Synthesis and Electrochemical Study of Three-Dimensional Graphene-Based Nanomaterials for Energy Applications
Abstract
:1. Introduction
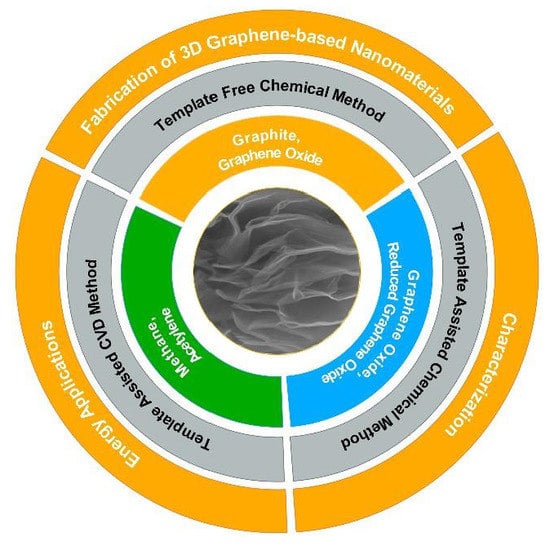
2. Fabrication of 3D Graphene-Based Nanomaterials
2.1. Template-Assisted Chemical Vapor Deposition Method
2.2. Template-Assisted Chemical Method
2.3. Template-Free Chemical Method
3. Surface Characterization of 3D Graphene-Based Nanomaterials
3.1. Morphology
3.2. Structural Characterization
4. Electrochemical Applications of 3D Graphene-Based Nanomaterials
4.1. Electrochemical Energy Storage Application
4.2. Electrochemical Energy Conversion Applications
4.2.1. Oxygen Reduction Performance of N-IC-rGO
4.2.2. Oxygen Reduction Performance of Pd-N-IC-rGO
5. Summary and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of grapheme. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azadmanjiri, J.; Srivastava, V.K.; Kumar, P.; Nikzad, M.; Wang, J.; Yu, A. Two- and three-dimensional graphene-based hybrid composites for advanced energy storage and conversion devices. J. Mater. Chem. A 2018, 6, 702–734. [Google Scholar] [CrossRef]
- Mao, B.; Sidhureddy, B.; Thiruppathi, A.R.; Wood, P.C.; Chen, A. Efficient dye removal and separation based on graphene oxide nanomaterials. New J. Chem. 2020, 44, 4519–4528. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; van der Zalm, J.; Chen, A. Graphene-oxide-based electrochemical sensors for the sensitive detection of pharmaceutical drug naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, T.S.; Panesar, D.K.; Sidhureddy, B.; Chen, A.; Wood, P.C. Nano-cement composite with graphene oxide produced from epigenetic graphite deposit. Compos. Part. B Eng. 2019, 159, 248–258. [Google Scholar] [CrossRef]
- Manikandan, V.; Sidhureddy, B.; Thiruppathi, A.; Chen, A. Sensitive Electrochemical Detection of Caffeic Acid in Wine Based on Fluorine-Doped Graphene Oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Min, S.H.; Gu, M.; Jung, Y.K.; Lee, W.; Lee, J.U.; Seong, D.G.; Kim, B.-S. Layer-by-Layer Assembly for Graphene-Based Multilayer Nanocomposites: Synthesis and Applications, Chem. Mater. 2015, 27, 3785–3796. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Liu, L.; Yao, S.; Wu, W.; Wang, Z.; Lv, P.; Zheng, J.; Yu, K.; Wei, W.; et al. Plasma enabled Fe2O3/Fe3O4 nano-aggregates anchored on nitrogen-doped graphene as anode for sodium-ion batteries. Nanomaterials 2020, 10, 782. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.-Y.; Wei, C.-Y.; Lin, M.-C.; Pan, C.-J.; Chou, H.-L.; Chen, H.-A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M.; et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Huang, S.-K.; Liao, H.-J.; Chen, Y.-M.; Wang, S.-W.; Kao, Y.-T.; An, J.-Y.; Lee, Y.-C.; Chuang, C.-H.; Huang, Y.-C.; et al. Insights into dynamic molecular intercalation mechanism for Al-C battery by operando synchrotron X-ray techniques. Carbon 2019, 146, 528–534. [Google Scholar] [CrossRef]
- Zhu, G.; Angell, M.; Pan, C.-J.; Lin, M.-C.; Chen, H.; Huang, C.-J.; Lin, J.; Achazi, A.J.; Kaghazchi, P.; Hwang, B.-J.; et al. Rechargeable aluminum batteries: Effects of cations in ionic liquid electrolytes. RSC Adv. 2019, 9, 11322–11330. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-S.; Patil, S.B.; Kao, Y.-T.; An, J.-Y.; Lee, Y.-C.; Lai, Y.-H.; Chang, C.-K.; Cheng, Y.-S.; Chuang, Y.-C.; Sheu, H.-S.; et al. Real-Time Observation of Anion Reaction in High Performance Al Ion Batteries. ACS Appl. Mater. Interfaces. 2020, 12, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lee, D.H.; Lee, W.W.; Park, W.I. Direct Synthesis of Graphene Meshes and Semipermanent Electrical Doping. J. Phys. Chem. Lett. 2013, 4, 2099–2104. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Sun, Z.; James, D.K.; Tour, J.M. Graphene Chemistry: Synthesis and Manipulation. J. Phys. Chem. Lett. 2011, 2, 2425–2432. [Google Scholar] [CrossRef]
