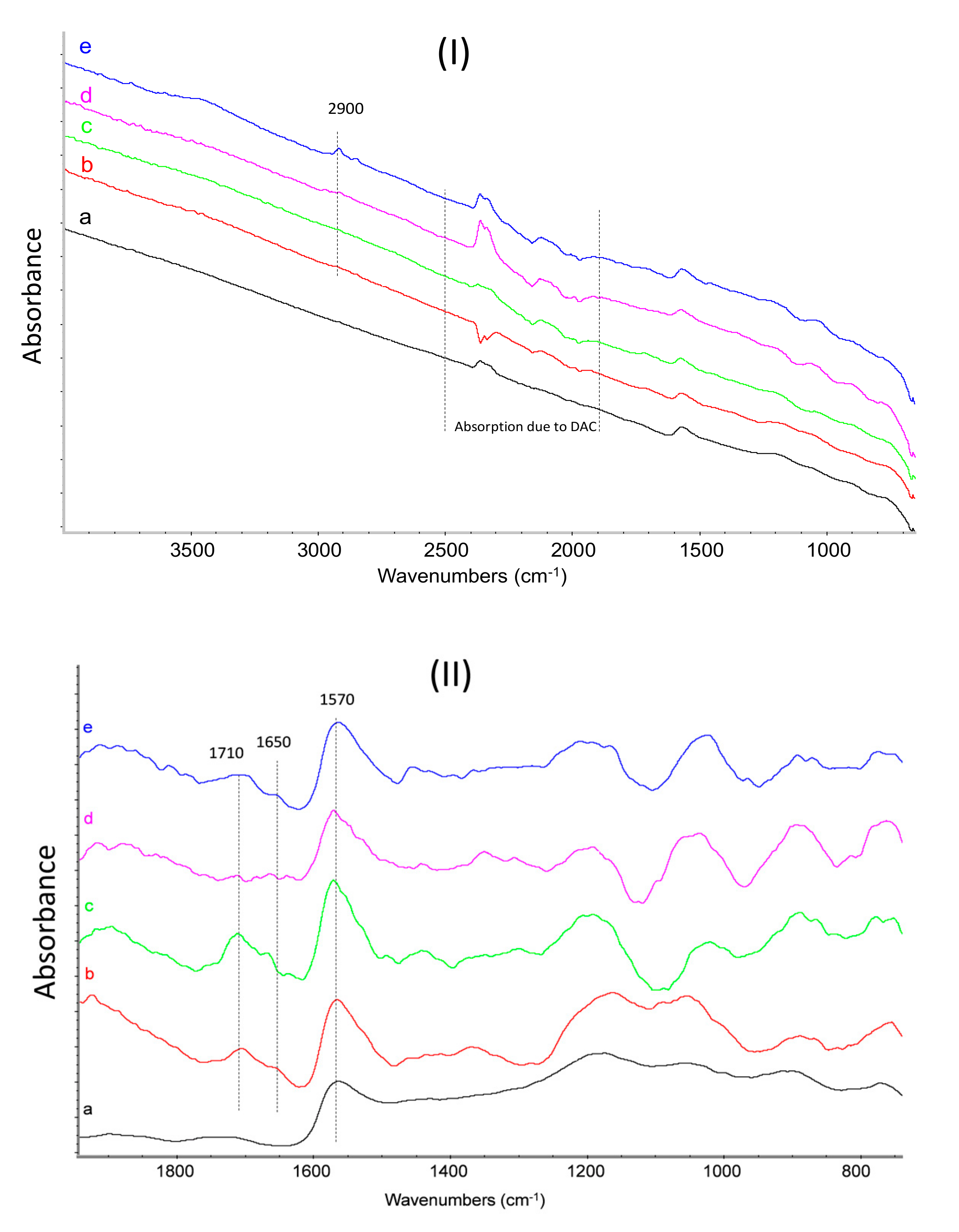
3.5. FT-IR Characterization
3.6. On the Mechanism of the Adducts’ Formation
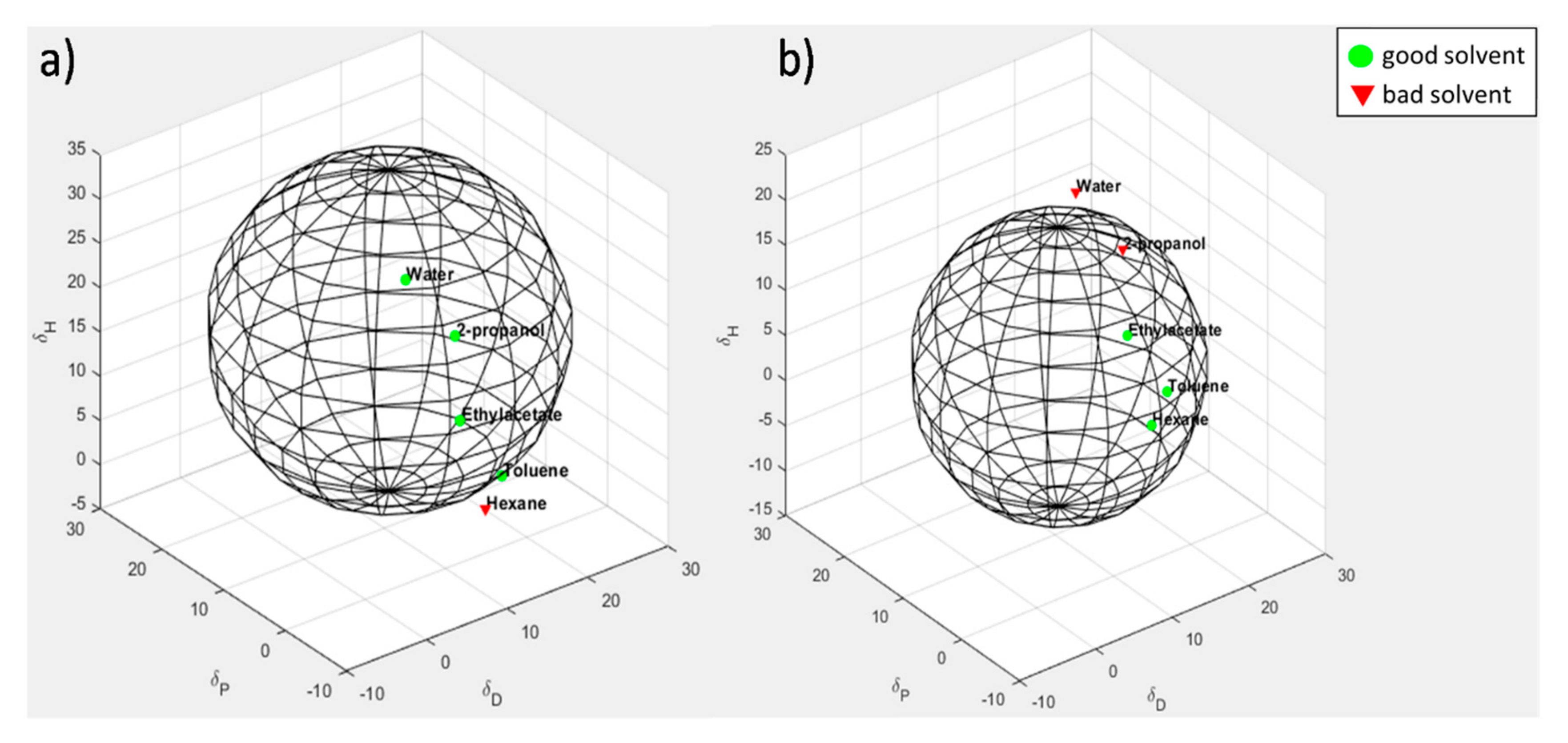
3.7. Evaluation of Solubility Parameters of CNTs and CNT-PyC Adducts
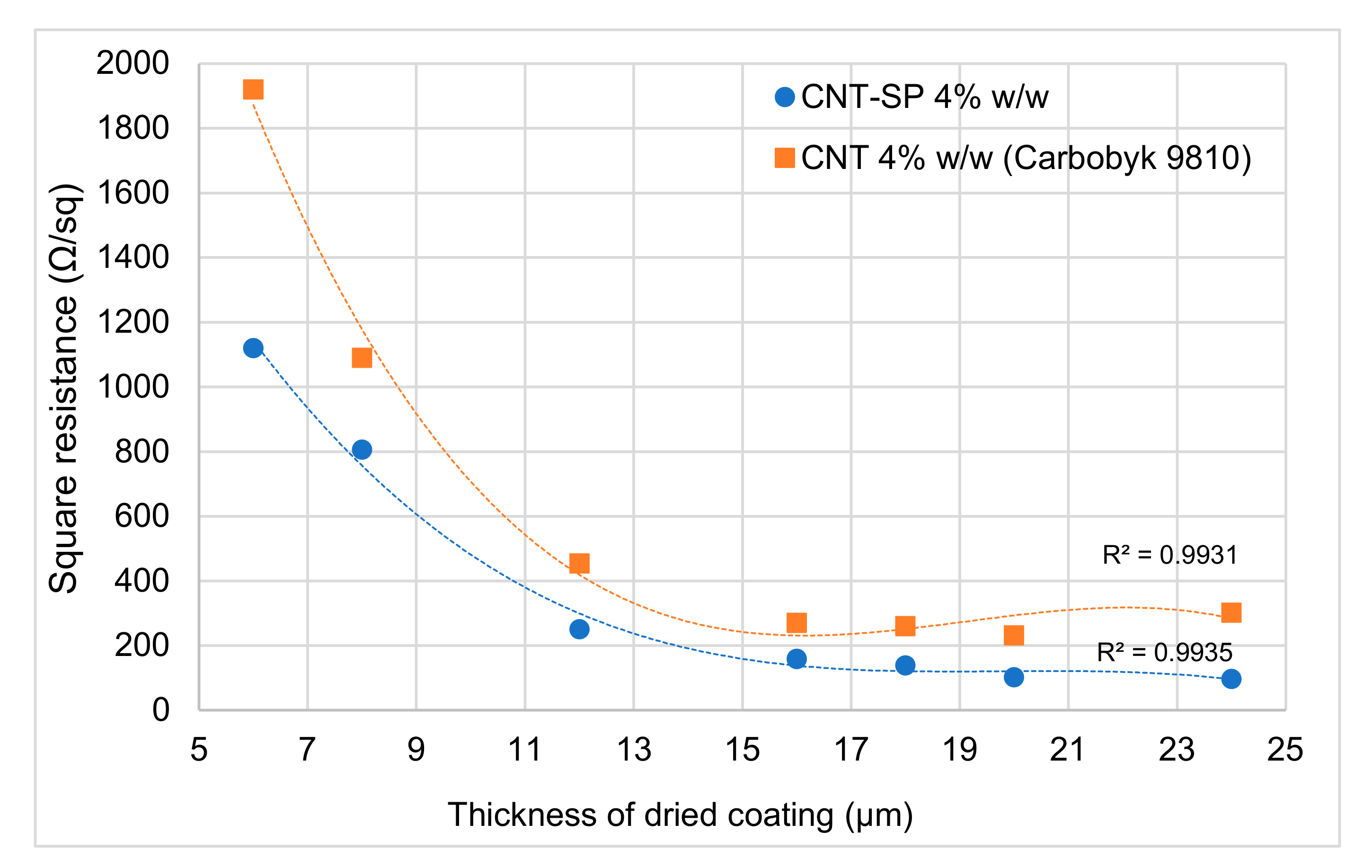
3.8. Electrically Conductive Coating Layer with CNT-SP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donnet, J.B. (Ed.) Carbon Black: Science and Technology; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Kroto, H.W.; McKay, K. The formation of quasi-icosahedral spiral shell carbon particles. Nature 1988, 331, 328–331. [Google Scholar] [CrossRef]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J.; Elías, A.L.; Muñoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.C.; et al. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; De Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who should be given the credit for the discovery of carbon nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, S.J.; Kim, J.K. Preparation of graphite nanoplatelets and graphene sheets. J. Coll. Interf. Sci. 2009, 336, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Yum, J.H.; Gratzel, M. Optically transparent cathode for dye-sensitized solar cells based on graphene nanoplatelets. ACS Nano 2011, 5, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Lahiri, D.; Agarwal, A. Synthesis and properties of bulk graphene nanoplatelets consolidated by spark plasma sintering. Carbon 2012, 50, 4068–4077. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Coleman, J.N. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624. [Google Scholar] [CrossRef]
- Zhang, J.; Terrones, M.; Park, C.R.; Mukherjee, R.; Monthioux, M.; Koratkar, N.; Kim, Y.S.; Hurt, R.; Frackowiak, E.; Enoki, T.; et al. Carbon science in 2016: Status, challenges and perspectives. Carbon 2016, 98, 708–732. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Satishkumar, B.C.; Govindaraj, A.; Nath, M. Nanotubes. ChemPhysChem 2001, 2, 78–105. [Google Scholar] [CrossRef]
- De Heer, W.A. Nanotubes and the pursuit of applications. MRS Bull. 2004, 29, 281–285. [Google Scholar] [CrossRef]
- Uchida, T.; Kumar, S. Single wall carbon nanotube dispersion and exfoliation in polymers. J. Appl. Polym. Sci. 2005, 98, 985–989. [Google Scholar] [CrossRef]
