Recent Advances in Applications of Carbon Nanotubes for Desalination: A Review
Abstract
:1. Introduction
2. CNT Membranes for Reverse Osmosis Desalination
2.1. Mechanisms and Influence Factors for Desalination
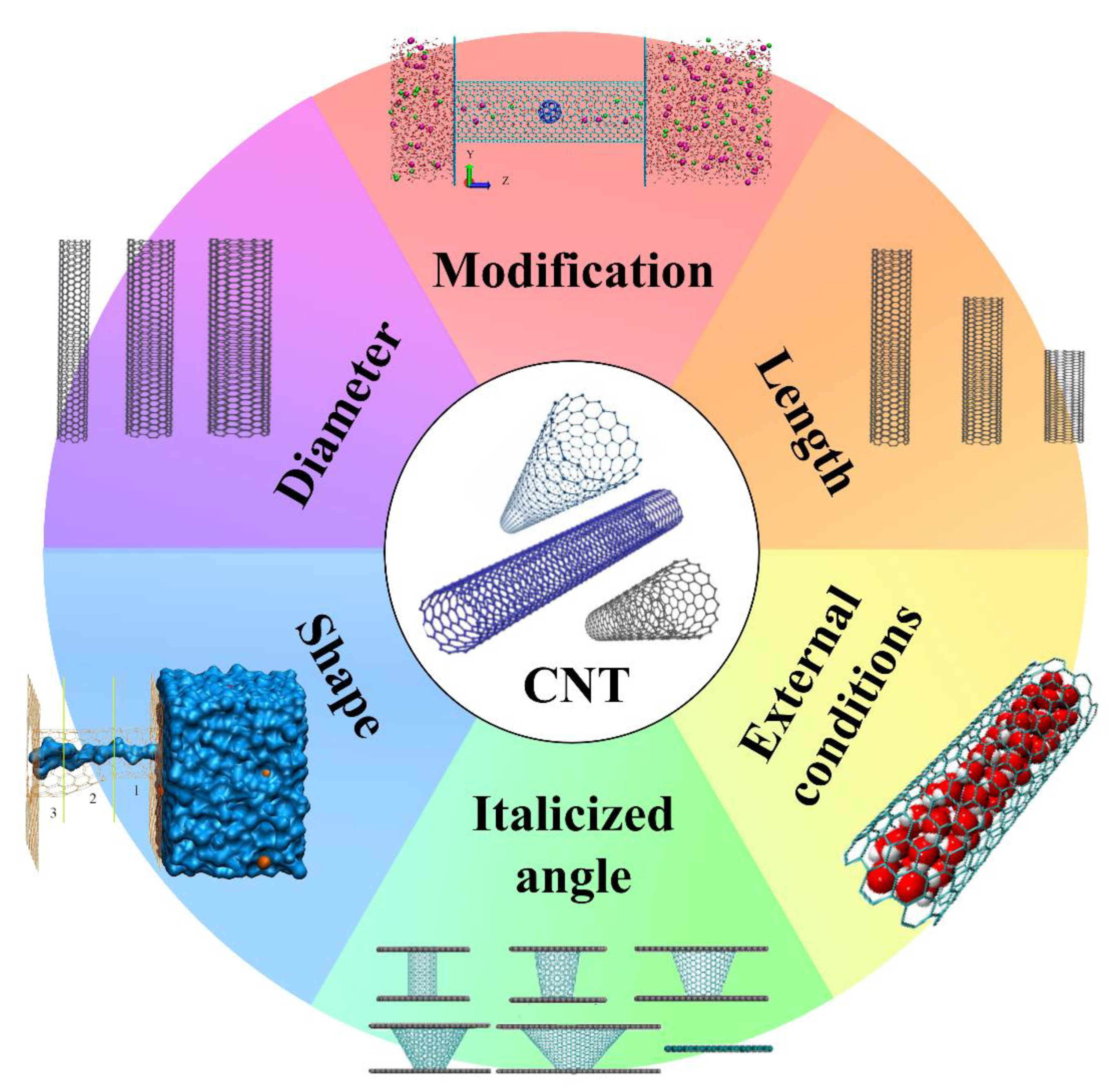
2.1.1. The Characteristics of CNTs Affecting the Water Flow Rate
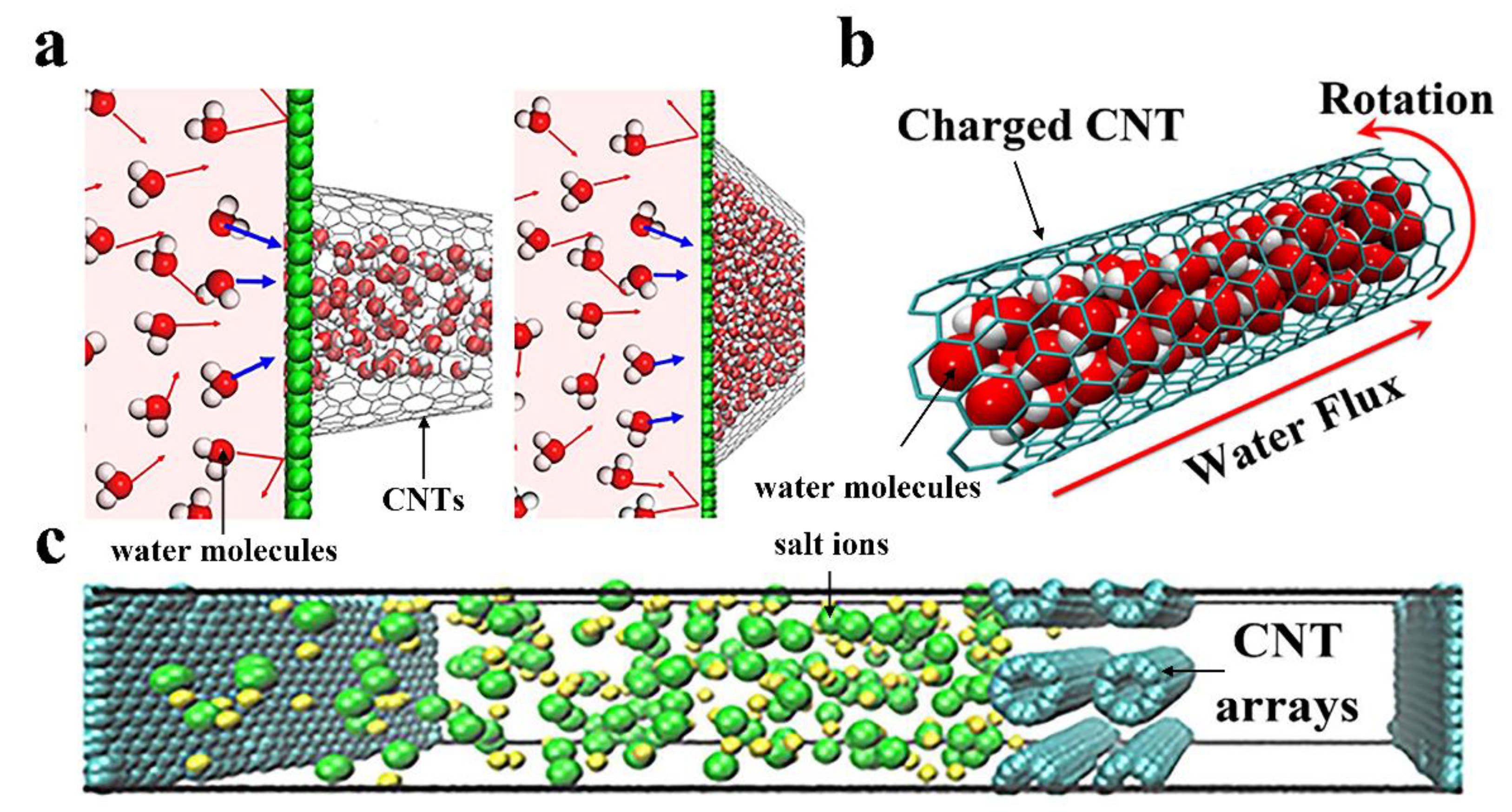
2.1.2. The External Conditions Affecting the Water Flow Rate of CNTs
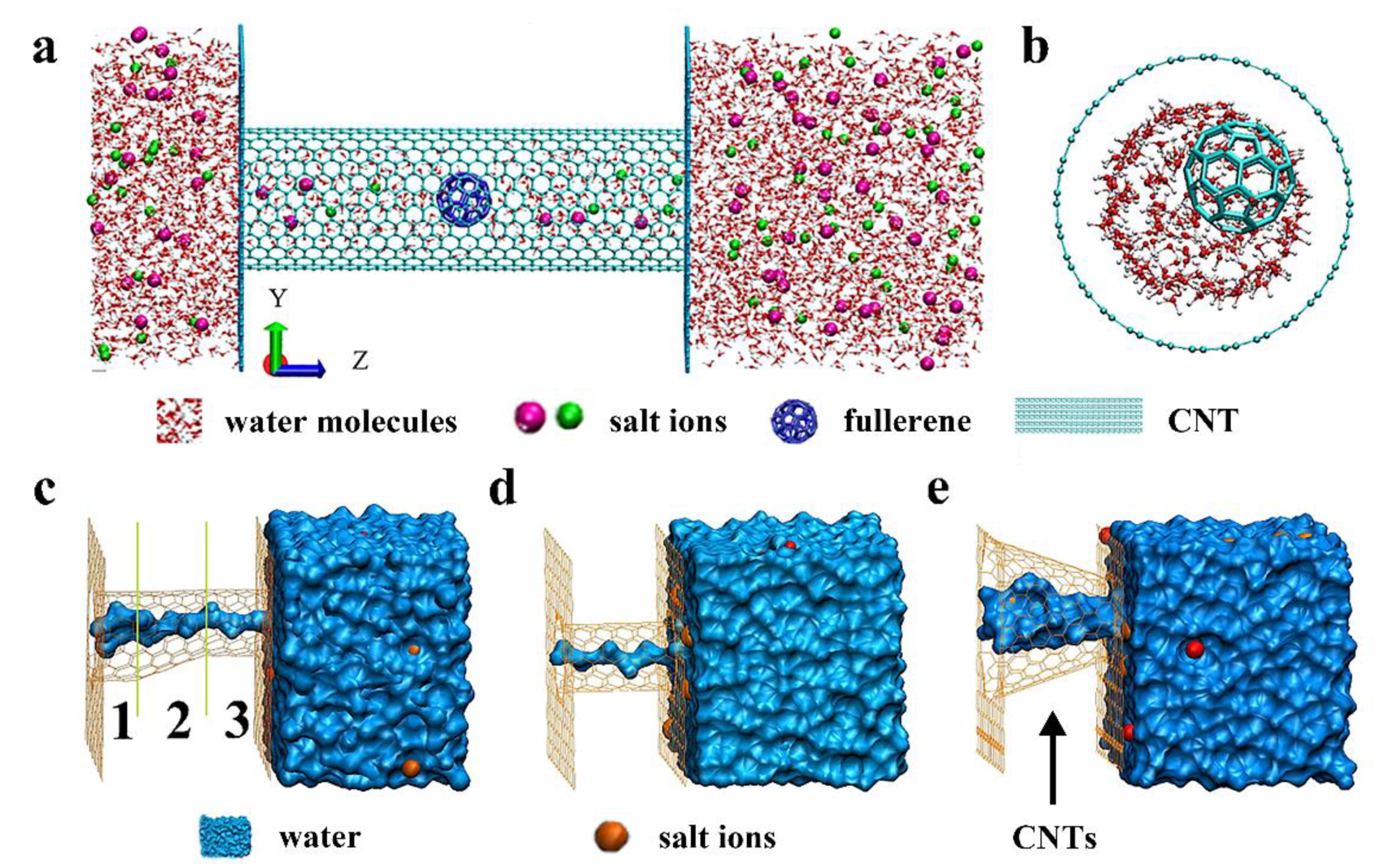
2.1.3. The Others Affecting the Water Flow Rate of CNTs
2.2. Effects of CNT Addition for Desalination
2.2.1. Water Permeability and Flux
2.2.2. Ion Selectivity
2.2.3. Fouling Resistance
2.2.4. Mechanical Performance
3. Challenges and Outlook
3.1. Cost
3.2. Scale-Up, Stability and Reproducibility
3.3. Simulation and Mechanism
3.4. Compatibility and Environmental Impact
3.5. Others
4. Conclusions
- Stacking multilayered horizontal aligned CNT (HACNT) membranes are an ideal replacement for sub-nano VACNT membranes and the scale-up and high-density preparation of this HACNT membrane is also feasible.
- The combination of CNTs with excellent mechanical properties and nanoporous materials (e.g., single-layer nanoporous two-dimensional nanomaterials) with atomic layer thickness can achieve ultra-thin, high-permeability desalination membranes.
- The modification of CNTs not only improves the dispersion in the polymer matrix, but also the desalination performance and anti-pollution performance of the membranes, so it is essential to simulate and optimize the modification of CNTs.
- Due to the excellent conductivity of CNTs, in addition to pressure-driven CNT membranes, extensive research is needed to explore the other possible applications of CNT-based membranes such as membrane distillation and capacitive deionization.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| N/A | Unavailable data |
| LMH | L/(m2 h) |
| DI | Deionized |
| PA | Aromatic polyamide |
| PSf | Polysulfone |
| CA | Cellulose acetate |
| PEG400 | Polyethylene glycol-400 |
| SE | Surface engineered |
| TMC | Trimesoyl chloride |
| MPD | m-Phenylenediamine |
| fCNT | Functionalized carbon nanotubes |
| PES | Polyethersulfone |
| MMMs | Mixed-matrix membranes |
| PDA | Polydopamine |
| PEI | Polyethyleneimine |
| PS-20 | Polysulfone |
| TNT | Titania |
| PS | Polysulfone |
| PA | Polyamide |
| c-MWCNT | Carboxylated multiwalled carbon nanotubes |
| PPy-raw or PPy-oxidized MWCNTs | Hydrophilic polypyrrole on raw or oxidized multiwalled carbon nanotubes |
| TA–MWNTs | Tannic acid-FeIII-functionalized multiwall carbon nanotubes |
| fMWCNTs | Functionalization of multiwalled carbon nanotubes |
| PAAm | Polyacrylamide |
| PVDF | Polyvinylidene fluoride |
| ZCNTs | Zwitterionization of multiwalled carbon nanotubes |
| DDA | Dodecylamine |
| SMWCNT | Sulfonated multiwall carbon nanotube |
| ZfCNTs | Zwitterion-functionalized carbon nanotubes |
| PVDF–HFP | Polyvinylidene fluoride-co-hexafluoropropene |
| ATRP | Atom-transfer radical polymerization |
| RGO | Reduced graphene oxide |
| SPSf | Sulfonated polysulfone |
References
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.B.A.; Ali, M.E.; Ismail, A.F.; Annuar, M.S.M.; Ramakrishna, S. Multifunctional carbon nanotubes in water treatment: The present, past and future. Desalination 2014, 354, 160–179. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.S.; Loh, C.H.; Permogorov, N.; Wang, R. Surfactant effects on water recovery from produced water via direct-contact membrane distillation. J. Membr. Sci. 2017, 528, 126–134. [Google Scholar] [CrossRef]
- Belfort, G. Membrane Filtration with Liquids: A Global Approach with Prior Successes, New Developments and Unresolved Challenges. Angew. Chem. Int. Ed. 2019, 58, 1892–1902. [Google Scholar] [CrossRef]
- Yu, W.Z.; Graham, N.J.D.; Fowler, G.D. Coagulation and oxidation for controlling ultrafiltration membrane fouling in drinking water treatment: Application of ozone at low dose in submerged membrane tank. Water Res. 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Ihsanullah. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, G.R. Graphene oxide (GO) as functional material in tailoring polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes. Desalination 2016, 394, 162–175. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.Y.; Farid, M.U.; Huang, H.O. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Wang, L.D.; Boutilier, M.S.H.; Kidambi, P.R.; Jang, D.; Hadjiconstantinou, N.G.; Karnik, R. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 2017, 12, 509–522. [Google Scholar] [CrossRef]
- Roy, K.; Mukherjee, A.; Maddela, N.R.; Chakraborty, S.; Shen, B.; Li, M.; Du, D.; Peng, Y.; Lu, F.; Cruzatty, L.C.G. Outlook on the bottleneck of carbon nanotube in desalination and membrane-based water treatment—A review. J. Environ. Eng. 2020, 8, 103572. [Google Scholar] [CrossRef]
- Elimelech, M. Materials for next generation desalination and water purification membranes. Abstr. Pap. Am. Chem. Soc. 2018, 255, 1. [Google Scholar] [CrossRef]
- Liu, G.P.; Jin, W.Q.; Xu, N.P. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Carbon Nanotube-Based Membranes for Water Purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–331. [Google Scholar] [CrossRef]
- Basu, A.; Debnath, B. Application of Carbon Nanotubes in Fluidic Waste treatment and Energy Harvesting. In Waste Management and Resource Efficiency; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1273–1285. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon 2016, 109, 694–710. [Google Scholar] [CrossRef]
