RETRACTED: Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. FLB TRF Preparation
2.3. Box–Behnken Design for FLB TRF Preparations
2.4. Vesicle Size Determination
2.5. Characterization of Optimized FLB TRFs
2.6. Preparation of Optimized FLB-TRF-Loaded Hydrogel
2.7. Optimized FLB TRF Gel Ex Vivo Permeation Study
2.8. In Vivo Pharmacokinetic Assessment
2.9. Statistical Analysis
3. Results
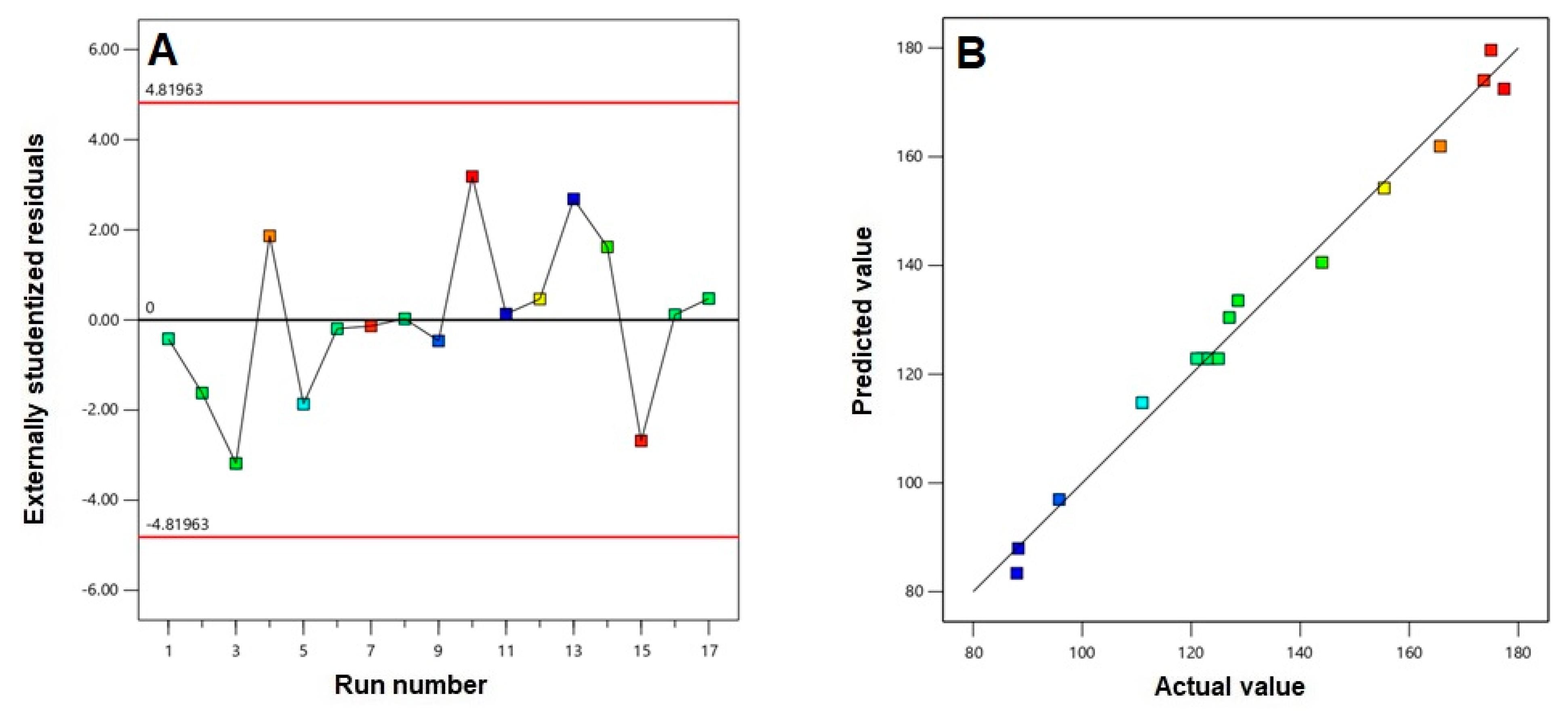
3.1. Polynomial Model Selection and Diagnostic Analysis
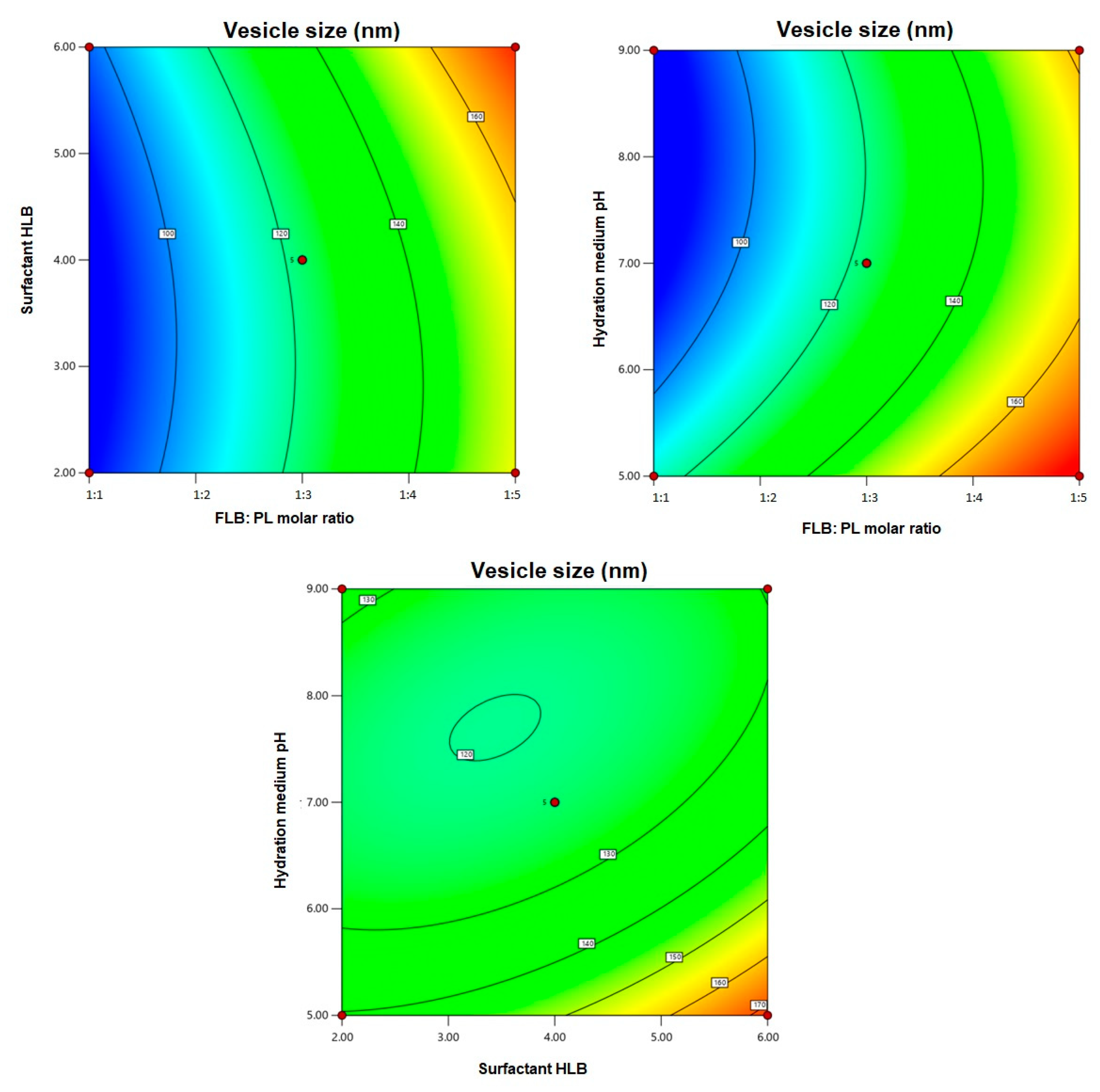
3.2. Statistical Analysis for the Effect of Variables on Vesicle Size (Y)
3.3. FLB TRF Optimization
3.4. Charactarization of the Optimized FLB TRFs
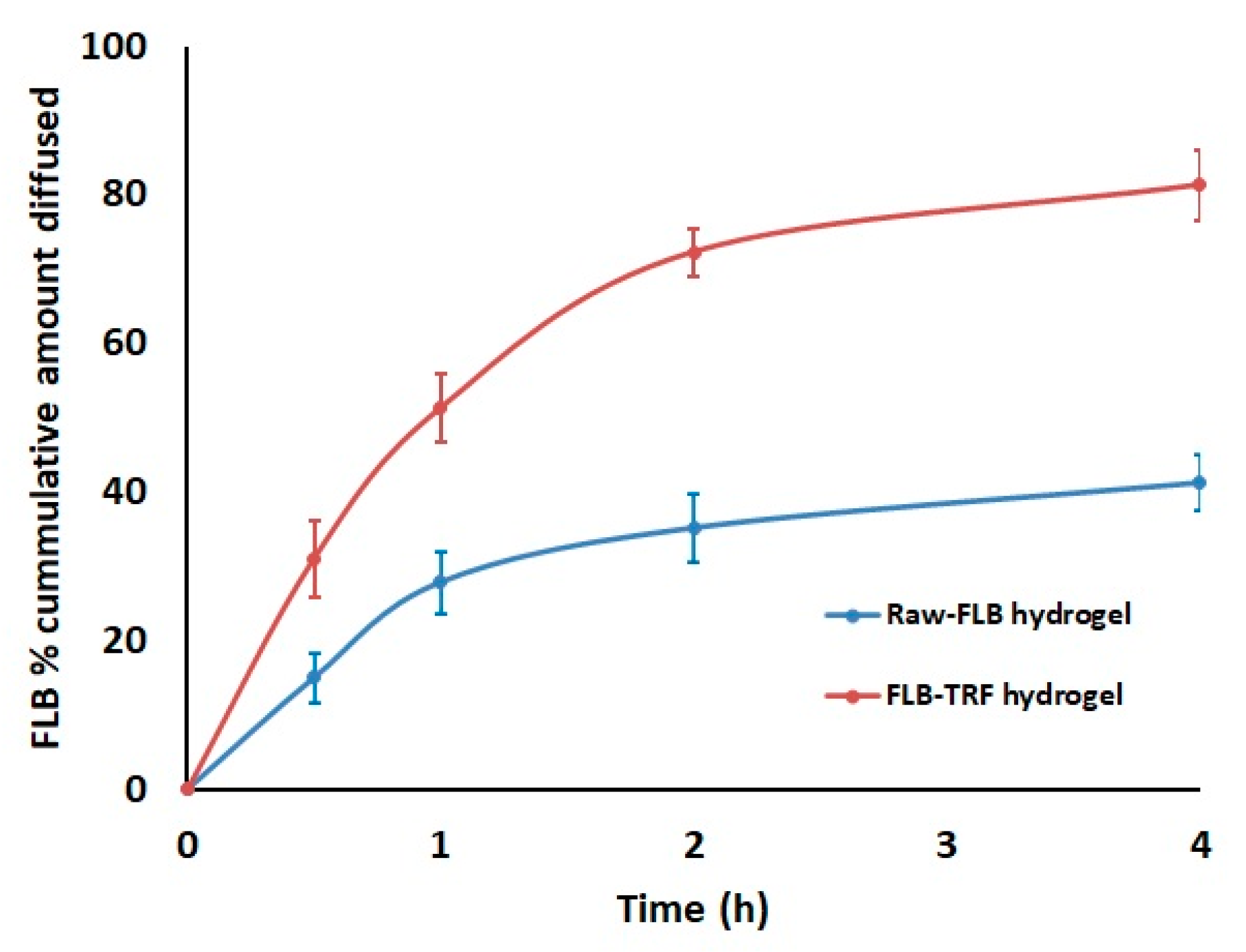
3.5. Optimized FLB TRF Gel Ex Vivo Permeation
3.6. In Vivo Pharmacokinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allers, K.A.; Dremencov, E.; Ceci, A.; Flik, G.; Ferger, B.; Cremers, T.I.F.H.; Ittrich, C.; Sommer, B. Acute and repeated flibanserin administration in female rats modulates monoamines differentially across brain areas: A microdialysis study. J. Sex. Med. 2010, 7, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Borsini, F. Method of using flibanserin for neuroprotection. US Patent App. 10/882, 1 July 2004. [Google Scholar]
- Kennedy, S. Flibanserin: Initial evidence of efficacy on sexual dysfunction, in patients with major depressive disorder. J. Sex. Med. 2010, 7, 3449–3459. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Derogatis, L.R.; Ackerman, R.; Hedges, P.; Lesko, L.; Garcia, M.; Sand, M. Efficacy of flibanserin in women with hypoactive sexual desire disorder: Results from the BEGONIA trial. J. Sex. Med. 2013, 10, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Lodise, N.M. Female sexual dysfunction: A focus on flibanserin. Int. J. Womens Health 2017, 9, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Dooley, E.M.; Miller, M.K.; Clayton, A.H. Flibanserin: From Bench to Bedside. Sex. Med. Rev. 2017, 5, 461–469. [Google Scholar] [CrossRef]