- Fan, Z.-J.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.-J.; Feng, J.; Ren, Y.-M.; Song, L.-P.; Wei, F. Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide. ACS Nano. 2011, 5, 191–198. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Huang, Y.; Zhang, F.; Yang, X.; Ma, Y.; Chen, Y. Preventing Graphene Sheets from Restacking for High-Capacitance Performance. J. Phys. Chem. C. 2011, 115, 23192–23197. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, N.; Kim, B.G.; Jung, D.S.; Im, K.; Hur, J.; Choi, J.W. Restacking-Inhibited 3D Reduced Graphene Oxide for High Performance Supercapacitor Electrodes. ACS Nano. 2013, 7, 9366–9374. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownson, D.A.C.; Figueiredo-Filho, L.C.S.; Ji, X.; Gómez-Mingot, M.; Iniesta, J.; Fatibello-Filho, O.; Kampouris, D.K.; Banks, C.E. Freestanding three-dimensional graphene foam gives rise to beneficial electrochemical signatures within non-aqueous media. J. Mater. Chem. A. 2013, 1, 5962. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Q.; Sun, Y.; Bai, H.; Shi, G. Three-Dimensional Self-Assembly of Graphene Oxide and DNA into Multifunctional Hydrogels. ACS Nano. 2010, 4, 7358–7362. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Huang, L. Carbon paper as current collectors in graphene hydrogel electrodes for high-performance supercapacitors. Nanomaterials 2020, 10, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.; Yan, P.; Tekik, H.; Elangovan, A.; Wang, J.; Lin, D.; Li, J. 3D printing of hybrid MoS2-graphene aerogels as highly porous electrode materials for sodium ion battery anodes. Mater. Des. 2019, 170, 107689. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Wang, Z.; Zhang, Y.; Tan, T.; Wang, G.; Bakenov, Z. Three-Dimensionally Hierarchical Graphene Based Aerogel Encapsulated Sulfur as Cathode for Lithium/Sulfur Batteries. Nanomaterials 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hu, C.; Song, L.; Wang, L.; Shi, G.; Dai, L.; Qu, L. Functional graphene nanomesh foam. Energy Environ. Sci. 2014, 7, 1913–1918. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.K.; Wang, M.; Kang, J.; Sun, Y.; Jung, J.W.; Kim, K.; Kim, S.M.; Nam, J.D.; Suhr, J. Experimental investigation on 3D graphene-CNT hybrid foams with different interactions. Nanomaterials 2018, 8, 694. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1408. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Guo, X.; Jin, K.; Wang, Y.; Li, L.; Zhang, Y.; Wang, Z.; Sun, L.; Zhang, T. N/S co-doped three-dimensional graphene hydrogel for high performance supercapacitor. Electrochim. Acta 2018, 278, 51–60. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Qi, P.; Liu, X.; Wei, G. Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review. Nanomaterials 2019, 9, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensafi, A.A.; Heydari-Soureshjani, E.; Rezaei, B. Three-dimensional graphene promoted by palladium nanoparticles, an efficient electrocatalyst for energy production and storage. Int. J. Hydrog. Energy. 2018, 43, 9652–9662. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Afzal, R.A.; Song, W.; An, K.S.; Farooq, A.; Park, J.Y.; Chun, S.H.; Jung, J. WS(1−x) Sex nanoparticles decorated three-dimensional graphene on nickel foam: A robust and highly efficient electrocatalyst for the hydrogen evolution reaction. Nanomaterials 2018, 8, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Liu, L.; Zhang, J.; Li, G.; Wang, B.; Zhao, J. Hole Defects and Nitrogen Doping in Graphene: Implication for Supercapacitor Applications. ACS Appl. Mater. Interfaces. 2013, 5, 11184–11193. [Google Scholar] [CrossRef] [PubMed]