- Jorio, A.; Dresselhaus, G.; Dresselhaus, M.S. Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Kasumov, A.Y.; Deblock, R.; Kociak, M.; Reulet, B.; Bouchiat, H.; Khodos, I.I.; Gorbatov, Y.B.; Volkov, V.T.; Journet, C.; Burghard, M. Supercurrents through single-walled carbon nanotubes. Science 1999, 284, 1508–1511. [Google Scholar] [CrossRef]
- Gross, A.J.; Hammond, J.L.; Holzinger, M.; Cosnier, S. Flotation Assembly of Large-Area Ultrathin MWCNT Nanofilms for Construction of Bioelectrodes. Nanomaterials 2017, 7, 342. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; De Rossi, D.; Rinzler, A.G.; et al. Carbon nanotube actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Bachtold, A.; Hadley, P.; Nakanishi, T.; Dekker, C. Logic circuits with carbon nanotube transistors. Science 2001, 294, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Ago, H.; Petritsch, K.; Shaffer, M.S.; Windle, A.H.; Friend, R.H. Composites of carbon nanotubes and conjugated polymers for photovoltaic devices. Adv. Mater. 1999, 11, 1281–1285. [Google Scholar] [CrossRef]
- Ajayan, P.M. Capillarity-induced filling of carbon nanotubes. Nature 1993, 361, 333–334. [Google Scholar] [CrossRef]
- Lepak-Kuc, S.; Podsiadły, B.; Skalski, A.; Janczak, D.; Jakubowska, M.; Lekawa-Raus, A. Highly conductive carbon nanotube-thermoplastic polyurethane nanocomposite for smart clothing applications and beyond. Nanomaterials 2019, 9, 1287. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Kepple, K.L.; Sanborn, G.P.; Lacasse, P.A.; Gruenberg, K.M.; Ready, W.J. Improved fracture toughness of carbon fiber composite functionalized with multi walled carbon nanotubes. Carbon 2008, 46, 2026–2033. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Dyke, C.A.; Tour, J.M. Covalent functionalization of single-walled carbon nanotubes for materials applications. J. Phys. Chem. A 2004, 108, 11151–11159. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Singh, P.; Campidelli, S.; Giordani, S.; Bonifazi, D.; Bianco, A.; Prato, M. Organic functionalisation and characterisation of single-walled carbon nanotubes. Chem. Soc. Rev. 2009, 38, 2214–2230. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manufact. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Setaro, A. Advanced carbon nanotubes functionalization. J. Phys. Condens. Matter 2017, 29, 423003. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Lin, M.; Zhong, W.; Feng, T.; Chen, X.; Chen, J.; Xue, F. Mechanical and thermal properties of epoxy nanocomposites reinforced with amino-functionalized multi-walled carbon nanotubes. Mater. Sci. Eng. A 2008, 492, 236–242. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Ayres, R.U.; Turton, H.; Casten, T. Energy efficiency, sustainability and economic growth. Energy 2007, 32, 634–648. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Guerra, S.; Conzatti, L.; Castiglioni, C.; Brambilla, L.; Serafini, A. Biobased Janus molecule for the facile preparation of water solutions of few layer graphene sheets. RSC Adv. 2015, 5, 81142–81152. [Google Scholar] [CrossRef]
- Barbera, V.; Bernardi, A.; Palazzolo, A.; Rosengart, A.; Brambilla, L.; Galimberti, M. Facile and sustainable functionalization of graphene layers with pyrrole compounds. Pure Appl. Chem. 2018, 90, 253–270. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Guerra, S.; Bernardi, A. Facile functionalization of sp2 carbon allotropes with a biobased Janus molecule. Rubber Chem. Technol. 2017, 90, 285–307. [Google Scholar] [CrossRef]
- Barbera, V.; Brambilla, L.; Milani, A.; Palazzolo, A.; Castiglioni, C.; Vitale, A.; Bongiovanni, R.; Galimberti, M. Domino reaction for the sustainable functionalization of few-layer graphene. Nanomaterials 2019, 9, 44. [Google Scholar] [CrossRef]
- Casagrande, C.; Fabre, P.; Raphael, E.; Veyssié, M. “Janus beads”: Realization and behaviour at water/oil interfaces. EPL 1989, 9, 251. [Google Scholar] [CrossRef]
- De Gennes, P.G. Soft matter. Rev. Mod. Phys. 1992, 64, 645. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Kumar, S. Effect of solvent solubility parameter on SWNT dispersion in PMMA. Polymer 2005, 46, 3419–3424. [Google Scholar] [CrossRef]
- Ham, H.T.; Choi, Y.S.; Chung, I.J. An explanation of dispersion states of single-walled carbon nanotubes in solvents and aqueous surfactant solutions using solubility parameters. J. Coll. Interf. Sci. 2005, 286, 216–223. [Google Scholar] [CrossRef]
- Bergin, S.D.; Sun, Z.; Rickard, D.; Streich, P.V.; Hamilton, J.P.; Coleman, J.N. Multicomponent solubility parameters for single-walled carbon nanotube–solvent mixtures. ACS Nano 2009, 3, 2340–2350. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, L. A new procedure for calculating Hansen solubility parameters of carbon nanotube/polymer composites. Polym. Bull. 2012, 68, 1053–1063. [Google Scholar] [CrossRef]