- Agrawal, K.V.; Drahushuk, L.W.; Strano, M.S. Observation and analysis of the Coulter effect through carbon nanotube and graphene nanopores. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 13. [Google Scholar] [CrossRef]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned multiwalled carbon nanotube membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Majumder, M.; Chopra, N.; Andrews, R.; Hinds, B. Erratum: Nanoscale hydrodynamics: Enhanced flow in carbon nanotubes. Nature 2005, 438, 930. [Google Scholar] [CrossRef] [Green Version]
- Ang, E.Y.M.; Toh, W.; Yeo, J.; Lin, R.M.; Liu, Z.S.; Geethalakshmi, K.R.; Ng, T.Y. A review on low dimensional carbon desalination and gas separation membrane designs. J. Membr. Sci. 2020, 598, 29. [Google Scholar] [CrossRef]
- Son, M.; Choi, H.G.; Liu, L.; Celik, E.; Park, H.; Choi, H. Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin-film composite membrane for desalination. Chem. Eng. J. 2015, 266, 376–384. [Google Scholar] [CrossRef]
- Ortiz-Medina, J.; Inukai, S.; Araki, T.; Morelos-Gomez, A.; Cruz-Silva, R.; Takeuchi, K.; Noguchi, T.; Kawaguchi, T.; Terrones, M.; Endo, M. Robust water desalination membranes against degradation using high loads of carbon nanotubes. Sci. Rep. 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Sianipar, M.; Kim, S.H.; Khoiruddin; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef] [Green Version]
- Tomanek, D.; Kyrylchuk, A. Designing an All-Carbon Membrane for Water Desalination. Phys. Rev. Appl. 2019, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Alshahrani, A.A.; Algamdi, M.S.; Alsohaimi, I.H.; Nghiem, L.D.; Tu, K.L.; Al-Illwajfeh, A.E.; Panhuis, M.I.H. The rejection of mono- and di-valent ions from aquatic environmental by MWNT/chitosan buckypaper composite membranes: Influences of chitosan concentrations. Sep. Purif. Technol. 2020, 234, 9. [Google Scholar] [CrossRef]
- Rashid, M.H.O.; Ralph, S.F. Carbon Nanotube Membranes: Synthesis, Properties, and Future Filtration Applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Biswas, P.; Fortner, J.D. A review of recent developments in graphene-enabled membranes for water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 915–922. [Google Scholar] [CrossRef]
- Heiranian, M.; Aluru, N.R. Nanofluidic Transport Theory with Enhancement Factors Approaching One. ACS Nano 2020, 14, 272–281. [Google Scholar] [CrossRef]
- Chan, Y.; Wu, B.S.; Ren, Y.; Xia, L. Analytical solution for Newtonian flow inside carbon nanotube taking into consideration van der Waals forces. J. Math. Chem. 2018, 56, 158–169. [Google Scholar] [CrossRef]
- Ma, M.; Grey, F.; Shen, L.M.; Urbakh, M.; Wu, S.; Liu, J.Z.; Liu, Y.L.; Zheng, Q.S. Water transport inside carbon nanotubes mediated by phonon-induced oscillating friction. Nat. Nanotechnol. 2015, 10, 692–695. [Google Scholar] [CrossRef]
- Sahu, P.; Ali, S.M.; Shenoy, K.T. Thermodynamics of fluid conduction through hydrophobic channel of carbon nanotubes: The exciting force for filling of nanotubes with polar and nonpolar fluids. J. Chem. Phys. 2015, 142, 11. [Google Scholar] [CrossRef]
- Thomas, M.; Corry, B. Thermostat choice significantly influences water flow rates in molecular dynamics studies of carbon nanotubes. Microfluid. Nanofluid. 2015, 18, 41–47. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razmkhah, M.; Ahmadpour, A.; Mosavian, M.T.H.; Moosavi, F. What is the effect of carbon nanotube shape on desalination process? A simulation approach. Desalination 2017, 407, 103–115. [Google Scholar] [CrossRef]
- Khodabakhshi, M.; Moosavi, A. Unidirectional Transport of Water through an Asymmetrically Charged Rotating Carbon Nanotube. J. Phys. Chem. C 2017, 121, 23649–23658. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.S.; Zheng, X.; Dong, Z.H.; Yan, Y.G.; Zhang, J. Molecular dynamics simulations of water flow enhancement in carbon nanochannels. Comput. Mater. Sci. 2017, 136, 60–66. [Google Scholar] [CrossRef]
- Foroutan, M.; Naeini, V.F.; Ebrahimi, M. Carbon nanotubes encapsulating fullerene as water nano-channels with distinctive selectivity: Molecular dynamics simulation. Appl. Surf. Sci. 2019, 489, 198–209. [Google Scholar] [CrossRef]
- Rikhtehgaran, S.; Lohrasebi, A. Water desalination by a designed nanofilter of graphene-charged carbon nanotube: A molecular dynamics study. Desalination 2015, 365, 176–181. [Google Scholar] [CrossRef]
- Varanasi, S.R.; Subramanian, Y.; Bhatia, S.K. High Interfacial Barriers at Narrow Carbon Nanotube-Water Interfaces. Langmuir 2018, 34, 8099–8111. [Google Scholar] [CrossRef]
- Ang, E.Y.M.; Ng, T.Y.; Yeo, J.J.; Lin, R.M.; Liu, Z.S.; Geethalakshmi, K.R. Effects of CNT size on the desalination performance of an outer-wall CNT slit membrane. Phys. Chem. Chem. Phys. 2018, 20, 13896–13902. [Google Scholar] [CrossRef]
- Zhao, K.W.; Wu, H.Y.; Han, B.S. Negative effect of nanoconfinement on water transport across nanotube membranes. J. Chem. Phys. 2017, 147, 4. [Google Scholar] [CrossRef]
- Sahu, P.; Ali, S.M.; Shenoy, K.T. Self Diffusion and Wetting Transition of Fluids in Carbon Nanotubes. In Proceedings of the Dae Solid State Physics Symposium 2015, Mumbai, India, 21 December 2015; Chitra, R., Bhattacharya, S., Sahoo, N.K., Eds.; AIP Publishing: Melville, NY, USA, 2016. [Google Scholar]
- Bernardina, S.D.; Paineau, E.; Brubach, J.B.; Judeinstein, P.; Rouziere, S.; Launois, P.; Roy, P. Water in Carbon Nanotubes: The Peculiar Hydrogen Bond Network Revealed by Infrared Spectroscopy. J. Am. Chem. Soc. 2016, 138, 10437–10443. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.B.; Song, X.Y.; Zhao, T.; Zhao, S.L.; Liu, H.L. Confinement effect on water transport in CNT membranes. Chem. Eng. Sci. 2018, 192, 1252–1259. [Google Scholar] [CrossRef]
