Analysis of Iron Oxide Reduction Kinetics in the Nanometric Scale Using Hydrogen
Abstract
1. Introduction
1.1. Background
1.2. Theory
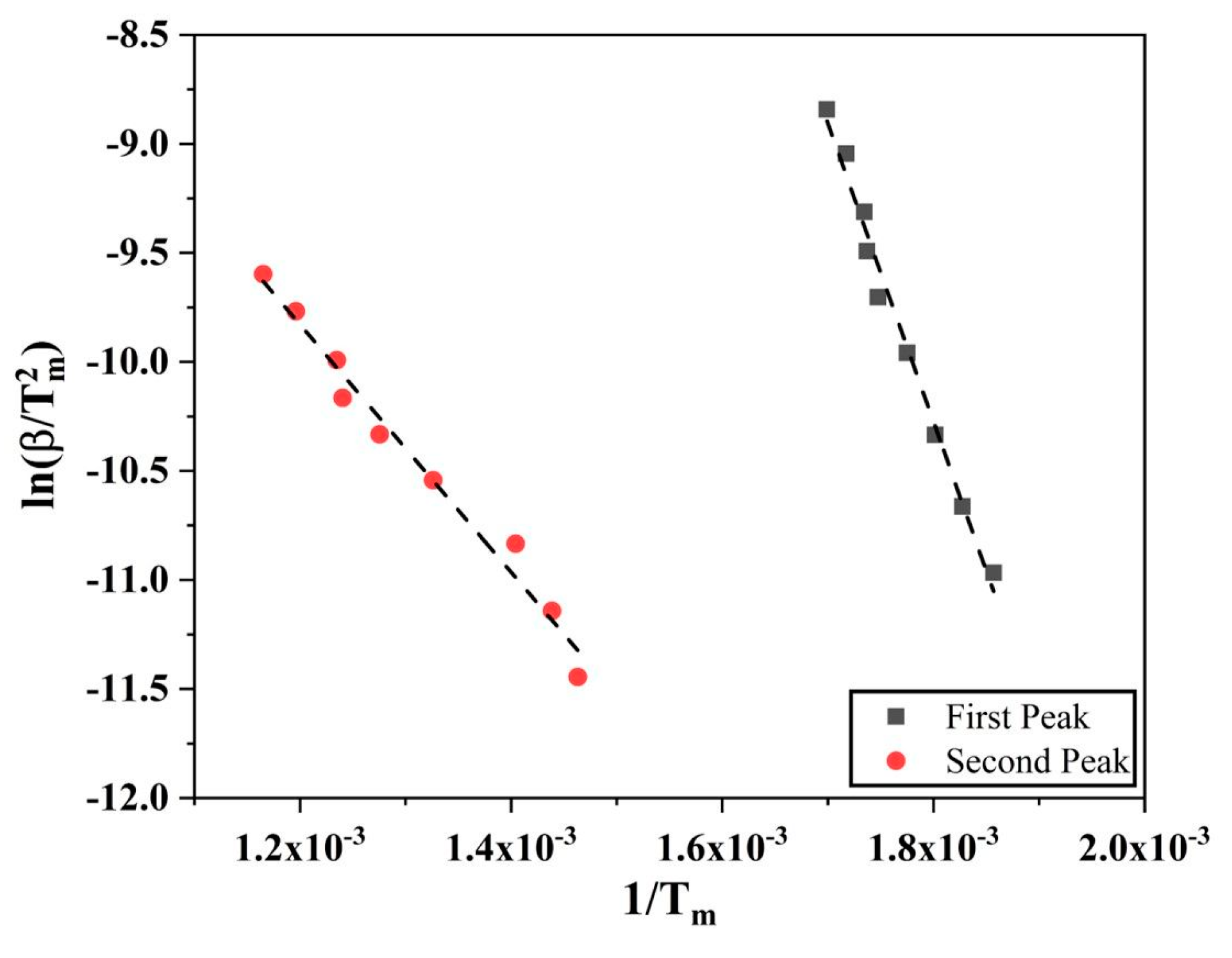
The Kissinger Method
2. Materials and Methods
3. Results and Analysis
3.1. Iron Nanopowder
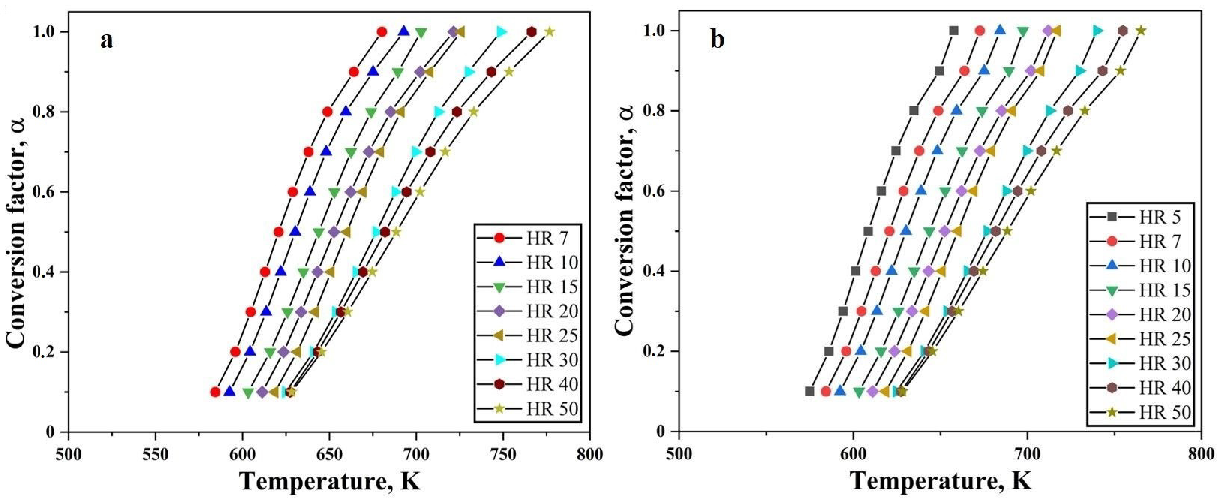
3.1.1. Non-Isothermal Reduction of Oxide Layer on Fe Nanopowder