- Pramanik, C.; Gissinger, J.R.; Kumar, S.; Heinz, H. Carbon nanotube dispersion in solvents and polymer solutions: Mechanisms, assembly, and preferences. ACS Nano 2017, 11, 12805–12816. [Google Scholar] [CrossRef]
- Launay, H.; Hansen, C.M.; Almdal, K. Hansen solubility parameters for a carbon fiber/epoxy composite. Carbon 2007, 45, 2859–2865. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Measurement of multicomponent solubility parameters for graphene facilitates solvent discovery. Langmuir 2010, 26, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Nonelectrolytes, 3rd ed.; Dover Publications: New York, NY, USA, 1964. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Paal, C. Synthese von Thiophen-und Pyrrolderivaten. Berichte Deutsch. Chem. Gesellsch. 1885, 18, 367–371. [Google Scholar] [CrossRef]
- Knorr, L. Einwirkung des Diacetbernsteinsäureesters auf Ammoniak und primäre Aminbasen. Berichte Deutsch. Chem. Gesellsch. 1885, 18, 299–311. [Google Scholar] [CrossRef]
- Barbera, V.; Citterio, A.; Galimberti, M.S.; Leonardi, G.; Sebastiano, R.; Shisoida, S.U.; Valerio, A.M. Process for the synthesis of 2-(2,5-dimethyl-1H-pyrrol-1-yl)-1,3-propanediol and its substituted derivatives. U.S. Patent 10,329,253, 25 June 2019. [Google Scholar]
- Mauro, M.; Cipolletti, V.; Galimberti, M.; Longo, P.; Guerra, G. Chemically reduced graphite oxide with improved shape anisotropy. J. Phys. Chem. C 2012, 116, 24809–24813. [Google Scholar] [CrossRef]
- Yang, K.; Han, H.; Pan, X.; Chen, N.; Gu, M. The effect of chemical treatment on the crystallinity of multi-walled carbon nanotubes. J. Phys. Chem. Solids 2008, 69, 222–229. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. Lond. A 2004, 362, 2271–2288. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Castiglioni, C.; Tommasini, M.; Zerbi, G. Raman spectroscopy of polyconjugated molecules and materials: Confinement effect in one and two dimensions. Philos. Trans. R. Soc. Lond. A 2004, 362, 2425–2459. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single-and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Ferrari, A.C. Raman Spectroscopy of Graphene Edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef]