- Zurutuza, A.; Marinelli, C. Challenges and opportunities in graphene commercialization. Nat. Nanotechnol. 2014, 9, 730–734. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D. Scalable production of graphene via wet chemistry: Progress and challenges. Mater. Today 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Sevilla, M.; Ferrero, G.A.; Diez, N.; Fuertes, A.B. One-step synthesis of ultra-high surface area nanoporous carbons and their application for electrochemical energy storage. Carbon 2018, 131, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Thind, S.S.; Chang, X.; Wentzell, J.S.; Chen, A. High-performance supercapacitor based on tantalum iridium oxides supported on tungsten oxide nanoplatelets. Electrochem. Commun. 2016, 67, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Chen, W.; Zhu, X.; Liu, Q. One-step preparation of one dimensional nickel ferrites/graphene composites for supercapacitor electrode with excellent cycling stability. J. Power Sources. 2018, 39, 41–48. [Google Scholar] [CrossRef]
- Dumont, J.H.; Martinez, U.; Artyushkova, K.; Purdy, G.M.; Dattelbaum, A.M.; Zelenay, P.; Mohite, A.; Atanassov, P.; Gupta, G. Nitrogen-Doped Graphene Oxide Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2019, 2, 1675–1682. [Google Scholar] [CrossRef]
- Konda, S.K.; Amiri, M.; Chen, A. Photoassisted Deposition of Palladium Nanoparticles on Carbon Nitride for Efficient Oxygen Reduction. J. Phys. Chem. C. 2016, 120, 14467–14473. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Amiri, M.; Konda, S.K.; Chen, A. Facile Synthesis of a Carbon Nitride/Reduced Graphene Oxide/Nickel Hydroxide Nanocomposite for Oxygen Reduction in Alkaline Media. ChemElectroChem 2017, 4, 997–1001. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, G.; Zhang, F. Multiple roles of graphene in heterogeneous catalysis. Chem. Soc. Rev. 2015, 44, 3023–3035. [Google Scholar] [CrossRef]
- Gnanaprakasam, P.; Jeena, S.E.; Premnath, D.; Selvaraju, T. Simple and Robust Green Synthesis of Au NPs on Reduced Graphene Oxide for the Simultaneous Detection of Toxic Heavy Metal Ions and Bioremediation Using Bacterium as the Scavenger. Electroanalysis 2016, 28, 1885–1893. [Google Scholar] [CrossRef]
- Xie, Y.L.; Zhao, S.Q.; Ye, H.L.; Yuan, J.; Song, P.; Hu, S.Q. Graphene/CeO2 hybrid materials for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II). J. Electroanal. Chem. 2015, 757, 235–242. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Xing, H.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Wang, W.; Zhang, Y.; Yang, T. Highly sensitive simultaneous determination of cadmium (II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. J. Electroanal. Chem. 2016, 760, 52–58. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Yin, H.; Hou, Y. Graphene-based nanocomposites for energy storage and conversion in lithium batteries, supercapacitors and fuel cells. J. Mater. Chem. A 2014, 2, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Han, M.; Zhao, Q.; Ren, Z.; Guo, X.; Xu, C.; Hu, N.; Lu, L. Hydrothermal synthesis of nanostructured graphene/polyaniline composites as high-capacitance electrode materials for supercapacitors. Sci. Rep. 2017, 7, 44562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, M.; Konda, S.K.; Keeler, W.; Chen, A. Superb Pseudocapacitance Based on Three-Dimensional Porous Nickel Oxide Modified with Iridium Oxide. J. Phys. Chem. C 2017, 121, 27274–27284. [Google Scholar] [CrossRef]