3.1.2. Reduction Kinetics for Removal of Oxide Layer on Fe Nanopowder
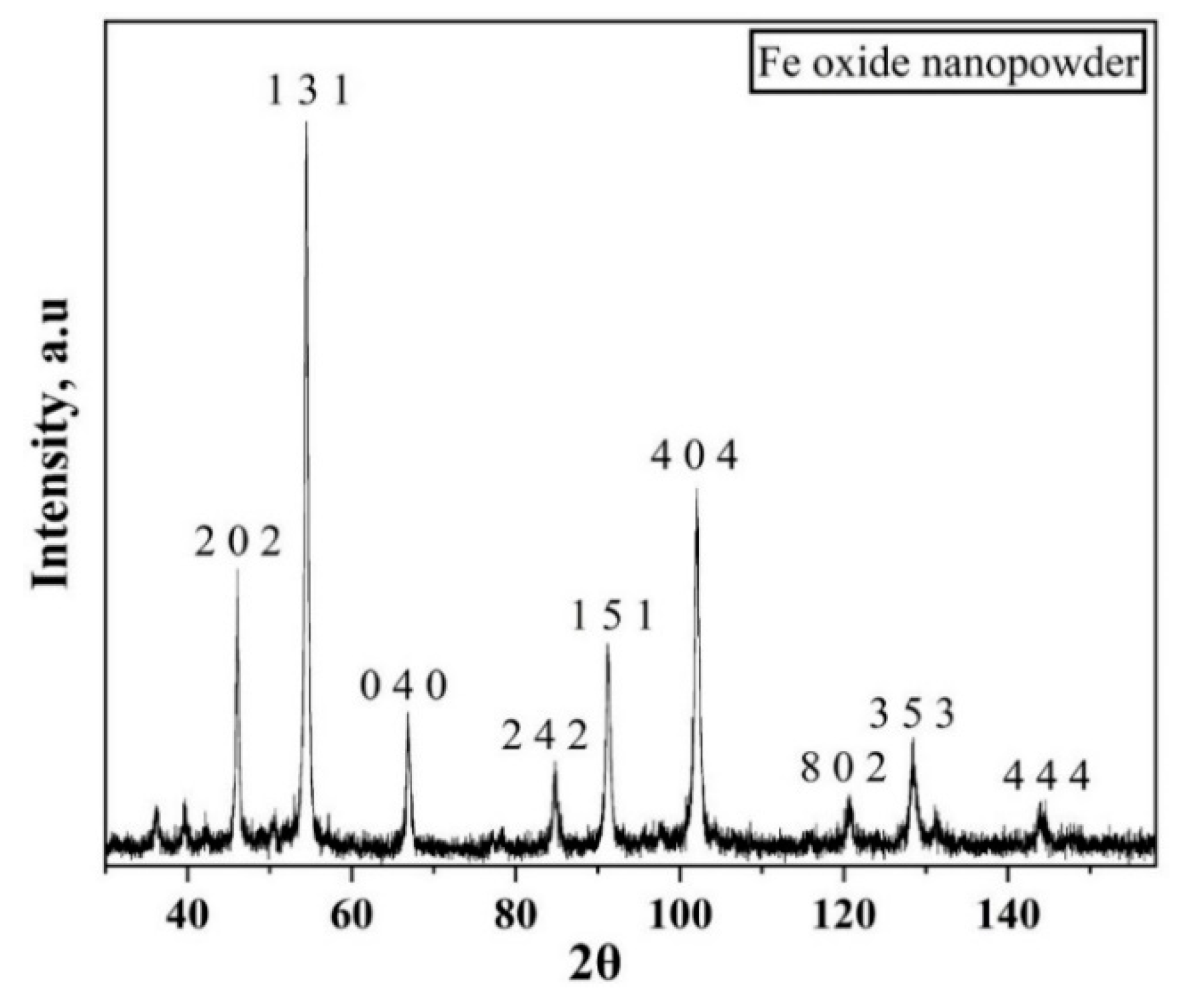
3.2. Iron Oxide Nanopowder
3.2.1. Non-Isothermal Reduction of Fe2O3 Nanopowder
3.2.2. Reduction Kinetics for Fe2O3 Nanopowder
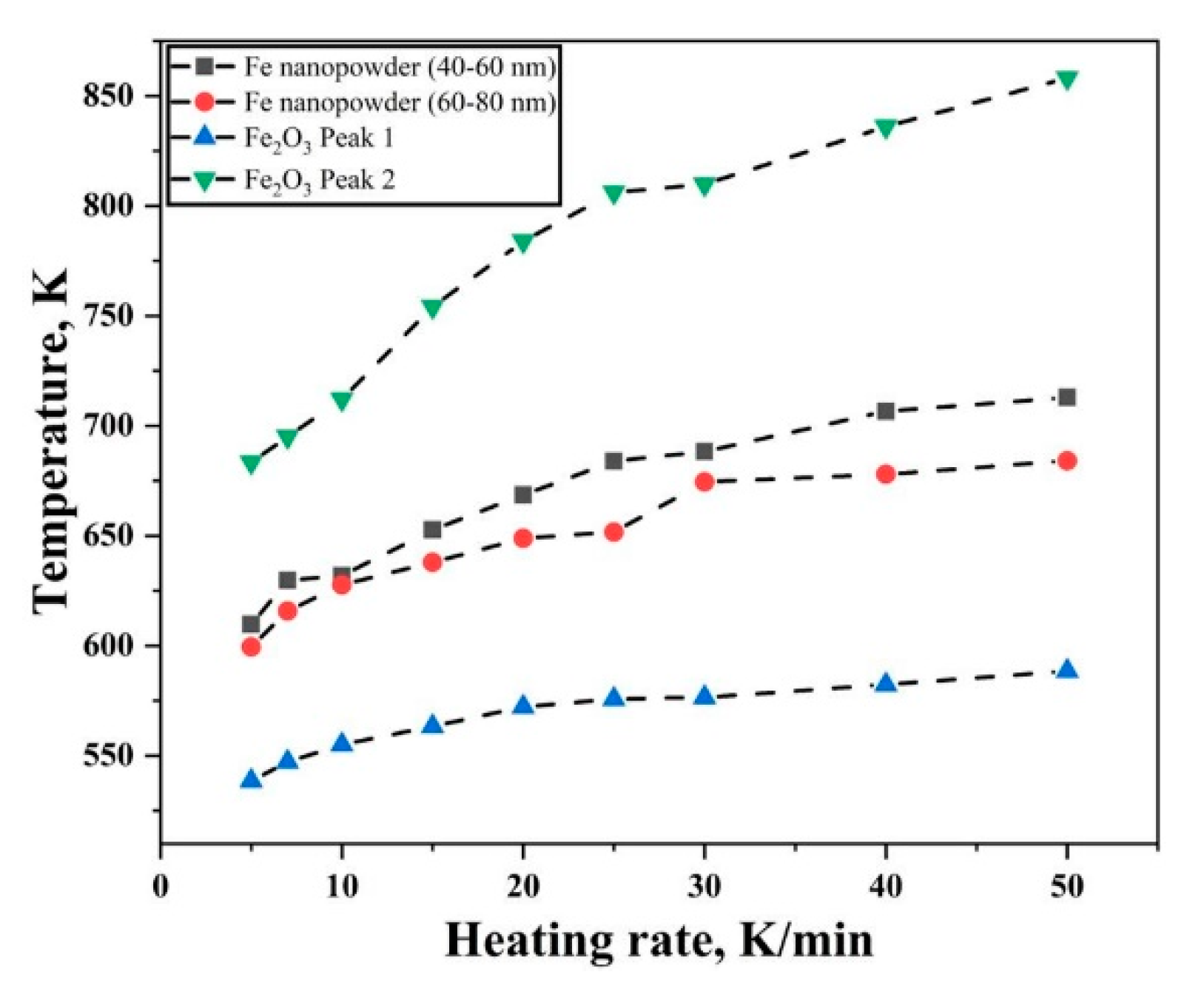
3.3. Kissinger Approach for Iron and Iron Oxide Reduction Kinetics
3.4. Proposed Model
- During the prereduction step, Fe2O3 is reduced to Fe3O4 completely. As the density of Fe3O4 is higher than that of Fe2O3, a porous Fe3O4 structure is expected upon transformation.
- The porous Fe3O4 is reduced to metallic Fe during the main reduction step, resulting in islands of metallic Fe.
- Catalytic reaction: The freshly formed metallic iron surfaces exhibit an auto-catalytic nature, supporting the chemisorption and disassociation of hydrogen molecules to yield active hydrogen atoms which are transferred or transported from metal surface to the metal/oxide interfaces through ‘portholes’ of water vapour [25,27].