- Radovic, L.R.; Bockrath, B. On the Chemical Nature of Graphene Edges: Origin of Stability and Potential for Magnetism in Carbon Materials. J. Am. Chem. Soc. 2005, 127, 5517–5927. [Google Scholar] [CrossRef]
- Rebelo, S.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araujio, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef] [PubMed]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Poschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Likodimos, V.; Steriotis, T.A.; Papageorgiou, S.K.; Romanos, G.E.; Marques, R.R.; Rocha, R.P.; Faria, J.L.; Pereira, M.F.R.; Figueiredo, J.L.; Silva, A.M.T.; et al. Controlled surface functionalization of multiwall carbon nanotubes by HNO3 hydrothermal oxidation. Carbon 2014, 69, 311–326. [Google Scholar] [CrossRef]
- Barbera, V.; Musto, S.; Citterio, A.; Conzatti, L.; Galimberti, M.S. Polyether from a biobased Janus molecule as surfactant for carbon nanotubes. eXPRESS Polym. Lett. 2016, 10, 548–558. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Citterio, A.; Sebastiano, R.; Truscello, A.; Valerio, A.M.; Conzatti, L.; Mendichi, R. Supramolecular interactions of carbon nanotubes with biosourced polyurethanes from 2-(2, 5-dimethyl-1H-pyrrol-1-yl)-1, 3-propanediol. Polymer 2015, 63, 62–70. [Google Scholar] [CrossRef]
- Barbera, V.; Brambilla, L.; Porta, A.; Bongiovanni, R.; Vitale, A.; Torrisi, G.; Galimberti, M. Selective edge functionalization of graphene layers with oxygenated groups by means of Reimer–Tiemann and domino Reimer–Tiemann / Cannizzaro reactions. J. Mater. Chem. A 2018, 6, 7749–7761. [Google Scholar] [CrossRef]
- Barbera, V.; Guerra, S.; Brambilla, L.; Maggio, M.; Serafini, A.; Conzatti, L.; Vitale, A.; Galimberti, M. Carbon papers and aerogels based on graphene layers and chitosan: Direct preparation from high surface area graphite. Biomacromolecules 2017, 18, 3978–3991. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, A.; Barbera, V.; Galimberti, M. Synthesis of adducts of sp2 carbon allotropes with pyrrole compounds. Effect of experimental conditions. 2020; unpublished. [Google Scholar]
- Meng, L.Y.; Park, S.J. Synthesis of Graphene Nanosheets via Thermal Exfoliation of Pretreated Graphite at Low Temperature. Adv. Mater. Res. 2010, 123–125, 787–790. [Google Scholar] [CrossRef]