- Ma, X.D.; Cambre, S.; Wenseleers, W.; Doorn, S.K.; Htoon, H. Quasiphase Transition in a Single File of Water Molecules Encapsulated in (6,5) Carbon Nanotubes Observed by Temperature-Dependent Photoluminescence Spectroscopy. Phys. Rev. Lett. 2017, 118, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.B.; Zhao, Y.Z.; Fang, C.; Su, J.Y. Transport of a simple liquid through carbon nanotubes: Role of nanotube size. Phys. Lett. A 2017, 381, 3487–3492. [Google Scholar] [CrossRef]
- Secchi, E.; Marbach, S.; Nigues, A.; Stein, D.; Siria, A.; Bocquet, L. Massive radius-dependent flow slippage in carbon nanotubes. Nature 2016, 537, 210–213. [Google Scholar] [CrossRef]
- Thomas, M.; Corry, B. A computational assessment of the permeability and salt rejection of carbon nanotube membranes and their application to water desalination. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tsuruoka, S.; Hayashi, Y.; Abe, K.; Hata, K.; Zhang, S.L.; Saito, Y.; Aiba, M.; Tokunaga, T.; Iijima, T.; et al. Water transport phenomena through membranes consisting of vertically-aligned double-walled carbon nanotube array. Carbon 2017, 120, 358–365. [Google Scholar] [CrossRef]
- Liu, L.; Patey, G.N. A molecular dynamics investigation of the influence of water structure on ion conduction through a carbon nanotube. J. Chem. Phys. 2017, 146, 9. [Google Scholar] [CrossRef]
- Liu, X.K.; Shu, L.S.; Jin, S.P. A modeling investigation on the thermal effect in osmosis with gap-filled vertically aligned carbon nanotube membranes. J. Membr. Sci. 2019, 580, 143–153. [Google Scholar] [CrossRef]
- Zaragoza, A.; Gonzalez, M.A.; Joly, L.; Lopez-Montero, I.; Canales, M.A.; Benavides, A.L.; Valeriani, C. Molecular dynamics study of nanoconfined TIP4P/2005 water: How confinement and temperature affect diffusion and viscosity. Phys. Chem. Chem. Phys. 2019, 21, 13653–13667. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Merabia, S.; Joly, L. Understanding Fast and Robust Thermo-osmotic Flows through Carbon Nanotube Membranes: Thermodynamics Meets Hydrodynamics. J. Phys. Chem. Lett. 2018, 9, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, S.; Zhang, S.L.; Aiba, M.; Inoue, H.; Hada, M.; Hayashi, Y.; Hata, K.; Tsuruoka, S.; Matsumoto, H. Temperature dependence of pressure-driven water permeation through membranes consisting of vertically-aligned double-walled carbon nanotube arrays. Carbon 2019, 146, 785–788. [Google Scholar] [CrossRef]
- Kohler, M.H.; da Silva, L.B. Size effects and the role of density on the viscosity of water confined in carbon nanotubes. Chem. Phys. Lett. 2016, 645, 38–41. [Google Scholar] [CrossRef]
- Meng, X.W. A remarkable enhancement of water molecules permeation across a combined carbon nanotube. EPL 2019, 125, 5. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Liu, Z. Progress and challenges of carbon nanotube membrane in water treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46, 999–1046. [Google Scholar] [CrossRef]
- Li, D.Y.; Shi, G.S.; Hong, F.; Fang, H.P. Potentials of classical force fields for interactions between Na+ and carbon nanotubes. Chin. Phys. B 2018, 27, 6. [Google Scholar] [CrossRef]
- Su, Z.L.; Chen, J.Y.; Zhao, Y.Z.; Su, J.Y. How ions block the single-file water transport through a carbon nanotube. Phys. Chem. Chem. Phys. 2019, 21, 11298–11305. [Google Scholar] [CrossRef]
- Wang, X.L.; Shi, G.S.; Liang, S.S.; Liu, J.; Li, D.Y.; Fang, G.; Liu, R.D.; Yan, L.; Fang, H.P. Unexpectedly High Salt Accumulation inside Carbon Nanotubes Soaked in Dilute Salt Solutions. Phys. Rev. Lett. 2018, 121, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Yang, D.F.; Xu, X.; Li, Q.; Ling, Q.; Liu, Q.Z. Tip and Inner Wall Modification of 4.9 nm Diameter Multi-Walled Carbon Nanotubes and Its Nanocomposite Polyamide Reverse Osmosis Membrane. J. Nanosci. Nanotechnol. 2019, 19, 5591–5600. [Google Scholar] [CrossRef]
- Qin, J.Y.; Zhu, B.E.; Liang, S.S.; Guo, P.; Liu, J. Impact of -C2H5 and -OH Functionalizations on the Water Flow Blockage in Carbon Nanotubes. J. Phys. Chem. C 2018, 122, 11807–11813. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.F.; Shi, J.S.; Wang, J.H.; Liu, Q.Z. Modification of (15,15) 2.034 nm diameter carbon nanotubes with long aliphatic chain and their desalination behavior. Desalin. Water Treat. 2018, 101, 61–69. [Google Scholar] [CrossRef]
- Lokesh, M.; Youn, S.K.; Park, H.G. Osmotic Transport across Surface Functionalized Carbon Nanotube Membrane. Nano Lett. 2018, 18, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, G.S.; Guo, P.; Yang, J.R.; Fang, H.P. Blockage of Water Flow in Carbon Nanotubes by Ions Due to Interactions between Cations and Aromatic Rings. Phys. Rev. Lett. 2015, 115, 6. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, J.C.; Zhu, C.Q.; Zeng, X.C.; Francisco, J.S. Water desalination through rim functionalized carbon nanotubes. J. Mater. Chem. A 2019, 7, 3583–3591. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Guan, C.Y.; Liu, C.X.; Lang, W.Z.; Wang, Y. Construction of SiO2@MWNTs incorporated PVDF substrate for reducing internal concentration polarization in forward osmosis. J. Membr. Sci. 2018, 564, 328–341. [Google Scholar] [CrossRef]
- Zhang, H.G.; Quan, X.; Chen, S.; Fan, X.F.; Wei, G.L.; Yu, H.T. Combined Effects of Surface Charge and Pore Size on Co-Enhanced Permeability and Ion Selectivity through RGO-OCNT Nanofiltration Membranes. Environ. Sci. Technol. 2018, 52, 4827–4834. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.F.; Shi, J.S.; Xu, X.; Yan, S.H.; Liu, Q.Z. Biomimetic modification of large diameter carbon nanotubes and the desalination behavior of its reverse osmosis membrane. Desalination 2016, 379, 164–171. [Google Scholar] [CrossRef]