- Balaji, S.S.; Elavarasan, A.; Sathish, M. High performance supercapacitor using N-doped graphene prepared via supercritical fluid processing with an oxime nitrogen source. Electrochim. Acta. 2016, 200, 37–45. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 3561, 361–365. [Google Scholar] [CrossRef]
- Pruna, A.; Cárcel, A.; Benedito, A.; Giménez, E. The Effect of Solvothermal Conditions on the Properties of Three-Dimensional N-Doped Graphene Aerogels. Nanomaterials 2019, 9, 350. [Google Scholar] [CrossRef] [Green Version]
- Maouche, C.; Zhou, Y.; Li, B.; Cheng, C.; Wu, Y.; Li, J.; Gao, S.; Yang, J. Thermal treated three-dimensional N-doped graphene as efficient metal free-catalyst for oxygen reduction reaction. J. Electroanal. Chem. 2019, 853, 113536. [Google Scholar] [CrossRef]
- Jukk, K.; Kongi, N.; Matisen, L.; Kallio, T.; Kontturi, K.; Tammeveski, K. Electroreduction of oxygen on palladium nanoparticles supported on nitrogen-doped graphene nanosheets. Electrochim. Acta. 2014, 137, 206–212. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Q.; Zhang, H.; Tian, W.; Tan, Y.; Qian, W. Low content Pt nanoparticles anchored on N-doped reduced graphene oxide with high and stable electrocatalytic activity for oxygen reduction reaction. Nat. Publ. Gr. 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liao, X.; Lin, Z.; Huang, F.; Jiang, Y.; Owusu, K.A.; Xu, L.; Liu, Z.; Li, J.; Zhao, Y.; et al. 3D Nitrogen—Doped Graphene Encapsulated Metallic Nickel–Iron Alloy Nanoparticles for Efficient Bifunctional Oxygen Electrocatalysis. Chem. A Eur. J. 2020. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Lai, W.; Wang, J.; Chou, S. Ultrafine Mn3O4 Nanowires/Three-Dimensional Graphene/Single-Walled Carbon Nanotube Composites: Superior Electrocatalysts for Oxygen Reduction and Enhanced Mg/Air Batteries. ACS Appl. Mater. Interfaces. 2016, 8, 27710–27719. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gastélum, M.; Salazar-Gastélum, M.I.; Flores-Hernández, J.R.; Botte, G.G.; Pérez-Sicairos, S.; Romero-Castañon, T.; Reynoso-Soto, E.; Félix-Navarro, R.M. Pt-Au nanoparticles on graphene for oxygen reduction reaction: Stability and performance on proton exchange membrane fuel cell. Energy 2019, 181, 1225–1234. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tanabe, Y.; Qiu, H.-J.; Sugawara, K.; Heguri, S.; Tu, N.H.; Huynh, K.K.; Fujita, T.; Takahashi, T.; Tanigaki, K.; et al. High-Quality Three-Dimensional Nanoporous Graphene. Angew. Chemie. 2014, 126, 4922–4926. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Schuchardt, A.; Mishra, Y.K.; Kaps, S.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. Aerographite: Ultra lightweight, flexible nanowall, carbon microtube material with outstanding mechanical performance. Adv. Mater. 2012, 24, 3486–3490. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Beechem, T.E.; Brumbach, M.T.; Lambert, T.N.; Davis, D.J.; Michael, J.R.; Washburn, C.M.; Wang, J.; Brozik, S.M.; Wheeler, D.R.; et al. Lithographically Defined Three-Dimensional Graphene Structures. ACS Nano. 2012, 6, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Nazarian-Samani, M.; Kim, H.K.; Park, S.H.; Youn, H.C.; Mhamane, D.; Lee, S.W.; Kim, M.S.; Jeong, J.H.; Haghighat-Shishavan, S.; Roh, K.C.; et al. Three-dimensional graphene-based spheres and crumpled balls: Micro- and nano-structures, synthesis strategies, properties and applications. RSC Adv. 2016, 6, 50941–50967. [Google Scholar] [CrossRef]
- Yang, Z.; Chabi, S.; Xia, Y.; Zhu, Y. Preparation of 3D graphene-based architectures and their applications in supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tao, Y.; Zheng, X.; Luo, J.; Kang, F.; Cheng, H.-M.; Yang, Q.-H. Ultra-thick graphene bulk supercapacitor electrodes for compact energy storage. Energy Environ. Sci. 2016, 9, 3135–3142. [Google Scholar] [CrossRef]