- Heald, C.G.; Wildgoose, G.G.; Jiang, L.; Jones, T.G.; Compton, R.G. Chemical derivatisation of multiwalled carbon nanotubes using diazonium salts. Chem. Phys. Chem. 2004, 5, 1794–1799. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Covalent chemistry on graphene. Chem. Soc. Rev. 2013, 42, 3222–3233. [Google Scholar] [CrossRef]
- Ata, S.; Mizuno, T.; Nishizawa, A.; Subramaniam, C.; Futaba, D.N.; Hata, K. Influence of matching solubility parameter of polymer matrix and CNT on electrical conductivity of CNT/rubber composite. Sci. Rep. 2015, 4, 7232. [Google Scholar] [CrossRef]
- Milliman, H.W.; Boris, D.; Schiraldi, D. Experimental Determination of Hansen Solubility Parameters for Select POSS and Polymer Compounds as a Guide to POSS Polymer Interaction Potentials. Macromolecules 2012, 45, 1931–1936. [Google Scholar] [CrossRef]
- Machui, F.; Langner, S.; Zhu, X.; Abbott, S.; Brabec, C.J. Determination of the P3HT: PCBM solubility parameters via a binary solvent gradient method: Impact of solubility on the photovoltaic performance. Sol. Energy Mater. Sol. Cells 2012, 100, 138–146. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, Conductive Carbon Nanotube Films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Gruner, G. Carbon nanotube thin films: Fabrication, properties, and applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef]
- Mirri, F.; Ma, A.W.; Hsu, T.T.; Behabtu, N.; Eichmann, S.L.; Young, C.C.; Tsentalovich, D.E.; Pasquali, M. High-performance carbon nanotube transparent conductive films by scalable dip coating. ACS Nano 2012, 6, 9737–9744. [Google Scholar] [CrossRef] [PubMed]
- Tasis, D.; Tagmatarchis, N.; Georgakilas, V.; Prato, M. Soluble carbon nanotubes. Chem. Eur. J. 2003, 9, 4000–4008. [Google Scholar] [CrossRef] [PubMed]
- Pennetta, C.; Floresta, G.; Graziano, A.C.E.; Cardile, V.; Rubino, L.; Galimberti, M.; Rescifina, A.; Barbera, V. Functionalization of Single and Multi-Walled Carbon Nanotubes with Polypropylene Glycol Decorated Pyrrole for the Development of Doxorubicin Nano-Conveyors for Cancer Drug Delivery. Nanomaterials 2020, 10, 1073. [Google Scholar] [CrossRef] [PubMed]

| Sample | Temperature Range | |||
|---|---|---|---|---|
| 0 < T < 200 °C | 200 < T < 700 °C | 700 < T < 900 °C | T > 900 °C | |
| SP | 6.0 | 94.0 a | 0 | 0 |
| CNTs | 0.8 | 2.6 | 0.1 | 96.5 |
| CNT-SP | 1.8 | 9.6 | 6.5 | 82.0 |
| CNT-TMP | 1.6 | 7.5 | 4.9 | 85.9 |