- Panahi, A.; Shomali, A.; Sabour, M.H.; Ghafar-Zadeh, E. Molecular dynamics simulation of electric field driven water and heavy metals transport through fluorinated carbon nanotubes. J. Mol. Liq. 2019, 278, 658–671. [Google Scholar] [CrossRef]
- Panahi, A.; Sabour, M.H. Electrokinetics desalination of water using fluorinated carbon nanotubes embedded in silicon membrane: Insights from molecular dynamics simulation. Chem. Eng. Sci. 2017, 173, 60–73. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed-matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Yu, X.; Zhao, J.; Li, S.; Liu, F.; Gao, P.; Zhang, Y.; Zhao, T.; Li, Q. Bio-inspired aggregation control of carbon nanotubes for ultra-strong composites. Sci. Rep. 2015, 5, 11533. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-X.; Choi, J.-W. Improved dispersion of carbon nanotubes in polymers at high concentrations. Nanomaterials 2012, 2, 329–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- de Lannoy, C.-F.; Soyer, E.; Wiesner, M.R. Optimizing carbon nanotube-reinforced polysulfone ultrafiltration membranes through carboxylic acid functionalization. J. Membr. Sci. 2013, 447, 395–402. [Google Scholar] [CrossRef]
- Li, J.P.; Kong, X.; Lu, D.N.; Liu, Z. Italicized carbon nanotube facilitating water transport: A molecular dynamics simulation. Sci. Bull. 2015, 60, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, W.; Xu, F.; Wei, M.J.; Wang, Y. Resistance of water transport in carbon nanotube membranes. Nanoscale 2018, 10, 13242–13249. [Google Scholar] [CrossRef]
- Ang, E.Y.M.; Ng, T.Y.; Yeo, J.J.; Lin, R.M.; Liu, Z.S.; Geethalakshmi, K.R. Carbon nanotube arrays as multilayer transverse flow carbon nanotube membrane for efficient desalination. J. Membr. Sci. 2019, 581, 383–392. [Google Scholar] [CrossRef]
- Sun, L.G.; He, X.Q.; Lu, J. Super square carbon nanotube network: A new promising water desalination membrane. npj Comput. Mater. 2016, 2, 7. [Google Scholar] [CrossRef]
- Tu, Q.S.; Yang, Q.; Wang, H.L.; Li, S.F. Rotating carbon nanotube membrane filter for water desalination. Sci. Rep. 2016, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Mistry, S.; Kammara, K.K.; Kumar, R. Co-axially rotating carbon nanotubes: A novel mechanism for nanoscale pumping of fluids. In Proceedings of the AIP Conference Proceedings, Glasgow, UK, 5 August 2019; AIP Publishing: Melville, NY, USA, 2019. [Google Scholar]
- Razmkhah, M.; Moosavi, F.; Mosavian, M.T.H.; Ahmadpour, A. Does electric or magnetic field affect reverse osmosis desalination? Desalination 2018, 432, 55–63. [Google Scholar] [CrossRef]
- Feng, J.W.; Ding, H.M.; Ma, Y.Q. Water desalination by electrical resonance inside carbon nanotubes. Phys. Chem. Chem. Phys. 2016, 18, 28290–28296. [Google Scholar] [CrossRef] [PubMed]
- Ang, E.Y.M.; Ng, T.Y.; Yeo, J.J.; Lin, R.M.; Geethalakshmi, K.R. Nanoscale Fluid Mechanics Working Principles of Transverse Flow Carbon Nanotube Membrane for Enhanced Desalination. Int. J. Appl. Mech. 2017, 9, 16. [Google Scholar] [CrossRef]
- Ang, E.Y.M.; Ng, T.Y.; Yeo, J.; Liu, Z.S.; Lin, R.M.; Geethalakshmi, K.R. Effects of oscillating pressure on desalination performance of transverse flow CNT membrane. Desalination 2019, 451, 35–44. [Google Scholar] [CrossRef]
- Macevele, L.E.; Moganedi, K.L.M.; Magadzu, T. Investigation of Antibacterial and Fouling Resistance of Silver and Multi-Walled Carbon Nanotubes Doped Poly(Vinylidene Fluoride-co-Hexafluoropropylene) Composite Membrane. Membranes 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Din, L.A.N.; El-Gendi, A.; Ismail, N.; Abed, K.A.; Ahmed, A.I. Evaluation of cellulose acetate membrane with carbon nanotubes additives. J. Ind. Eng. Chem. 2015, 26, 259–264. [Google Scholar] [CrossRef]
- Jin, H.Y.; Huang, Y.B.; Wang, X.; Yu, P.; Luo, Y.B. Preparation of modified cellulose acetate membranes using functionalized multi-walled carbon nanotubes for forward osmosis. Desalin. Water Treat. 2016, 57, 7166–7174. [Google Scholar] [CrossRef]
- Choi, H.G.; Yoon, S.H.; Son, M.; Celik, E.; Park, H.; Choi, H. Efficacy of synthesis conditions on functionalized carbon nanotube blended cellulose acetate membrane for desalination. Desalin. Water Treat. 2016, 57, 7545–7554. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.J.; Wang, T.; Wang, S.Z.; Wang, Z.N.; Gao, C.J. Fabrication and characterization of polyethersulfone/carbon nanotubes (PES/CNTs) based mixed-matrix membranes (MMMs) for nanofiltration application. Appl. Surf. Sci. 2015, 330, 118–125. [Google Scholar] [CrossRef]
- Khalid, A.; Al-Juhani, A.A.; Al-Hamouz, O.C.; Laoui, T.; Khan, Z.; Atieh, M.A. Preparation and properties of nanocomposite polysulfone/multi-walled carbon nanotubes membranes for desalination. Desalination 2015, 367, 134–144. [Google Scholar] [CrossRef]
- Mangukiya, S.; Prajapati, S.; Kumar, S.; Aswal, V.K.; Murthy, C.N. Polysulfone-based composite membranes with functionalized carbon nanotubes show controlled porosity and enhanced electrical conductivity. J. Appl. Polym. Sci. 2016, 133, 8. [Google Scholar] [CrossRef]
- Al-Hobaib, A.S.; Al-Sheetan, K.M.; Shaik, M.R.; Al-Suhybani, M.S. Modification of thin-film polyamide membrane with multi-walled carbon nanotubes by interfacial polymerization. Appl. Water Sci. 2017, 7, 4341–4350. [Google Scholar] [CrossRef] [Green Version]