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Statement
References
- Song, J.; Lee, G.-Y.; Hong, E.-J.; Lee, C.S.; Lee, J.-S. Sintering behavior of bimodal iron nanopowder agglomerates. J. Am. Ceram. Soc. 2019, 102, 3791–3801. [Google Scholar] [CrossRef]
- Choi, J.-P.; Lyu, H.-G.; Lee, W.-S.; Lee, J.-S. Densification and microstructural development during sintering of powder injection molded Fe micro–nanopowder. Powder Technol. 2014, 253, 596–601. [Google Scholar] [CrossRef]
- Dominguez, O.; Champion, Y.; Bigot, J. Liquidlike Sintering Behavior of Nanometric Fe and Cu Powders: Experimental Approach. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1998, 29, 2941–2949. [Google Scholar] [CrossRef]
- Puri, P.; Yang, V.J. Effect of Particle Size on Melting of Aluminum at Nano Scales. Phys. Chem. C 2007, 111, 11776–11783. [Google Scholar] [CrossRef]
- Sun, J.; Simon, S.L. The melting behavior of aluminum nanoparticles. Mater. Acta 2007, 463, 32–40. [Google Scholar] [CrossRef]
- Alavi, S.; Thompson, D.L. Molecular Dynamics Simulations of the Melting of Aluminum Nanoparticles. J. Phys. Chem. A 2006, 110, 1518–1523. [Google Scholar] [CrossRef]
- Manchili, S.K.; Shvab, R.; Zehri, A.; Ye, L.; Hryha, E.; Liu, J.; Nyborg, L. Surface analysis of iron and steel nanopowder. Surf. Interface Anal. 2018, 50, 1083–1088. [Google Scholar] [CrossRef]
- Wendel, J.; Manchili, S.K.; Hryha, E.; Nyborg, L.J. Oxide reduction and oxygen removal in water-atomized iron powder: A kinetic study. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Pourghahramani, P.; Forssberg, E. Reduction kinetics of mechanically activated hematite concentrate with hydrogen gas using nonisothermal methods. Thermochim. Acta 2007, 454, 69–77. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Role of isoconversional methods in varying activation energies of solid-state kinetics: II. Nonisothermal kinetic studies. Thermochim. Acta 2005, 436, 101–112. [Google Scholar] [CrossRef]
- Vyazovkin, S.J. Advanced isoconversional method. Therm. Anal. 1997, 49, 1493–1499. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Opfermann, J.; Kaisersberger, E. An advantageous variant of the Ozawa-Flynn-Wall analysis. Thermochim. Acta 1992, 203, 167–175. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S.J. Modification of the integral isoconversional method to account for variation in the activation energy. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Wendel, J.; Shvab, R.; Cao, Y.; Hryha, E.; Nyborg, L. Surface analysis of fine water-atomized iron powder and sintered material. Surf. Interface Anal. 2018, 50, 1065–1071. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe 2+ and Fe 3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Sastri, M.V.C.; Viswanath, R.P.; Viswanathan, B. Studies on the reduction of iron oxide with hydrogen. Int. J. Hydrogen Energy 1982, 7, 951–955. [Google Scholar] [CrossRef]
- Jung, S.S.; Lee, J.S. In-situ kinetic study of hydrogen reduction of Fe2O3 for the production of Fe nanopowder. Mater. Trans. 2009, 50, 2270–2276. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chen, Y.-W.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar]
- Lee, G.Y.; Song, J.l.; Lee, J.S. Reaction kinetics and phase transformation during hydrogen reduction of spherical Fe2O3nanopowder agglomerates. Powder Technol. 2016, 302, 215–221. [Google Scholar] [CrossRef]