- El-Kattan, A.; Varm, M. Oral Absorption, Intestinal Metabolism and Human Oral Bioavailability. In Topics on Drug Metabolism; Paxton, J., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Lungare, S.; Bowen, J.; Badhan, R. Development and Evaluation of a Novel Intranasal Spray for the Delivery of Amantadine. J. Pharm. Sci. 2016, 105, 1209–1220. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. BBA Biomembr. 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, O.; Abdel-Ghany, M.; Abdel-Hamid, M.; Cairo, I. Development, evaluation and application of Transfersomal Green tea extract (Camellia sinensis) formulations. Am. J. Med. Pharm. Res. 2020, 2, 21–39. [Google Scholar]
- Piumitali, B.; Neeraj, U.; Jyotivardhan, J. Transfersomes—A Nanoscience in Transdermal Drug Delivery and Its Clinical Advancements. Int. J. Nanosci. 2020. [Google Scholar] [CrossRef]
- Aboud, H.M.; Ali, A.A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of carvedilol for intranasal delivery: Formulation, characterization and in vivo evaluation. Drug Deliv. 2016, 23, 2471–2481. [Google Scholar]
- Pitta, S.K.; Dudhipala, N.; Narala, A.; Veerabrahma, K. Development of zolmitriptan transfersomes by Box–Behnken design for nasal delivery: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2018, 44, 484–492. [Google Scholar] [CrossRef]
- Mouez, M.A.; Nasr, M.; Abdel-Mottaleb, M.; Geneidi, A.S.; Mansour, S. Composite chitosan-transfersomal vesicles for improved transnasal permeation and bioavailability of verapamil. Int. J. Biol. Macromol. 2016, 93, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Wahdan, S.A. Neuroprotective effects of novel nanosystems simultaneously loaded with vinpocetine and piracetam after intranasal administration. Life Sci. 2019, 226, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A.S. Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J. Liposome Res. 2012, 22, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.M.; Eleraky, N.E.; El Sisi, A.M.; Hasan, O.A. Development and evaluation of in-situ nasal gel formulations of nanosized transferosomal sumatriptan: Design, optimization, in vitro and in vivo evaluation. Drug Des. Dev. Ther. 2019, 13, 4413–4430. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kishida, T.; Hasegawa, U.; Ueda, Y.; Imanishi, J.; Yamagishi, H.; Akiyoshi, K.; Otsuji, E.; Mazda, O. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem. Biophys. Res. Commun. 2008, 367, 330–335. [Google Scholar] [CrossRef]
- Sarei, F.; Dounighi, N.; Zolfagharian, H.; Khaki, P.; Bidhendi, S. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef]
- Salatin, S.; Jelvehgari, M.; Maleki-Dizaj, S.; Adibkia, K. A sight on protein-based nanoparticles as drug/gene delivery systems. Ther. Deliv. 2015, 6, 1017–1029. [Google Scholar] [CrossRef]
- Joysa Ruby, J.; Pandey, V.P. Chitosan nanoparticles as a nasal drug delivery for memantine hydrochloride. Int. J. Pharm. Pharm. Sci. 2015, 7, 34–37. [Google Scholar]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Milani, M.A.; Jelvehgari, M. Hydrogel nanoparticles and nanocomposites for nasal drug/vaccine delivery. Arch. Pharm. Res. 2016, 39, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Rigogliuso, S.; Sabatino, M.A.; Adamo, G.; Grimaldi, N.; Dispenza, C.; Ghersi, G. Polymeric nanogels: Nanocarriers for drug delivery application. Chem. Eng. Trans. 2012, 27, 247–252. [Google Scholar]
- Bhavna; Sharma, V.; Ali, M.; Baboota, S.; Ali, J. Preparation and characterization of chitosan nanoparticles for nose to brain delivery of a cholinesterase inhibitor. Indian J. Pharm. Sci. 2007, 69, 712–713. [Google Scholar]