- Azelee, I.W.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wong, K.C.; Subramaniam, M.N. Enhanced desalination of polyamide thin film nanocomposite incorporated with acid treated multiwalled carbon nanotube-titania nanotube hybrid. Desalination 2017, 409, 163–170. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Delnavaz, M.; Vatanpour, V. Investigation of raw and oxidized multiwalled carbon nanotubes in fabrication of reverse osmosis polyamide membranes for improvement in desalination and antifouling properties. Desalination 2017, 410, 1–9. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, H.J.; Kim, S.H.; Lee, J.C.; Yoon, J. Evaluation of carbon nanotube-polyamide thin-film nanocomposite reverse osmosis membrane: Surface properties, performance characteristics and fouling behavior. J. Ind. Eng. Chem. 2017, 56, 327–334. [Google Scholar] [CrossRef]
- Chan, W.F.; Marand, E.; Martin, S.M. Novel zwitterion functionalized carbon nanotube nanocomposite membranes for improved RO performance and surface anti-biofouling resistance. J. Membr. Sci. 2016, 509, 125–137. [Google Scholar] [CrossRef]
- Wu, H.; Sun, H.; Hong, W.; Mao, L.; Liu, Y. Improvement of polyamide thin film nanocomposite membrane assisted by tannic acid–FeIII functionalized multiwall carbon nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 32255–32263. [Google Scholar] [CrossRef]
- Zheng, J.F.; Li, M.; Yu, K.; Hu, J.H.; Zhang, X.; Wang, L.J. Sulfonated multiwall carbon nanotubes assisted thin-film nanocomposite membrane with enhanced water flux and anti-fouling property. J. Membr. Sci. 2017, 524, 344–353. [Google Scholar] [CrossRef]
- Wu, M.B.; Lv, Y.; Yang, H.C.; Liu, L.F.; Zhang, X.; Xu, Z.K. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Song, X.J.; Wang, L.; Tang, C.Y.; Wang, Z.N.; Gao, C.J. Fabrication of carbon nanotubes incorporated double-skinned thin film nanocomposite membranes for enhanced separation performance and antifouling capability in forward osmosis process. Desalination 2015, 369, 1–9. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Ji, Y.L.; Weng, X.D.; Mi, Y.F.; Ye, C.C.; An, Q.F.; Gao, C.J. High-Flux Positively Charged Nanocomposite Nanofiltration Membranes Filled with Poly(dopamine) Modified Multiwall Carbon Nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 6693–6700. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.H.; Wang, P.; Zhou, Z.Y.; Hu, Y.X. New Insights into the Role of an Interlayer for the Fabrication of Highly Selective and Permeable Thin-Film Composite Nanofiltration Membrane. ACS Appl. Mater. Interfaces 2019, 11, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Inukai, S.; Cruz-Silva, R.; Ortiz-Medina, J.; Morelos-Gomez, A.; Takeuchi, K.; Hayashi, T.; Tanioka, A.; Araki, T.; Tejima, S.; Noguchi, T.; et al. High-performance multi-functional reverse osmosis membranes obtained by carbon nanotube.polyamide nanocomposite. Sci. Rep. 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Silva, R.; Inukai, S.; Araki, T.; Morelos-Gomez, A.; Ortiz-Medina, J.; Takeuchi, K.; Hayashi, T.; Tanioka, A.; Tejima, S.; Noguchi, T.; et al. High Performance and Chlorine Resistant Carbon Nanotube/Aromatic Polyamide Reverse Osmosis Nanocomposite Membrane. MRS Adv. 2016, 1, 1469–1476. [Google Scholar] [CrossRef]
- Gumbi, N.N.; Li, J.X.; Mamba, B.B.; Nxumalo, E.N. Relating the performance of sulfonated thin-film composite nanofiltration membranes to structural properties of macrovoid-free polyethersulfone/sulfonated polysulfone/O-MWCNT supports. Desalination 2020, 474, 14. [Google Scholar] [CrossRef]
- Choi, H.G.; Son, M.; Choi, H. Integrating seawater desalination and wastewater reclamation forward osmosis process using thin-film composite mixed-matrix membrane with functionalized carbon nanotube blended polyethersulfone support layer. Chemosphere 2017, 185, 1181–1188. [Google Scholar] [CrossRef]
- Ma, X.H.; Guo, H.; Yang, Z.; Yao, Z.K.; Qing, W.H.; Chen, Y.L.; Xu, Z.L.; Tang, C.Y.Y. Carbon nanotubes enhance permeability of ultrathin polyamide rejection layers. J. Membr. Sci. 2019, 570, 139–145. [Google Scholar] [CrossRef]
- Sabir, A.; Shafiq, M.; Islam, A.; Sarwar, A.; Dilshad, M.R.; Shafeeq, A.; Butt, M.T.Z.; Jamil, T. Fabrication of tethered carbon nanotubes in cellulose acetate/polyethylene glycol-400 composite membranes for reverse osmosis. Carbohydr. Polym. 2015, 132, 589–597. [Google Scholar] [CrossRef]
- Vatanpour, V.; Zoqi, N. Surface modification of commercial seawater reverse osmosis membranes by grafting of hydrophilic monomer blended with carboxylated multiwalled carbon nanotubes. Appl. Surf. Sci. 2017, 396, 1478–1489. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Fu, S.C.; Zhang, H.F.; Huang, H.; Wei, Y.Y.; Zhang, Y.S. Enhanced separation and antifouling performance of reverse osmosis membrane incorporated with carbon nanotubes functionalized by atom transfer radical polymerization. RSC Adv. 2017, 7, 46969–46979. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.F.; Liu, Y.M.; Quan, X. A novel reduced graphene oxide/carbon nanotube hollow fiber membrane with high forward osmosis performance. Desalination 2019, 451, 117–124. [Google Scholar] [CrossRef]
- Zhao, X.Z.; Li, J.; Liu, C.K. A novel TFC-type FO membrane with inserted sublayer of carbon nanotube networks exhibiting the improved separation performance. Desalination 2017, 413, 176–183. [Google Scholar] [CrossRef]
- Gao, S.J.; Zhu, Y.Z.; Gong, Y.Q.; Wang, Z.Y.; Fang, W.X.; Jin, J. Ultrathin Polyamide Nanofiltration Membrane Fabricated on Brush-Painted Single-Walled Carbon Nanotube Network Support for Ion Sieving. ACS Nano 2019, 13, 5278–5290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Xie, W.; Gao, S.J.; Zhang, F.; Zhang, W.B.; Liu, Z.Y.; Jin, J. Single-Walled Carbon Nanotube Film Supported Nanofiltration Membrane with a Nearly 10 nm Thick Polyamide Selective Layer for High-Flux and High-Rejection Desalination. Small 2016, 12, 5034–5041. [Google Scholar] [CrossRef]