- Colombo, U.; Gazzarrini, F.; Lanzavecchia, G. Mechanisms of Iron Oxides Reduction at Temperatures below 400 °C. Mater. Sci. Eng. 1967, 2, 125. [Google Scholar] [CrossRef]
- Viswanathan, R.P.; Viswanathan, B.; Sastri, M.V.C. Kinetics of reduction of Fe2O3 to Fe3O4 by the constant temperature differential thermal anaiysis method. Thermochim. A 1976, 16, 240–244. [Google Scholar] [CrossRef]
- Wimmers, O.J.; Arnoldy, P.; Moulijn, J.A. Determination of the Reduction Mechanism by Temperature-Programmed Reduction: Application to Small Fe2O3 Particles. J. Phys. Chem. 1986, 632, 1331–1337. [Google Scholar]
- Tiernan, M.J.; Barnes, P.A.; Parkes, G.M.B. Reduction of Iron Oxide Catalysts: The Investigation of Kinetic Parameters Using Rate Perturbation and Linear Heating Thermoanalytical Techniques. J. Phys. Chem. B 2001, 105, 220–228. [Google Scholar] [CrossRef]













| Iron Oxide | Activation Energy, kJ/mol | Reduction | Temperature Range, °C |
|---|---|---|---|
| Granulated Fe2O3 nanopowder [23] α-Fe2O3 → Fe2O3 → Fe | 75–125 | Hydrogen reduction, Isoconversional approach | 320–500 |
| Ball milled Fe2O3 nanopowder agglomerates [21] α-Fe2O3 → Fe2O3 → Fe | 20–46 | Hygrometry method, Hydrogen reduction Isoconversional approach | 270–580 |
| Both natural and laboratory iron oxide α-Fe2O3 →Fe3O4 [24] | 108 | Hydrogen reduction | 250–400 |
| 99.8% pure iron oxide α-Fe2O3 → Fe3O4, [19] | 76 | Hydrogen reduction | 220–683 |
| α-Fe2O3 → Fe3O4, [19] | 95 | H2–N2 | 337–604 |
| α-Fe2O3 → Fe3O4, [19] | 114 | CO | 265–482 |
| Fe3O4 → Fe, [19] | 39–88 | Hydrogen reduction | 220–683 |
| Fe3O4 → Fe, [19] | 36–103 | H2–N2 | 337–604 |
| Fe3O4 → Fe, [19] | 40–114 | CO | 265–482 |
| α-Fe2O3 →Fe3O4, [22] | 90 | Temperature programmed reduction | 230–380 |
| Fe3O4 →Fe, [22] | 70 | Temperature programmed reduction | 330–730 |
| Iron oxide nanopowder α-Fe2O3 → Fe3O4, this work | 105–120 | Hydrogen reduction Isoconversional approach | 230–430 |
| Fe3O4 → Fe, this work | 45–55 | Hydrogen reduction Isoconversional approach | 430–680 |
| Reaction | Activation Energy, Isoconversional Approach, kJ/mol | Activation Energy, Kissinger Approach, kJ/mol |
|---|---|---|
| Fe nanopowder surface oxide reduction (40–60 nm) | 118–65 | 62–70 |
| Fe nanopowder surface oxide reduction (60–80 nm) | 115–71 | 78–84 |
| Fe2O3 nanopowder, Fe2O3 → Fe3O4 | 105–120 | 109–117 |
| Fe2O3 nanopowder, Fe3O4 → Fe | 55–45 | 45–55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manchili, S.K.; Wendel, J.; Hryha, E.; Nyborg, L. Analysis of Iron Oxide Reduction Kinetics in the Nanometric Scale Using Hydrogen. Nanomaterials 2020, 10, 1276. https://doi.org/10.3390/nano10071276
Manchili SK, Wendel J, Hryha E, Nyborg L. Analysis of Iron Oxide Reduction Kinetics in the Nanometric Scale Using Hydrogen. Nanomaterials. 2020; 10(7):1276. https://doi.org/10.3390/nano10071276
Chicago/Turabian StyleManchili, Swathi K., Johan Wendel, Eduard Hryha, and Lars Nyborg. 2020. "Analysis of Iron Oxide Reduction Kinetics in the Nanometric Scale Using Hydrogen" Nanomaterials 10, no. 7: 1276. https://doi.org/10.3390/nano10071276
APA StyleManchili, S. K., Wendel, J., Hryha, E., & Nyborg, L. (2020). Analysis of Iron Oxide Reduction Kinetics in the Nanometric Scale Using Hydrogen. Nanomaterials, 10(7), 1276. https://doi.org/10.3390/nano10071276