- de Luna, M.S.; Wang, Y.; Zhai, T.; Verdolotti, L.; Buonocore, G.G.; Lavorgna, M.; Xia, H. Nanocomposite polymeric materials with 3D graphene-based architectures: From design strategies to tailored properties and potential applications. Prog. Polym. Sci. 2019, 89, 213–249. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Feng, J.; Wu, P. Graphene-Oxide-Sheet-Induced Gelation of Cellulose and Promoted Mechanical Properties of Composite Aerogels. J. Phys. Chem. C 2012, 116, 8063–8068. [Google Scholar] [CrossRef]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; de Heer, W.; Bongiorno, A.; Riedo, E. Room-temperature metastability of multilayer graphene oxide films. Nat. Mater. 2012, 11, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef]
- Sudeep, P.M.; Narayanan, T.N.; Ganesan, A.; Shaijumon, M.M.; Yang, H.; Ozden, S.; Patra, P.K.; Pasquali, M.; Vajtai, R.; Ganguli, S.; et al. Covalently Interconnected Three-Dimensional Graphene Oxide Solids. ACS Nano 2013, 7, 7034–7040. [Google Scholar] [CrossRef] [PubMed]
- Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. From graphite to interconnected reduced graphene oxide: One-pot synthesis and supercapacitor application. Chem. Commun. 2017, 53, 7828–7831. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano. 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Boopathi, S.; Narayanan, T.N.; Kumar, S.S. Improved heterogeneous electron transfer kinetics of fluorinated graphene derivatives. Nanoscale 2014, 6, 10140–10146. [Google Scholar] [CrossRef] [Green Version]
- Thiruppathi, A.R.; Sidhureddy, B.; Salverda, M.; Wood, P.C.; Chen, A. Novel three-dimensional N-doped interconnected reduced graphene oxide with superb capacitance for energy storage. J. Electroanal. Chem. 2020, 113911. [Google Scholar] [CrossRef]
- Serov, A.; Andersen, N.I.; Kabir, S.A.; Roy, A.; Asset, T.; Chatenet, M.; Maillard, F.; Atanassov, P. Palladium Supported on 3D Graphene as an Active Catalyst for Alcohols Electrooxidation. J. Electrochem. Soc. 2015, 162, F1305–F1309. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-S.; Ren, W.; Gao, L.; Liu, B.; Jiang, C.; Cheng, H.-M. Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/graphene composite paper for sodium-ion battery electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seehra, M.S.; Narang, V.; Geddam, U.K.; Stefaniak, A.B. Correlation between X-ray diffraction and Raman spectra of 16 commercial graphene–based materials and their resulting classification. Carbon 2017, 111, 380–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.P.; Kesavan, T.; Kalita, G.; Ragupathy, P.; Narayanan, T.N.; Pattanayak, D.K. On the large capacitance of nitrogen doped graphene derived by a facile route. RSC Adv. 2014, 4, 38689–38697. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Mazánek, V.; Jankovský, O.; Luxa, J.; Sedmidubský, D.; Janoušek, Z.; Šembera, F.; Mikulics, M.; Sofer, Z. Tuning of fluorine content in graphene: Towards large-scale production of stoichiometric fluorographene. Nanoscale 2015, 7, 13646–13655. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Jha, P.K.; Gupta, K.; Debnath, A.K.; Rana, S.; Sharma, R.; Ballav, N. 3D mesoporous reduced graphene oxide with remarkable supercapacitive performance. Carbon 2019, 148, 354–360. [Google Scholar] [CrossRef]
- Liu, X.; Zou, S.; Liu, K.; Lv, C.; Wu, Z.; Yin, Y.; Liang, T. Highly compressible three-dimensional graphene hydrogel for foldable all- solid-state supercapacitor. J. Power Sources. 2018, 384, 214–222. [Google Scholar] [CrossRef]