| CNT-DDcP | 1.1 | 8.4 | 8.2 | 82.2 |
| CNT-APTESP | 1.5 | 7.8 | 3.7 | 87.0 |
| Adduct | PyC in the Adduct (phc) b | Functionalization Yield (%) c |
|---|---|---|
| CNT-TMP | 10.9 | 73 |
| CNT-DDcP | 13.3 | 92 |
| CNT-SP | 13.5 | 90 |
| CNT-APTESP | 9.8 | 65 |
| Solvent | Samples | ||||
|---|---|---|---|---|---|
| CNTs | CNT/TMP | CNT/DDcP | CNT/SP | CNT/APTESP | |
| Alkanes: hexane heptane | GOOD n.d. | BAD n.d. | GOOD GOOD | BAD BAD | BAD BAD |
| Halogenated alkanes: chloroform | n.d. | BAD | BAD | n.d. | n.d. |
| Arenes: toluene | GOOD | GOOD | GOOD | GOOD | GOOD |
| Alcohols: 2-propanol 2-butanol methanol | BAD n.d. BAD | GOOD GOOD n.d. | BAD n.d. n.d. | GOOD n.d. n.d. | GOOD n.d. n.d. |
| Polar solvents: acetone water | BAD BAD | GOOD BAD | n.d. BAD | GOOD GOOD | GOOD BAD |
| Others: ethyl acetate dichloromethane | BAD BAD | GOOD n.d. | GOOD BAD | GOOD BAD | GOOD GOOD |
| Sample | δD | δP | δH | Radius | δTb |
|---|---|---|---|---|---|
| CNTs | 17.0 | 1.7 | 1.3 | 3.3 | 17.1 |
| CNT-TMP | 8.7 | 12.0 | 5.2 | 15.8 | 15.7 |
| CNT-DDcP | 8.5 | 7.5 | 5.2 | 15.4 | 12.5 |
| CNT-SP | 11.9 | 11.4 | 15.7 | 18.1 | 22.7 |
| CNT-APTESP | 6.7 | 12.0 | 5.2 | 15.8 | 14.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locatelli, D.; Barbera, V.; Brambilla, L.; Castiglioni, C.; Sironi, A.; Galimberti, M. Tuning the Solubility Parameters of Carbon Nanotubes by Means of Their Adducts with Janus Pyrrole Compounds. Nanomaterials 2020, 10, 1176. https://doi.org/10.3390/nano10061176
Locatelli D, Barbera V, Brambilla L, Castiglioni C, Sironi A, Galimberti M. Tuning the Solubility Parameters of Carbon Nanotubes by Means of Their Adducts with Janus Pyrrole Compounds. Nanomaterials. 2020; 10(6):1176. https://doi.org/10.3390/nano10061176
Chicago/Turabian StyleLocatelli, Daniele, Vincenzina Barbera, Luigi Brambilla, Chiara Castiglioni, Annalisa Sironi, and Maurizio Galimberti. 2020. "Tuning the Solubility Parameters of Carbon Nanotubes by Means of Their Adducts with Janus Pyrrole Compounds" Nanomaterials 10, no. 6: 1176. https://doi.org/10.3390/nano10061176
APA StyleLocatelli, D., Barbera, V., Brambilla, L., Castiglioni, C., Sironi, A., & Galimberti, M. (2020). Tuning the Solubility Parameters of Carbon Nanotubes by Means of Their Adducts with Janus Pyrrole Compounds. Nanomaterials, 10(6), 1176. https://doi.org/10.3390/nano10061176