- Mahdavi, M.R.; Delnavaz, M.; Vatanpour, V.; Farahbakhsh, J. Effect of blending polypyrrole coated multiwalled carbon nanotube on desalination performance and antifouling property of thin film nanocomposite nanofiltration membranes. Sep. Purif. Technol. 2017, 184, 119–127. [Google Scholar] [CrossRef]
- Zheng, J.F.; Li, M.; Yao, Y.J.; Zhang, X.; Wang, L.J. Zwitterionic carbon nanotube assisted thin-film nanocomposite membranes with excellent efficiency for separation of mono/divalent ions from brackish water. J. Mater. Chem. A 2017, 5, 13730–13739. [Google Scholar] [CrossRef]
- Wang, M.; Li, T.; Yao, Y.; Lu, H.; Li, Q.; Chen, M.; Li, Q. Wafer-scale transfer of vertically aligned carbon nanotube arrays. J. Am. Chem. Soc. 2014, 136, 18156–18162. [Google Scholar] [CrossRef]
- Trivedi, S.; Alameh, K. Effect of vertically aligned carbon nanotube density on the water flux and salt rejection in desalination membranes. SpringerPlus 2016, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Eslami, J.; Abdi, Y.; Khamsavi, A.; EbrahimNataj, Z.; Kazemi, A.S. Effect of surface area of carbon nanotubes on membrane performance for effective water desalination. Appl. Phys. A Mater. Sci. Process. 2018, 124, 9. [Google Scholar] [CrossRef]
- Lee, K.J.; Park, H.D. The most densified vertically-aligned carbon nanotube membranes and their normalized water permeability and high pressure durability. J. Membr. Sci. 2016, 501, 144–151. [Google Scholar] [CrossRef]
- Yu, M.; Funke, H.H.; Falconer, J.L.; Noble, R.D. High density, vertically-aligned carbon nanotube membranes. Nano Lett. 2009, 9, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Nurkhamidah, S.; Rahmawati, Y.; Gumilang, R.A.; Riswanda, M.I.; Fahrizal, Y.E.; Ramadhan, I.H. Enhanced Performance of Celluloce Acetate/Polyethylene Glycol (CA/PEG) with the Addition of Functionalized Carbon Nanotube (CNT) Prepared by Wet-Dry Method. In Proceedings of the Pps-34: The 34th International Conference of the Polymer Processing Society–Conference Papers, Taipei, Taiwan, 6 February 2019; Liu, S.J., Ed.; AIP Publishing: Melville, NY, USA, 2018; Volume 2065. [Google Scholar] [CrossRef]
- Li, K.Z.; Lee, B.; Kim, Y. High performance reverse osmosis membrane with carbon nanotube support layer. J. Membr. Sci. 2019, 592, 6. [Google Scholar] [CrossRef]
- Luan, H.Y.; Teychene, B.; Huang, H.O. Efficient removal of As(III) by Cu nanoparticles intercalated in carbon nanotube membranes for drinking water treatment. Chem. Eng. J. 2019, 355, 341–350. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.Y.; Liu, Y.; Jian, M.P.; Gao, L.; Wang, H.T.; Zhang, X.W. Thin-Film Nanocomposite Forward-Osmosis Membranes on Hydrophilic Microfiltration Support with an Intermediate Layer of Graphene Oxide and Multiwall Carbon Nanotube. ACS Appl. Mater. Interfaces 2018, 10, 34464–34474. [Google Scholar] [CrossRef]
- Yang, G.; Xie, Z.L.; Gran, M.; Ng, D.; Gray, S. Enhanced desalination performance of poly (vinyl alcohol)/carbon nanotube composite pervaporation membranes via interfacial engineering. J. Membr. Sci. 2019, 579, 40–51. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Delnavaz, M.; Vatanpour, V. Simulation and characterization of novel reverse osmosis membrane prepared by blending polypyrrole coated multiwalled carbon nanotubes for brackish water desalination and antifouling properties using artificial neural networks. J. Membr. Sci. 2019, 581, 123–138. [Google Scholar] [CrossRef]
- Al Aani, S.; Haroutounian, A.; Wright, C.J.; Hilal, N. Thin Film Nanocomposite (TFN) membranes modified with polydopamine coated metals/carbon-nanostructures for desalination applications. Desalination 2018, 427, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Li, D.; Liu, B.; Yao, J. Enhancing the permeability of TFC membranes based on incorporating polyamide matrix into MWCNTs framework. Appl. Surf. Sci. 2019, 496, 7. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Water desalination via novel positively charged hybrid nanofiltration membranes filled with hyperbranched polyethyleneimine modified MWCNT. J. Ind. Eng. Chem. 2019, 69, 127–140. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Safarpour, M.; Ghadimi, A.; Adabi, J. Synergistic effect of carboxylated-MWCNTs on the performance of acrylic acid UV-grafted polyamide nanofiltration membranes. React. Funct. Polym. 2019, 134, 74–84. [Google Scholar] [CrossRef]
- Vatanpour, V.; Haghighat, N. Improvement of polyvinyl chloride nanofiltration membranes by incorporation of multiwalled carbon nanotubes modified with triethylenetetramine to use in treatment of dye wastewater. J. Environ. Manag. 2019, 242, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Shokouhian, M.; Solouki, S. p-Phenylenediamine-grafted multi-walled carbon nanotubes as a hydrophilic modifier in thin-film nanocomposite membrane. Polym. Bull. 2019, 1–14. [Google Scholar] [CrossRef]
- Zou, S.Q.; Smith, E.D.; Lin, S.H.; Martin, S.M.; He, Z. Mitigation of bidirectional solute flux in forward osmosis via membrane surface coating of zwitterion functionalized carbon nanotubes. Environ. Int. 2019, 131, 9. [Google Scholar] [CrossRef]
- Araki, T.; Cruz-Silva, R.; Tejima, S.; Ortiz-Medina, J.; Morelos-Gomez, A.; Takeuchi, K.; Hayashi, T.; Terrones, M.; Endo, M. Water Diffusion Mechanism in Carbon Nanotube and Polyamide Nanocomposite Reverse Osmosis Membranes: A Possible Percolation-Hopping Mechanism. Phys. Rev. Appl. 2018, 9, 13. [Google Scholar] [CrossRef]