- Wang, D.; Min, Y.; Yu, Y.; Peng, B. A general approach for fabrication of nitrogen-doped graphene sheets and its application in supercapacitors. J. Colloid Interface Sci. 2014, 417, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qin, Y.; Tan, W.; Tao, Y.; Kong, Y. Smartly designed 3D N-doped mesoporous graphene for high-performance supercapacitor electrodes. Electrochim. Acta. 2017, 241, 1–9. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.-M.; Du, Y.; et al. Nitrogen-Superdoped 3D Graphene Networks for High-Performance Supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef]
- Huo, J.; Zheng, P.; Wang, X.; Guo, S. Three-dimensional sulphur/nitrogen co-doped reduced graphene oxide as high-performance supercapacitor binder-free electrodes. Appl. Surf. Sci. 2018, 442, 575–580. [Google Scholar] [CrossRef]
- Akhter, T.; Islam, M.M.; Faisal, S.N.; Haque, E.; Minett, A.I.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Self-Assembled N/S Codoped Flexible Graphene Paper for High Performance Energy Storage and Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces. 2016, 8, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lu, L.; Sun, C. Green Synthesis of Three-Dimensional MnO2 /Graphene Hydrogel Composites as a High-Performance Electrode Material for Supercapacitors. ACS Appl. Mater. Interfaces. 2018, 10, 16474–16481. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Dou, H.; Zhang, B.; Fan, W.; Wang, X. Three-dimensional graphene combined with hierarchical CuS for the design of flexible solid-state supercapacitors. Electrochim. Acta. 2017, 237, 109–118. [Google Scholar] [CrossRef]
- Zhou, R.; Han, C.; Wang, X. Hierarchical MoS2 coated three-dimensional graphene network for enhanced supercapacitor performances. J. Power Sources. 2017, 352, 99–110. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Hu, N.; Yang, C.; Li, M.; Wei, H.; Yang, Z.; Su, Y.; Zhang, Y. Three-dimensional structures of graphene/polyaniline hybrid films constructed by steamed water for high-performance supercapacitors. J. Power Sources. 2017, 342, 1–8. [Google Scholar] [CrossRef]
- Govindhan, M.; Chen, A. Simultaneous synthesis of gold nanoparticle/graphene nanocomposite for enhanced oxygen reduction reaction. J. Power Sources. 2015, 274, 928–936. [Google Scholar] [CrossRef]
- Liao, Y.; Gao, Y.; Zhu, S.; Zheng, J.; Chen, Z.; Yin, C.; Lou, X.; Zhang, D. Facile Fabrication of N-Doped Graphene as Efficient Electrocatalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces. 2015, 7, 19619–19625. [Google Scholar] [CrossRef]
- Xue, Q.; Ding, Y.; Xue, Y.; Li, F.; Chen, P.; Chen, Y. 3D nitrogen-doped graphene aerogels as efficient electrocatalyst for the oxygen reduction reaction. Carbon 2018, 139, 137–144. [Google Scholar] [CrossRef]
- Kabir, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Nitrogen-Doped Three-Dimensional Graphene-Supported Palladium Nanocomposites: High-Performance Cathode Catalysts for Oxygen Reduction Reactions. ACS Catal. 2017, 7, 6609–6618. [Google Scholar] [CrossRef]
- Chen, W.; Xu, L.; Tian, Y.; Li, H.; Wang, K. Boron and nitrogen co-doped graphene aerogels: Facile preparation, tunable doping contents and bifunctional oxygen electrocatalysis. Carbon 2018, 137, 458–466. [Google Scholar] [CrossRef]
- Chabu, J.M.; Wang, L.; Tang, F.; Zeng, K.; Sheng, J.; Walle, M.D.; Deng, L.; Liu, Y.-N. Synthesis of Three-Dimensional Nitrogen and Sulfur Dual-Doped Graphene Aerogels as an Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. ChemElectroChem 2017, 4, 1885–1890. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Z.; Wang, X.; Qiao, H.; Huang, H. Silver-modified porous 3D nitrogen-doped graphene aerogel: Highly efficient oxygen reduction electrocatalyst for Zn−Air battery. Electrochim. Acta. 2019, 302, 216–224. [Google Scholar] [CrossRef]