- Gyu Kong, I.; Sato, A.; Yuki, Y.; Nochi, T.; Takahashi, H.; Sawada, S.; Mejima, M.; Kurokawa, S.; Okada, K.; Sato, S.; et al. Nanogel-based pspa intranasal vaccine prevents invasive disease and nasal colonization by streptococcus pneumoniae. Infect. Immun. 2013, 81, 1625–1634. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Jerca, F.A.; Anghelache, A.M.; Ghibu, E.; Cecoltan, S.; Stancu, I.C.; Trusca, R.; Vasile, E.; Teodorescu, M.; Vuluga, D.M.; Hoogenboom, R.; et al. Poly(2-isopropenyl-2-oxazoline) Hydrogels for Biomedical Applications. Chem. Mater. 2018, 30, 7938–7949. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. Covalent Poly(2-Isopropenyl-2-Oxazoline) Hydrogels with Ultrahigh Mechanical Strength and Toughness through Secondary Terpyridine Metal-Coordination Crosslinks. Adv. Funct. Mater. 2019, 29, 1904886. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, X.; Hu, Q.; Liu, S.; Guan, S. PVA/PEG hybrid hydrogels prepared by freeze-thawing and high energy electron beam irradiation. Chem. Res. Chin. Univ. 2017, 33, 995–999. [Google Scholar] [CrossRef]
- Joshi, S.C. Sol-gel behavior of hydroxypropyl methylcellulose (HPMC) in ionic media including drug release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.; Lee, P.I. Probing the Mechanisms of Drug Release from Hydroxypropylmethyl Cellulose Matrices. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1994, 11, 1379–1384. [Google Scholar]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd ElHady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Escudero, J.J.; Ferrero, C.; Jiménez-Castellanos, M.R. Compaction properties, drug release kinetics and fronts movement studies from matrices combining mixtures of swellable and inert polymers. II. Effect of HPMC with different degrees of methoxy/hydroxypropyl substitution. Int. J. Pharm. 2010, 387, 56–64. [Google Scholar] [CrossRef]