- Song, X.J.; Wang, L.; Mao, L.L.; Wang, Z.N. Nanocomposite Membrane with Different Carbon Nanotubes Location for Nanofiltration and Forward Osmosis Applications. ACS Sustain. Chem. Eng. 2016, 4, 2990–2997. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Hu, Y.X.; Boo, C.; Liu, Z.Y.; Li, J.Q.; Deng, L.Y.; An, X.C. High-Performance Thin-Film Composite Membrane with an Ultrathin Spray-Coated Carbon Nanotube Interlayer. Environ. Sci. Technol. Lett. 2018, 5, 243–248. [Google Scholar] [CrossRef]
- Kiani, F.; Khosravi, T.; Moradi, F.; Rahbari, P.; Aghaei, M.; Arabi, M.; Tajik, H.; Kalantarinejad, R. Computational Investigation of Carbon NanotubesEnhanced Membrane for Water Desalination Based on Flux and Rejection Characteristics. J. Comput. Theor. Nanosci. 2014, 11, 1237–1243. [Google Scholar] [CrossRef]
- Araki, T.; Cruz-Silva, R.; Tejima, S.; Takeuchi, K.; Hayashi, T.; Inukai, S.; Noguchi, T.; Tanioka, A.; Kawaguchi, T.; Terrones, M.; et al. Molecular Dynamics Study of Carbon Nanotubes/Polyamide Reverse Osmosis Membranes: Polymerization, Structure, and Hydration. ACS Appl. Mater. Interfaces 2015, 7, 24566–24575. [Google Scholar] [CrossRef]
- Guo, Y.S.; Mi, Y.F.; Zhao, F.Y.; Ji, Y.L.; An, Q.F.; Gao, C.J. Zwitterions functionalized multi-walled carbon nanotubes/polyamide hybrid nanofiltration membranes for monovalent/divalent salts separation. Sep. Purif. Technol. 2018, 206, 59–68. [Google Scholar] [CrossRef]
- Farid, M.U.; Khanzada, N.K.; An, A.K. Understanding fouling dynamics on functionalized CNT-based membranes: Mechanisms and reversibility. Desalination 2019, 456, 74–84. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 33. [Google Scholar] [CrossRef]
- Fan, X.F.; Liu, Y.M.; Quan, X.; Chen, S. Highly Permeable Thin-Film Composite Forward Osmosis Membrane Based on Carbon Nanotube Hollow Fiber Scaffold with Electrically Enhanced Fouling Resistance. Environ. Sci. Technol. 2018, 52, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.; Ayoub, G.M.; Malaeb, L. Antibacterial activity of chitosan nano-composites and carbon nanotubes: A review. Sci. Total Environ. 2019, 668, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, B.; Engel, M.; Polubesova, T.; Wu, W.; Xing, B.; Chefetz, B. Dual functionality of an Ag-Fe3O4-carbon nanotube composite material: Catalytic reduction and antibacterial activity. J. Environ. Eng. 2018, 6, 4103–4113. [Google Scholar] [CrossRef]
- Cioffi, N.; Rai, M. Nano-Antimicrobials: Progress and Prospects; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Ajiki, H.; Ando, T. Aharonov-Bohm effect in carbon nanotubes. Phys. B Condens. Matter 1994, 201, 349–352. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Ihsanullah; Laoui, T.; Al-Amer, A.M.; Khalil, A.B.; Abbas, A.; Khraisheh, M.; Atieh, M.A. Novel anti-microbial membrane for desalination pretreatment: A silver nanoparticle-doped carbon nanotube membrane. Desalination 2015, 376, 82–93. [Google Scholar] [CrossRef]
- Dong, X.; Al Awak, M.; Wang, P.; Sun, Y.-P.; Yang, L. Carbon dot incorporated multi-walled carbon nanotube coated filters for bacterial removal and inactivation. RSC Adv. 2018, 8, 8292–8301. [Google Scholar] [CrossRef]
- Takizawa, Y.; Inukai, S.; Araki, T.; Cruz-Silva, R.; Ortiz-Medina, J.; Morelos-Gomez, A.; Tejima, S.; Yamanaka, A.; Obata, M.; Nakaruk, A.; et al. Effective Antiscaling Performance of Reverse-Osmosis Membranes Made of Carbon Nanotubes and Polyamide Nanocomposites. ACS Omega 2018, 3, 6047–6055. [Google Scholar] [CrossRef]
- Takeuchi, K.; Takizawa, Y.; Kitazawa, H.; Fujii, M.; Hosaka, K.; Ortiz-Medina, J.; Morelos-Gomez, A.; Cruz-Silva, R.; Fujishige, M.; Akuzawa, N.; et al. Salt rejection behavior of carbon nanotube-polyamide nanocomposite reverse osmosis membranes in several salt solutions. Desalination 2018, 443, 165–171. [Google Scholar] [CrossRef]
- Shan, M.J.; Kang, H.; Xu, Z.W.; Li, N.; Jing, M.L.; Hu, Y.L.; Teng, K.Y.; Qian, X.M.; Shi, J.; Liu, L.Y. Decreased cross-linking in interfacial polymerization and heteromorphic support between nanoparticles: Towards high-water and low-solute flux of hybrid forward osmosis membrane. J. Colloid Interface Sci. 2019, 548, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.B.; Yang, X.D.; Liang, L.; Gao, Y.Y.; Cheng, H.Y.; Li, X.M.; Zou, M.C.; Cao, A.Y.; Ma, R.Z.; Yuan, Q.; et al. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration. Science 2019, 364, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wang, Z.X.; Lin, S.H.; Jin, H.L.; Gao, S.J.; Zhu, Y.Z.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Manawi, Y.M.; Wang, K.; Kochkodan, V.; Johnson, D.J.; Atieh, M.A.; Khraisheh, M.K. Engineering the Surface and Mechanical Properties of Water Desalination Membranes Using Ultralong Carbon Nanotubes. Membranes 2018, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idarraga-Mora, J.A.; Childress, A.S.; Friedel, P.S.; Ladner, D.A.; Rao, A.M.; Husson, S.M. Role of Nanocomposite Support Stiffness on TFC Membrane Water Permeance. Membranes 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsuhybani, M.; Alshahrani, A.; Haidyrah, A.S. Synthesis, Characterization, and Evaluation of Evaporated Casting MWCNT/Chitosan Composite Membranes for Water Desalination. J. Chem. 2020, 2020, 9. [Google Scholar] [CrossRef]
- Mehwish, N.; Kausar, A.; Siddiq, M.; Raheel, M. Design and properties of polyvinylidene fluoride/poly(styrene-butadienestyrene)/functionalized multi- walled carbon nanotube nanocomposite membranes. J. Plast. Film Sheeting 2015, 31, 118–143. [Google Scholar] [CrossRef]
- Farid, M.U.; Luan, H.Y.; Wang, Y.F.; Huang, H.; An, A.K.; Khan, R.J. Increased adsorption of aqueous zinc species by Ar/O-2 plasma-treated carbon nanotubes immobilized in hollow-fiber ultrafiltration membrane. Chem. Eng. J. 2017, 325, 239–248. [Google Scholar] [CrossRef]