- Tong, X.; Chen, S.; Guo, C.; Xia, X.; Guo, X. Mesoporous NiCo2O4 Nanoplates on Three-Dimensional Graphene Foam as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 28274–28282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Z.; Wang, L.; Sun, P.; Zhang, Z.; Wang, S. Spinel MnCo2O4 Nanoparticles Supported on Three—Dimensional Graphene with Enhanced Mass Transfer as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. ChemSusChem 2018, 11, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Z.; Wang, L.; Zhang, Z.; Wang, S. ScienceDirect Spinel CoFe2O4 supported by three dimensional graphene as high-performance bi-functional electrocatalysts for oxygen reduction and evolution reaction. Int. J. Hydrogen Energy. 2018, 44, 1610–1619. [Google Scholar] [CrossRef]











| Sample | C=C (%) | C–C (%) | C–O (%) | C=O (%) | O–C=O (%) | π–π* (%) |
|---|---|---|---|---|---|---|
| Graphite | 88.71 | 10.64 | 0.00 | 0.00 | 0.00 | 0.65 |
| GO | 27.93 | 7.04 | 32.04 | 21.17 | 11.80 | 0.00 |
| IC-rGO | 54.80 | 11.70 | 15.90 | 7.80 | 9.80 | 0.00 |
| N-IC-rGO | 57.19 | 20.40 | 7.65 | 5.85 | 8.91 | 0.00 |
| Material | Method/Precursor/Doping Level | Electrolyte/Cell Configuration, 3E or 2E * | Specific Capacitance | Ref. |
|---|---|---|---|---|
| 3D mesoporous rGO | CuCl reduction mesoporous rGO | 10 wt% H2SO4 PVA gel/2E | 310 F g−1 at 1 A g−1 | [92] |
| 3D graphene hydrogel | Hydrothermal self-assembly/phytic acid | 1 M H2SO4/3E PVA- H2SO4 gel/2E | 248.8 F g−1 at 1 A g−1 170.6 F g−1 at 1 A g−1 | [93] |
| N-graphene | Supercritical fluid processing/NA | 1 M H2SO4/3E | 286 F g−1 at 0.5 A g−1 | [54] |
| N-graphene | Hydrothermal/N = 3.77 at % | 6 M KOH /3E | 243.5 F g−1 at 1 A g−1 | [91] |
| N-graphene | Hydrothermal/N = 6.85 at % | 1 M H2SO4/2E | 242 F g−1 at 1 A g−1 | [94] |
| 3D N-graphene | Hydrothermal assembly through amidation/N-9.25 at % | 1 M H2SO4/3E | 408 F g−1 at 1.0 A g−1 | [95] |
| N-super-doped rGO aerogels | Hydrothermal and annealing/ammonia gas/N = 15.8 at % | 6M KOH/3E 6M KOH/2E | 380 F g−1 at 0.6 A g−1 297 F g−1 at 0.3 A g−1 | [96] |
| N, S-graphene aerogel | Solvothermal/N = 0.32 at % | 1 M KOH/3E | 254 F g−1 at 1 A g−1 | [97] |
| N, S-graphene hydrogel | Hydrothermal/ammonia and thiourea | 6M KOH/3E PVA-KOH gel/2E | 1063 C g−1 at 1 A g−1 45 C g−1 at 1 A g−1 | [31] |
| N, S-3D graphene | Thermal treatment by self-assembly approach | 6 M KOH/3E 6 M KOH/2E | 362 F g−1 at 100 mV s−1 62.5 F g−1 at 1 A g−1 | [98] |
| MnO2/graphene hydrogel | Self-assembly using glucose via the hydrothermal method | 1 M Na2SO4/3E 1 M Na2SO4/2E | 200.6 F g−1 at 1 A g−1 132 F g−1 at 1 A g−1 | [99] |
| 3D graphene/CuS | 3D graphene prepared via CVD/solution chemistry method | 3M KOH/3E PVA-KOH gel/2E | 249 F g−1 at 4 A g−1 32 F g−1 at 1 A g−1 | [100] |
| MoS2-3D graphene network | 3D graphene from CVD/liquid phase exfoliation | 3M KOH/3E 3M KOH/2E | 1972.58 F g−1 at 1 A g−1 102.46 F g−1 at 1 A g−1 | [101] |
| 3D graphene/polyaniline hybrid | Hydrothermal method | 1 M H2SO4/3E 1 M H2SO4/2E | 1182 F g−1 at 1 A g−1 808 F g−1 at 1 A g−1 | [102] |
| IC-rGO | Streamlined Hummers method | 0.5 M H2SO4/3E | 212 F g−1 at 1.0 A g−1 | [78] |
| N-IC-rGO | Hydrothermal/NH4F/N – 4.74 at % | 0.1 M KOH/3E 0.5 M H2SO4/3E | 259 F g−1 at 5.0 A g−1 319 F g−1 at 10 A g−1 | [81] |
| Material | Method | Electrolyte | Eonset (V)/E ½ (V) @ 1600 RPM | Jl/n @ 1600 RPM | Ref. |
|---|---|---|---|---|---|