- Colombo, P. Swelling-controlled release in hydrogel matrices for oral route. Adv. Drug Deliv. Rev. 1993, 11, 37–57. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.S.; Ahmed, O.A.A. Optimized nano-transfersomal films for enhanced sildenafil citrate transdermal delivery: Ex vivo and in vivo evaluation. Drug Des. Devel. Ther. 2016, 10, 1323–1333. [Google Scholar] [CrossRef]
- Galgatte, U.C.; Kumbhar, A.B.; Chaudhari, P.D. Development of in situ gel for nasal delivery: Design, optimization, in vitro and in vivo evaluation. Drug Deliv. 2014, 21, 62–73. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Badr-Eldin, S.M. In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: Formulation, characterization, and in vivo performance. Int. J. Nanomed. 2018, 13, 6325–6335. [Google Scholar] [CrossRef]
- Al Asmari, A.K.; Ullah, Z.; Tariq, M.; Fatani, A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des. Dev. Ther. 2016, 10, 205–215. [Google Scholar]
- Young, J. Histopathologic examination of the rat nasal cavity. Fundam. Appl. Toxicol. 1981, 1, 309–312. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar] [CrossRef]
- Abourehab, M.; Ahmed, O.; Balata, G.; Almalki, W. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int. J. Nanomed. 2018, 13, 3679–3687. [Google Scholar] [CrossRef]
- El Zaafarany, G.G.M.; Awad, G.G.A.S.; Holayel, S.S.M.; Mortada, N.D.N. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- El-Laithy, H.M.; Shoukry, O.; Mahran, L.G. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: Preclinical and clinical studies. Eur. J. Pharm. Biopharm. 2011, 77, 43–55. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Ahmad, S.; Mahmood, M.A.; Rehman, M.; Ashfaq, M. Formulation design and characterization of a non-ionic surfactant based vesicular system for the sustained delivery of a new chondroprotective agent. Braz. J. Pharm. Sci. 2015, 51, 607–616. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Al-Ghananeem, A.M.; Saeed, H.; Florence, R.; Yokel, R.A.; Malkawi, A.H. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; An attractive route against infections caused by aids viruses. J. Drug Target. 2010, 18, 381–388. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Nanosized Transferosome-Based Intranasal In Situ Gel for Brain Targeting of Resveratrol: Formulation, Optimization, In Vitro Evaluation, and In Vivo Pharmacokinetic Study. AAPS PharmSciTech 2019, 20, 181. [Google Scholar] [CrossRef] [PubMed]




| Experimental Run Number | Independent Variables | Vesicle Size (nm) ± SD | ||
|---|---|---|---|---|
| FLB:PL Molar Ratio * | Surfactant HLB | Hydration Medium pH | ||
| T1 | 3.00 | 4.00 | 7.00 | 121 ± 2.81 |
| T2 | 3.00 | 6.00 | 9.00 | 127 ± 1.78 |
| T3 | 3.00 | 2.00 | 9.00 | 128 ± 3.11 |
| T4 | 5.00 | 4.00 | 9.00 | 166 ± 1.12 |
| T5 | 1.00 | 4.00 | 5.00 | 111 ± 1.65 |
| T6 | 3.00 | 4.00 | 7.00 | 122 ± 1.27 |
| T7 | 5.00 | 6.00 | 7.00 | 174 ± 2.18 |
| T8 | 3.00 | 4.00 | 7.00 | 123 ± 2.09 |
| T9 | 1.00 | 6.00 | 7.00 | 96 ± 1.03 |
| T10 | 3.00 | 6.00 | 5.00 | 177 ± 1.99 |
| T11 | 1.00 | 2.00 | 7.00 | 89 ± 0.99 |
| T12 | 5.00 | 2.00 | 7.00 | 155 ± 1.45 |
| T13 | 1.00 | 4.00 | 9.00 | 88 ± 0.86 |
| T14 | 3.00 | 2.00 | 5.00 | 144 ± 2.56 |
| T15 | 5.00 | 4.00 | 5.00 | 175 ± 2.43 |
| T16 | 3.00 | 4.00 | 7.00 | 123 ± 1.49 |
| T17 | 3.00 | 4.00 | 7.00 | 125 ± 1.66 |
| Factor | Optimum Level | Low Level | High Level | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1: FLB:PL molar ratio | 1:1.2 | 1:1 | 1:5 | ||||||
| X2: Surfactant HLB | 2.3 | 2 | 6 | ||||||
| X3: Hydration medium pH | 7.2 | 5 | 9 | ||||||
| Response | Predicted | Actual | Residual error % | ||||||
| Vesicle size (nm) | 87.89 | 89.71 | 2.07% | ||||||
| Statistical analysis output of TRF vesicle size (Quadratic model) | R2 | Adjusted R2 | Predicted R2 | Adequate precision | |||||
| 0.9885 | 0.9738 | 0.8262 | 26.6354 | ||||||