- Shaban, M.; Ashraf, A.M.; AbdAllah, H.; Abd El-Salam, H.M. Titanium dioxide nanoribbons/multi-walled carbon nanotube nanocomposite blended polyethersulfone membrane for brackish water desalination. Desalination 2018, 444, 129–141. [Google Scholar] [CrossRef]
- Azelee, I.W.; Goh, P.S.; Lau, W.J.; Ismail, A.F. Facile acid treatment of multiwalled Carbon nanotube-titania nanotube thin film nanocomposite membrane for reverse osmosis desalination. J. Clean Prod. 2018, 181, 517–526. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
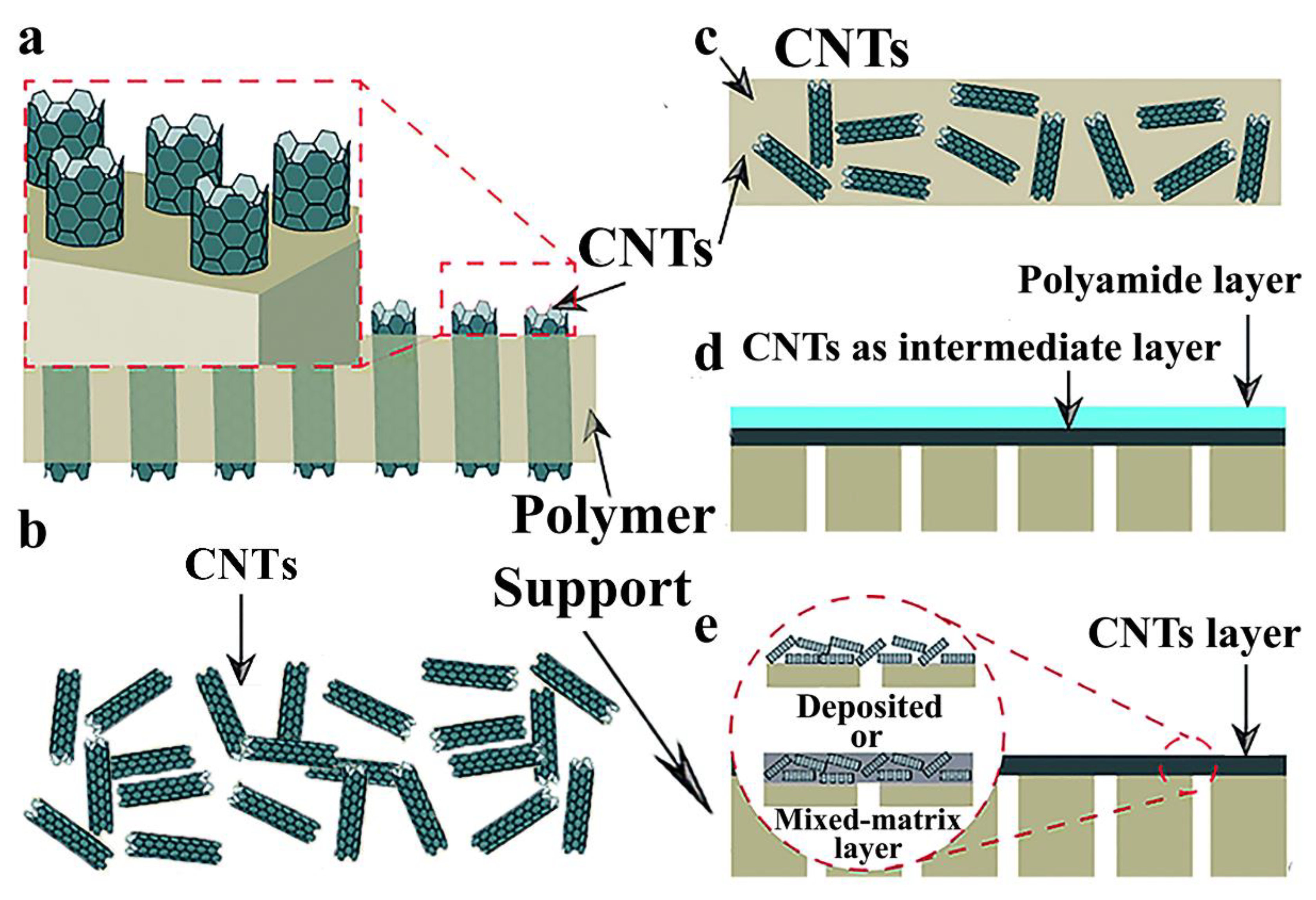

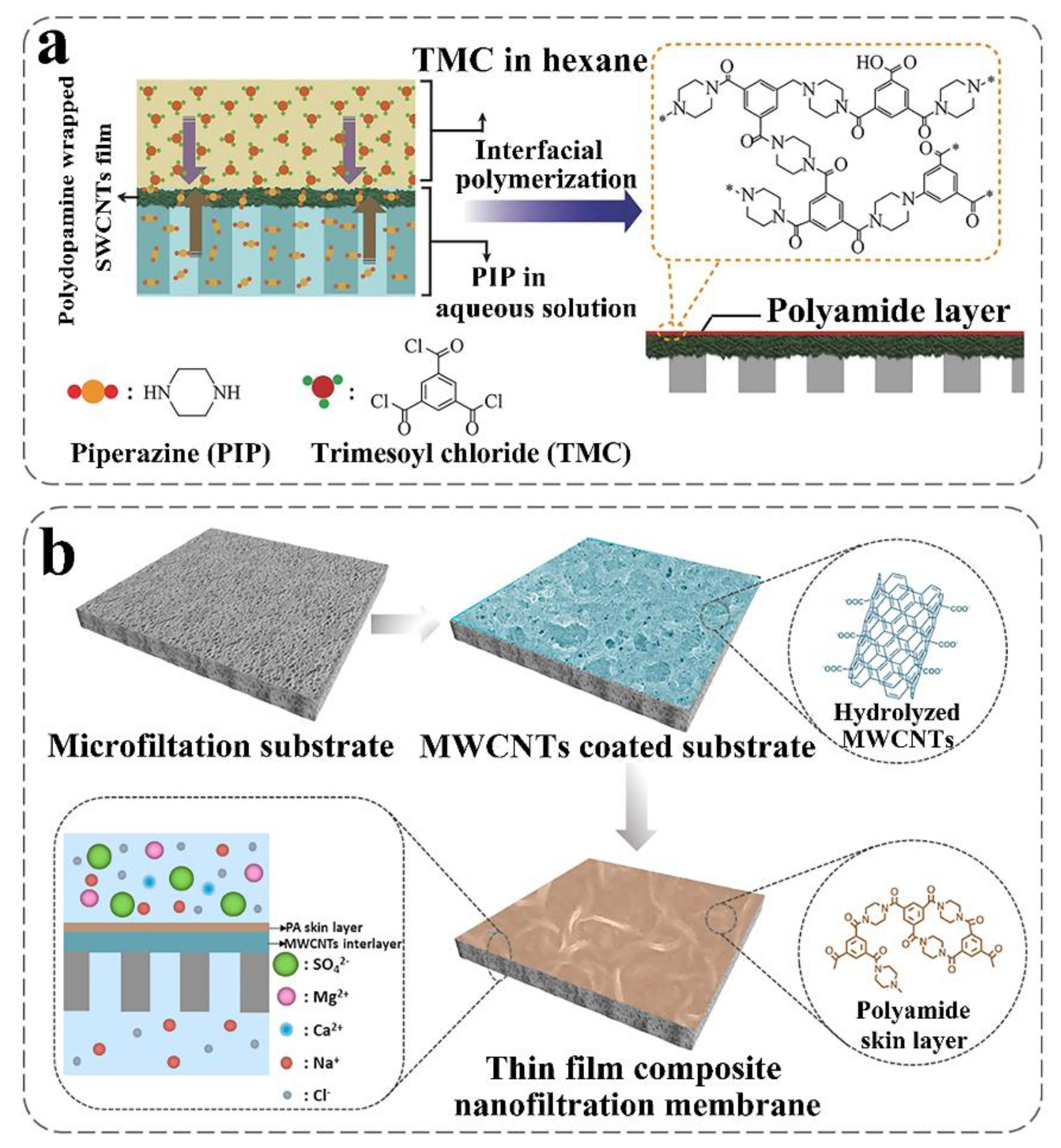
| Preparation Method | Composition of Membrane | Feed System | Applied Pressure | Water Flux | Membrane Rejection | The Effect of CNT Addition | Ref. |
|---|---|---|---|---|---|---|---|
| Phase inversion | MWCNT doped PVDF–HFP | 2-g/L NaCl | N/A | N/A | N/A | Improved hydrophilicity | [89] |
| CA + CNT | 5-g/L NaCl | 35 bar | N/A | 94% | Increased pure water flux | [90] | |
| CA + CNT | 58.5-g/L NaCl | N/A | 18.1 LMH | N/A | Increased water flux | [91] | |
| CA + CNT | MgSO4 | N/A | 69.5 LMH | 90.6% | Enhance permeability | [92] | |
| PES/CNTs + MMMs | 0.2-g/L Na2SO4 | 4 bar | ∼45.2 LMH | ∼87.2% | Enhanced the water flux and salt rejection | [93] | |
| PSf + DDA–MWCNTs | DI water | 1 bar | N/A | N/A | Improved fouling resistance | [94] | |
| functionalized CNT/PS | N/A | 2.06 bar | 600 LMH | N/A | Enhanced pure water permeation | [95] | |
| Interfacial polymerization | PS-20 + MPD + TMC + MWCNT | 2-g/L NaCl | 225 psi | 43 LMH | 99% | Enhanced water flux and pure water permeance | [96] |
| MWCNT–TNT hybrid + TMC + MPD + PS | 2-g/L NaCl | 15 bar | 0.74 LMH/bar | 97.97% | Experienced high-water permeability | [97] | |
| MWCNTs + PA | 2-g/L NaCl | 15 bar | 28.9 LMH | 96.7%–97.8% | Improved water flux and better antifouling performance | [98] | |
| PA + CNT + PSf | 2-g/L NaCl | 15.5 bar | 46.2 LMH | 97% | Enhanced water flux | [99] | |
| ZfCNTs + PA | 2-g/L Na+ | 2.41 MPa | 34.7 LMH | Above 98% | Improved surface biofouling resistance and salt rejection | [100] | |
| TA–MWNTs + PA | 0.71-g/L Na2SO4 | 0.6 MPa | 31.4 LMH | N/A | Optimized water flux and salt rejection | [101] | |
| SMWCNT | 1-g/L Na2SO4 | 0.6 MPa | 13.2 LMH/bar | 96.8% | Enhanced water flux and antifouling ability | [102] | |
| PES + CNT | 1-g/L Na2SO4 | 0.6 MPa | 105.4 LMH | ~95% | Provided a robust mechanical support | [103] | |
| PDA/CNTs + TMC + PSf | 0.3-g/L alginate | N/A | 12.4 LMH | N/A | Antifouling capacity | [104] | |
| Modified PDA-MWCNTs + PEI + TMC | 0.5-g/L ZnCl2 | 6 bar | 15.32 LMH/bar | 93% | Improved water permeability | [105] | |
| PES + CNT + PA | Na2SO4 and MgSO4 solution | 5 bar | 21 LMH/bar | 98.3% | Exhibited an excellent water flux and comparable salt rejection | [106] | |
| PA + MWCNT | 3.5 wt% saline water | 5 MPa | 1.7 (m3/m2·day−1) | 90 wt% | Antifouling nature, chlorine resistance | [107] | |
| PA + MWCNT | N/A | N/A | N/A | N/A | Improved chlorine resistance, antifouling and desalination performance | [108] | |
| PES/SPSf/O–MWCNT | brackish waters | 3 bar | 30.2 LMH/bar | 25 (NaCl/Na2SO4 selectivity) | Enhanced water permeability and salt rejection | [109] | |
| Interfacial polymerization + phase inversion | fCNT + PES | 35.1-g/L NaCl | N/A | 11.98 LMH | N/A | Contributed to the water flux and diminish of alginate fouling | [110] |
| TMC + MPD + fCNT | 1-g/L NaCl | 10 bar | 20.23 LMH | 72.3% | Enhanced water permeability | [22] | |
| Electrospray-assisted interfacial polymerization | CNTs + PA | 1-g/L NaCl | 4 bar | 96.8 LMH | 89.6% | Improved in water flux | [111] |
| Dissolution casting method | CA/PEG400 + SE–MWCNT | 1-g/L NaCl | 4 bar | 0.61 LMH | 99.8% | Improved the salt rejection performance | [112] |
| Surface Grafting and nanoparticle incorporation | Hydrophilic monomer + c-MWCNT | 2-g/L NaCl | from 20 bar to 15 bar | N/A | Above 96% | Reduced fouling | [113] |
| ATRP + Interfacial polymerization | fMWCNTs + PAAm + PA | 2-g/L NaCl | 1.55 MPa | 66.9 LMH | 95.3% | Improved membrane separation and antifouling capability | [114] |
| Electrophoretic deposition + chemical reduction | RGO + CNTs | 5.85-g/L NaCl | 1 bar | 40.4 ± 3.7 LMH | 94.0% ± 1.9% | Increase water flux | [115] |
| Filtration-assisted interfacial polymerization | PVDF + CNT + PA | 117-g/L NaCl | 0.3 MPa | 0.54 ± 0.08 LMH | (91.3 ± 0.4)% | Improved the water flux | [116] |
| Brush painting | SWCNT + PA + PES | 1-g/L Na2SO4 | 6 bar | 40 LMH | 96.5% | Improved permeability | [117] |
| Polymerization | PDA/SWCNTs | 1-g/L Na2SO4 | 6 bar | 32 LMH/bar | 95.9% | Long-term chlorine tolerance property | [118] |
| Chemically synthesized | PPy-raw or PPy-oxidized MWCNTs | 2-g/L Na2SO4 | 1 MPa | 88.9 LMH | 96.6 wt% | Improved in the flux | [119] |
| ATRP | ZCNTs + poly(4-styrenesulfonic acid) + P4VP | Model brackish water | 0.6 MPa | 14.9 ± 0.5 LMH | 5.6 ± 0.8 (MgSO4/NaCl selectivity) | Enhanced water permeability | [120] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Chen, D.; Wang, Q.; Ying, Y.; Gao, W.; Xie, L. Recent Advances in Applications of Carbon Nanotubes for Desalination: A Review. Nanomaterials 2020, 10, 1203. https://doi.org/10.3390/nano10061203
Wang R, Chen D, Wang Q, Ying Y, Gao W, Xie L. Recent Advances in Applications of Carbon Nanotubes for Desalination: A Review. Nanomaterials. 2020; 10(6):1203. https://doi.org/10.3390/nano10061203
Chicago/Turabian StyleWang, Ruiqian, Dinghao Chen, Qi Wang, Yibin Ying, Weilu Gao, and Lijuan Xie. 2020. "Recent Advances in Applications of Carbon Nanotubes for Desalination: A Review" Nanomaterials 10, no. 6: 1203. https://doi.org/10.3390/nano10061203