| 3D Pd/rGO | Hydrothermal/PdCl2 and glucose | 0.1 M KOH | Eonset = 0.90 V Vs RHE | Jl = −3.7 mA cm−2 | [33] |
| 3D N-graphene | Hydrothermal self-assembly (ascorbic acid)/C3N4 and urea/N = 4.94 at % | 0.1 M KOH | Eonset = 0.88 V/E1/2 = 0.81 Vs RHE | Jl = 5.21 mA cm−2/ n = ~3.4 (catalyst loading 0.1 mg cm−2) | [58] |
| 3D N-graphene aerogel | Ascorbic acid-hydrothermal assembly/Pyrolysis, Polyallylamine/N = 2.07 at % | 0.1 M KOH | E1/2 = −0.77 V Vs RHE | Jl = 5.7 mA cm−2/(catalyst loading 0.102 mg cm−2) | [105] |
| Pd−N/3D graphene | Silica template assisted/surfactant free Pd deposition/N=3.5 at %/30 wt % Pd (XPS = 2.2 at %) | 0.1 M NaOH | Eonset = 1.0 V Vs RHE | Jl = 5.8 mA cm−2/n = ~ 4 | [106] |
| N, B-graphene aerogel | Hydrothermal (NH4B5O8) | 0.1 M KOH | Eonset = −0.05 V/E1/2 = −0.2 V Vs Ag/AgCl | Jl = 5.7 mA cm−2 (catalyst loading 0.14 mg cm−2) | [107] |
| N, S-flexible 3D graphene | Thermal treatment by self-assembly approach/3-Amino- benzenesulfonic acid | 0.1 M KOH | Eonset = −0.13 V Vs Ag/AgCl | Jl = −3.72 mA cm−2 | [98] |
| 3D N-S-graphene aerogel | Hydrothermal self-assembly (ammonia and sulfur powder)/N-5.7 at %; S-4.5 at % | 0.1 M KOH | E1/2 = −0.21 V Vs Ag/AgCl | NA | [108] |
| Ag/3D N-G-Aerogel | Hydrothermal self-assembly | 0.1 M KOH | Eonset = 0.97 V/E1/2 = 0.81 Vs RHE | Jl = 5.25 mA cm−2 (catalyst loading 0.128 mg cm−2) | [109] |
| 3D N- graphene encapsulated Ni-Fe | Hydrothermal assembly/pyrrole/N-6.4 at % | 0.1 M KOH | Eonset = 0.93 V/E1/2 = 0.80 V Vs RHE | NA | [61] |
| Mn3O4 nanowire/3D graphene/SWCNT | CTAB assisted microwave irradiation method | 0.1 M KOH | Eonset = −0.048V/E1/2 = −0.15 V Vs Ag/AgCl | n = ~4 | [62] |
| NiCo2O4 on graphene foam | Ni foam template-assisted CVD/Chemical synthesis (NH4F and urea) | 0.1 M KOH | E1/2 = 0.86 V Vs RHE | Jl = 6.25 mA cm−2/n = 4 (catalyst loading 0.4 mg cm−2) | [110] |
| MnCo2O4 NP/3D graphene | Template method/Coal tar pitch/hydrothermal method | 0.1 M KOH | Eonset = 0.98 V Vs RHE/E1/2 = 0.81 /n = 4 | NA | [111] |
| Spinel CoFe2O4/3D graphene | Templated method/coal tar pitch /Hydrothermal | 0.1 M KOH | E1/2 = 0.80 V Vs RHE | n = ~4 | [112] |
| N-IC-rGO | Streamlined Hummers method/hydrothermal method (NH4F)/N = 4.74 at % | 0.1 M KOH | Eonset = −0.05 V/ E1/2 = −0.18 V Vs Ag/AgCl | Jl = 2.9 mA cm−2/n = approximately 4 (catalyst loading 0.21 mg cm−2) | This work |
| Pd-N-IC-rGO | Streamlined Hummers method/hydrothermal method (NH4F)/surfactant-free Pd deposition | 0.1 M KOH | Eonset = 0.02 V/ E1/2 = −0.16 V Vs Ag/AgCl | Jl = 3.34 mA cm−2/n = approximately 4 (catalyst loading 0.21 mg cm−2) | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiruppathi, A.R.; Sidhureddy, B.; Boateng, E.; Soldatov, D.V.; Chen, A. Synthesis and Electrochemical Study of Three-Dimensional Graphene-Based Nanomaterials for Energy Applications. Nanomaterials 2020, 10, 1295. https://doi.org/10.3390/nano10071295
Thiruppathi AR, Sidhureddy B, Boateng E, Soldatov DV, Chen A. Synthesis and Electrochemical Study of Three-Dimensional Graphene-Based Nanomaterials for Energy Applications. Nanomaterials. 2020; 10(7):1295. https://doi.org/10.3390/nano10071295
Chicago/Turabian StyleThiruppathi, Antony R., Boopathi Sidhureddy, Emmanuel Boateng, Dmitriy V. Soldatov, and Aicheng Chen. 2020. "Synthesis and Electrochemical Study of Three-Dimensional Graphene-Based Nanomaterials for Energy Applications" Nanomaterials 10, no. 7: 1295. https://doi.org/10.3390/nano10071295
APA StyleThiruppathi, A. R., Sidhureddy, B., Boateng, E., Soldatov, D. V., & Chen, A. (2020). Synthesis and Electrochemical Study of Three-Dimensional Graphene-Based Nanomaterials for Energy Applications. Nanomaterials, 10(7), 1295. https://doi.org/10.3390/nano10071295