| p-value | X1 | X2 | X3 | X2 × 3 | X22 | X32 | |||
| 0.0001 | 0.0035 | 0.0002 | 0.0075 | 0.0148 | 0.0005 | ||||
| Pharmacokinetic Parameter | Plasma Data | Brain Data | ||
|---|---|---|---|---|
| Raw FLB Hydrogel | FLB TRF Hydrogel | Raw FLB Hydrogel | FLB TRF Hydrogel | |
| Cmax (ng/mL, plasma) (ng/g, brain) | 122.89 ± 4.01 | 406.81 ± 76.15 # | 9.70 ± 1.32 | 20.81 ± 2.30 # |
| AUC0–∞ (ng.hr/mL, plasma) (ng.hr/ng, brain) | 296.87 ± 15.18 | 1188.13 ± 287.16 # | 85.52 ± 4.34 | 148.82 ± 12.4 # |
| Tmax (h) | 1.0 | 0.5 | 4.0 | 4.0 |
| Relative bioavailability | ---- | 400.22% | ---- | 174.02% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, O.A.A.; Fahmy, U.A.; Badr-Eldin, S.M.; Aldawsari, H.M.; Awan, Z.A.; Asfour, H.Z.; Kammoun, A.K.; Caruso, G.; Caraci, F.; Alfarsi, A.; et al. RETRACTED: Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route. Nanomaterials 2020, 10, 1270. https://doi.org/10.3390/nano10071270
Ahmed OAA, Fahmy UA, Badr-Eldin SM, Aldawsari HM, Awan ZA, Asfour HZ, Kammoun AK, Caruso G, Caraci F, Alfarsi A, et al. RETRACTED: Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route. Nanomaterials. 2020; 10(7):1270. https://doi.org/10.3390/nano10071270
Chicago/Turabian StyleAhmed, Osama A. A., Usama A. Fahmy, Shaimaa M. Badr-Eldin, Hibah M. Aldawsari, Zuhier A. Awan, Hani Z. Asfour, Ahmed K. Kammoun, Giuseppe Caruso, Filippo Caraci, Anas Alfarsi, and et al. 2020. "RETRACTED: Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route" Nanomaterials 10, no. 7: 1270. https://doi.org/10.3390/nano10071270
APA StyleAhmed, O. A. A., Fahmy, U. A., Badr-Eldin, S. M., Aldawsari, H. M., Awan, Z. A., Asfour, H. Z., Kammoun, A. K., Caruso, G., Caraci, F., Alfarsi, A., A. Al-Ghamdi, R., A. Al-Ghamdi, R., & Alhakamy, N. A. (2020). RETRACTED: Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route. Nanomaterials, 10(7), 1270. https://doi.org/10.3390/nano10071270






